Abstract
During four years (2006–2009), methane (CH4) emission was measured at different biomes (dry, wet grasslands, lake and lake vegetation) of mature thermokarst depression located at the most typical thermokarst terrain on the east bank of the Lena River, Central Yakutia, Russia (62°08′N, 130°30′E). To estimate total CH4 emission from the whole thermokarst depression ecosystem, CH4 emissions via plant bodies and ebullition were measured in addition to diffusive CH4 flux measurement. The lake area increased twice from 20.4 ha in 2006 to 43.3 ha in 2007 and then did not change significantly in 2008 and 2009 (46.5 and 44.4 ha, respectively). Ebullition was considered to be a minor source for CH4 emission from the lake in the studied thermokarst depression. CH4 emissions from the lake water surface and via the plant body of lake vegetation (hygrophyte and hydrophyte vegetation) were the main sources of CH4 and these increased by flooding both CH4 emission rate and area. Using spatial changes of these biomes, the annual emission of CH4 was calculated taking into account different sources of CH4. Total CH4 emission from the studied alas (63.7 ha) was 5.7, 5.2, 20.1 and 50.1 Mg carbon (C) in 2006–2009, respectively, and its difference during this period reached about 10 times. An extreme increase in CH4 emission from the lake occurred in the second year of continuous flooding (2008), which might have been caused by the decomposition of flooded organic C. So, the lake water ecosystem is the main source of CH4 in thermokarst depression controlled by the duration of flooding. Under future global climate change, thermokarst depressions in Central Yakutia have potential for lake expansion, causing significant increase in CH4 emission in the studied region.
INTRODUCTION
Regions with permafrost distribution are considered to play a significant role in the global methane (CH4) cycle because they involve a large source of CH4, such as tundra wetlands and thermokarst lakes (Zimov et al. Citation1997; Nakano et al. Citation2000; Morishita et al. Citation2003; Walter et al. Citation2006; Takakai et al. Citation2008). The Central Yakutia is situated in the East Siberia region of the Russian Federation. The main features of the region are continuous permafrost and the presence of 45% of all Russian taiga forest.
Fluctuations in thermal conditions during the early Holocene led to thermokarst formation caused by degradation of the ice wedge complex in Central Yakutia (Bosikov Citation1991). The volumetric content of the ice wedge in Central Yakutia is estimated at 50% of the total upper permafrost volume (Ivanov Citation1984). The thermokarst process is initiated with thawing of the ice-wedge complex as a result of soil heating after climate change or human activity, such as forest fire or clear-cutting (Desyatkin Citation1993). This process has four different stages, finally forming a stable thermokarst depression with a lake and grassland vegetation inside which is called “alas” (Bosikov Citation1991; Desyatkin et al. Citation2009). Approximately 16,000 alases are located in the Central Yakutia lowland, with a total area of 440,000 ha, which is approximately 17 to 30% of the total land area of the Central Yakutia lowland (Bosikov Citation1991; Suzuki et al. Citation2001). Thus, there is a risk of future thermokarst formation in the Central Yakutia lowland because about 50% of ice wedges still remain in upper permafrost (Ivanov Citation1984), and those ice wedges have a potential to thaw under the projected climate warming.
The inter-annual variation of air temperature and precipitation in Central Yakutia causes fluctuation of the lake areas in alases. In years with high precipitation, a thermokarst depression is filled by the lake water (flood) and in years with low precipitation these lakes can completely disappear. In such dry years the main feature of Central Yakutia thermokarst is the presence of grassland. Grassland area in the thermokarst depression is divided into two different belts of vegetation around the lake: wet grassland and dry grassland (Desyatkin et al. Citation2007; Citation2009). It was reported that flooded wet grasslands distributed around the lake in an alas are an important CH4 source (Takakai et al. Citation2008; Desyatkin et al. Citation2009). CH4 emission from the alas could be controlled by the level of soil moisture, and especially by flooding due to the expansion of lake water. To assess the amount of CH4 emission from the whole alas ecosystem, it is necessary to account for different sources such as emission by diffusion, via plant stem and ebullition from a lake.
The amount of CH4 emitted as bubbles from the lakes of Central Yakutia is still not clear. Takakai et al. (Citation2008) estimated CH4 emission from the whole alas ecosystem in the region based on flux measurement throughout the growing period. However, they did not include CH4 ebullition at the time of lake ice melting in spring. Some studies carried out in boreal regions reported that high values of ebullition accounted for 52–64% of total CH4 emission from the lakes (Huttunen et al. Citation2001; Walter et al. Citation2006). Therefore, CH4 emission with ebullition from the thermokarst lakes should be included for precise estimation of CH4 budget in Central Yakutia.
So, it is important to observe seasonal and inter-annual changes of CH4 flux in flooding area in the alas ecosystem. From a review of the previous literature, it is considered that the thermokarst depression CH4 budget in the Central Yakutia lowland greatly depends on the lake area change (Morishita et al. Citation2003; Takakai et al. Citation2008). The objective of the present study is to evaluate annual CH4 emission from the whole alas ecosystem and its annual fluctuation (from 2006 to 2009) related to lake area change.
MATERIALS AND METHODS
Study site
The studied thermokarst depression is located at the most typical thermokarst terrain on the east bank of the Lena River, about 50 km east of Yakutsk city (), Russia (62°08′N, 130°30′E). The grassland area in the thermokarst depression was divided into wet and dry grassland by the soil moisture and vegetation cover. Also divided was the forest edge which has shrub vegetation and dead tree remainders, the emissions from which were similar to those of a larch forest (Larix gmelini Rupr.) location and were not used for CH4 estimation. In this study, for more precise estimation of CH4 emission, the lake vegetation area was also distinguished from the lake water surface (no vegetation). The depth of the depression from the surrounding forest level to the bottom of the lake was 1680 cm, and the lake depth was 180 cm. Under the depression of this site, the ice-wedge complex is completely absent. The approximate age of this depression is 8500 years (Katamura et al. Citation2006). The soil carbon (C) storage in the topsoil (0–40 cm, including organic layer) amounted to 49.7, 9.5 and 214 Mg C ha−1 in the surrounding forest, dry and wet grasslands, respectively (Desyatkin et al. Citation2007).
The forest surrounding of all studied thermokarst is dominated by mature larch (Larix gmelinii Rupr.) with Vaccinium vitis-idaea L. on the forest floor. Poa pratensis L. and Elytrigia repens L. are the dominant vegetation of the dry grassland belt, and Alopecurus arundinaceus Poir., Beckmannia syzigachne (Steud.) Fernald and Carex orthostachys C.A.Mey. belong to the wet grassland belt (Nikolaeva and Desyatkin Citation2004).
This study was carried out from 2006 to 2009. For 100 years the annual mean temperature of this region has been –9.7°C with average temperatures in January and July of –40.7 and 19.0°C, respectively. The annual mean precipitation during the last 100 years is 223, 151 mm of which occurs as summer (May–Sept.) precipitation. The monthly average air temperature of the summer period (Yakutsk weather station) was 6.7, 15.7, 19.0, 15.1 and 6.2°C in May, June, July, August and September, respectively (). During the study years, the monthly temperature of May and June was higher than the 100-year average from 0.8°C in 2006 May and up to 3.5°C in 2009 June. In July of 2006 and 2007, it was lower than the long-term average, which was 18.6 and 16.7°C, respectively. The monthly temperature in August during all four years was higher than the long-term average, but in 2009 it was almost the same (15.2°C). The monthly temperature in September except for 2008 (5.4°C) was also higher than the 100-year average. It shows that in the warmest summer months (June, July) air temperatures were increasing over the last two years. The annual air temperature from 2006 to 2009 was –8.8, –7.0, –7.1 and –7.7°C, respectively. The gradual increase of annual air temperature in comparison with the 100-year average amounted to 0.9, 2.7, 2.6 and 2.0°C for the observation period (2006–2009).
Table 1 Annual air temperature and precipitation
Annual precipitation during the study years was decreasing (). It was 326, 288, 260 and 197 mm each year from 2006 to 2009. Maximum precipitation occurred in summer, mostly in July and August. Precipitation of the summer period (May–September) amounted to 256, 191, 146 and 131 mm for 2006, 2007, 2008 and 2009, respectively. The long-term average (100 years) of summer precipitation is 151 mm for Central Yakutia. In this connection, 2008–2009 had a deficiency of precipitation during these years in the summer periods.
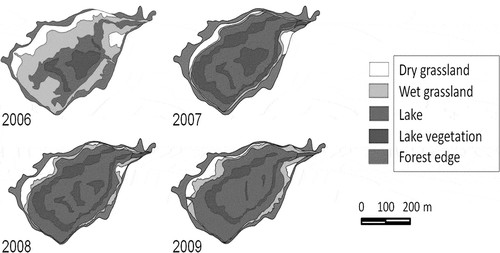
During four years (2006–2009), the field survey was conducted to estimate the spatial distribution of biomes inside the thermokarst depression using theodolite (2T30P, UOMZ, Russia) at the beginning of each July from 2006 to 2009 (). July was chosen because at this time the difference in vegetation cover can be observed most clearly among the biomes. Total area of the studied depression was 63.7 ha including dry, wet grasslands and lake area. Total depth of the depression was about 16.8 m including 1.8 m of lake depth. The lake area inside the depression is a controlling factor for allocation of two grasslands. The minimum area of the lake was observed in the first year (July of 2006) and it was 20.4 ha. In subsequent years, lake area was affected by precipitation and increased by almost two times. It became 43.3, 46.5 and 44.4 ha in 2007, 2008 and 2009, respectively. Consequently, the area of dry and wet grasslands significantly changed, especially in the second year (2007). The area of dry grassland decreased gradually from 19.2 to 10.7 ha during study years, because of the expansion of the lake and wet grassland. Wet grassland at the beginning of measurement in 2006 occupied 24.1 ha, and, after expansion of the lake area in 2007, abruptly decreased to 3.3 ha. In 2008, when soil moisture increased and was stabilized due to lateral flow, the wet grassland expanded to 6.2 ha. In 2009, the area of wet grassland increased further and reached 8.7 ha, while the dry grassland area decreased (). These floods occur during years with high precipitation and have a temporary character, and during the years with precipitation less than the long-term average the lake size is significantly reduced. So, the dynamics of the lake area has a cyclical character.
Table 2 Inter-annual fluctuation of biome spatial distributions in the studied thermokarst depression
Inside the lake, there was growing hygrophyte (Alopecurus arundinaceus, Beckmannia syzigachne, Carex orthostachys) and hydrophyte (Glyceria triflora (Korsh.), Eleocharis palustris (L.), Bolboschoenus compactus (Hoffm.) T.V. Egorova) vegetation which makes a large contribution to CH4 emission via stems. In 2006, their area was 10.2 ha, and with the lake expanding, in 2007, it increased to 26.0 ha due to flooded wet grassland hygrophyte vegetation. In 2008 and 2009, the lake area did not have a large fluctuation, and the area occupied by lake vegetation decreased slightly due to a reduction of the hygrophyte vegetation ().
CH4 emission from whole alas ecosystem
For the estimation of net CH4 emission, the different sources were observed, because the CH4 produced in flooded soils and sediments can migrate to the flood surface and be emitted into the atmosphere by one of three different pathways. First, diffusion can take place in solution towards the surface, in the course of which a substantial proportion of the CH4 is oxidized (Bastviken et al. Citation2002; Liikanen et al. Citation2002; Kankaala et al. Citation2004). In our studied alas, the fluxes by diffusion were measured at all locations (forest, dry and wet grasslands and lake water surface). Second, sufficient gas may be produced for bubbles to form in the water layer and force their way to the surface, which is the process of ebullition. The speed of this process prevents any significant oxidation (Conrad Citation1989). Ebullition was measured at the lake surface. The third route is through aerenchyma, which is the continuous air spaces of vascular plants adapting to life in flooded environments (Thomas et al. Citation1996; Terazawa et al. Citation2007; Gauci et al. Citation2010). These structures have evolved to transport oxygen that is necessary for root respiration and cell division, but also serve as channels for the transport of CH4 from the root environment to the atmosphere. Emission of CH4 via plant stems was measured at the wet grassland and lake, because CH4 production occurred only in wet or flooded soil.
CH4 flux measurement
The CH4 flux from each biome (surrounding forest, dry and wet grasslands and lake water surface) was measured by the closed chamber method. Diffusion CH4 flux from soil and water surfaces was measured during the growing season (once a month from May to September) during four years (2006–2009). For the measurement, six cylindrical stainless-steel chambers with detachable lids, 25 cm in height and 18.5–21.0 cm in diameter, were used. To prevent pressure affecting the inside of the chamber, a plastic bag which has a direct connection to the outside air was used for pressure compensation. The chambers were installed to a depth of 3 cm into the soil after removing the vegetation and remained in place overnight to eliminate any fluctuations resulting from the disturbance. The next day, a 20-mL gas sample was taken into a 10-mL vacuum vial sealed with a butyl rubber stopper (SVF-10; Nichiden-Rika, Kobe, Japan) at time intervals of 0, 30 and 60 min after the chamber was covered with the lid. At the pond surface plots, the CH4 flux was measured using floating chambers equipped with floats made of polystyrene foam (Takakai et al. Citation2008). The air temperature at a height of 1 m was measured with a thermistor thermometer (CT-410W; Custom, Tokyo, Japan) during the flux measurements. There were three replicates for each flux measurement. Additionally, CH4 flux was measured in April and October (2008 and 2009, respectively) to evaluate the surface flux during the snow cover and frozen soil period.
To estimate CH4 emission via plant bodies on the wet grassland and in the lake, the CH4 emission from the hygrophyte and hydrophyte vegetation area was also measured except in 2006, because of the absence of the transparent acryl chambers in that year. These grasses were distributed with high spatial variation during the observation period. Measurement of CH4 emission via plant bodies could not be measured in May and September due to grass fading. Consequently, at these sites, measurement of CH4 via plant bodies was conducted during the three months June, July and August, and all plants inside the chamber were retained during the measurement. This CH4 flux was determined as whole ecosystem (including both soil and plants) flux. The transparent acryl chambers (30 × 30 cm × 60 cm) in three replications for each location were used for CH4 flux measurement. The time interval of sampling was 0, 10 and 20 min after inserting the chamber into the collar and closing the top. During sampling, the temperature inside the chamber was measured. A detailed description of this method was given in Takakai et al. (Citation2008) and Desyatkin et al. (Citation2009).
Using the difference between the results of CH4 emission from the whole ecosystem (transparent chamber) and CH4 emission from the soil/water surface (stainless-steel chamber), CH4 emission via the plant body was estimated as reported for a similar ecosystem (Takakai et al. Citation2008).
CH4 concentration was analyzed using a gas chromatograph (GC-8A, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector. The minimum detectable concentration of CH4 was ± 0.1 ppmv. The CH4 flux was calculated by a linear regression of three samples as follows:
Because of the small number of measurements, the cumulative CH4 flux was calculated from the flux values as follows:
in which Fi is the mean gas flux (kg C ha−1 d−1) between two sampling times (i.e., for time interval i), Di is the number of days in the sampling interval, and n is the number of sampling times.
Ebullition
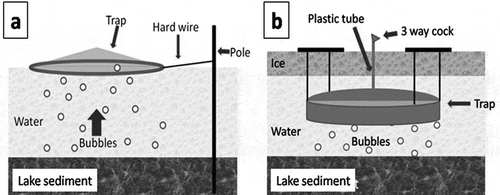
Estimation of CH4 ebullition from this thermokarst depression lake was carried out using bubble traps. Traps were set up at three locations from the edge across the middle to the center of the lake, along a transect, with three replications for winter measurement and five replications for summer measurement, respectively.
Winter traps were fixed in the lake water at a depth of 50 cm at the edge of the lake, at a depth of 100 cm in the middle of the lake, and at a depth of 150–250 cm at the center of the lake. These depths were chosen to prevent the trap from coming into contact with the lake sediment, because the ice depth in winter can reach more than 1 m. Winter ebullition was measured during the 2008–2009 winter season (from October to April; 210 days). At the beginning of October 2008, the basin-shaped bubble traps with a volume of 34 L (diameter: 54 cm, depth: 15 cm) were set up and fixed under the ice cover in three replications. To prevent sediment disturbance, they were hung up using hard wires at 20 cm above the lake sediment (). To take gas samples from under ice, a plastic tube with a three-way cock was attached to the top of the trap. The trap-tube system was filled with water by letting the air flow out through the stopcock. After that, helium gas was injected in the tube to prevent ice formation inside the tube. However, at the edge and middle locations, the trap was entirely frozen without any headspace in water during winter (ice depth was 1 m). Therefore, air samples were taken only from the traps in the center of the lake, and bubble volume and its CH4 concentration in the ice from the trap was measured at the edge and middle locations in April 2009. A cubic block of ice, 10 × 10 × 10 cm, was taken from the center of each given ice thickness, and placed in a thick plastic bag with double fastener (Ziplock pack, L size 26.8 × 27.9 cm). The plastic bag was equipped with a cock with rubber sealant for complete removal of air from inside. After melting of the sampled ice block, a 20-mL sample of headspace air was taken using a syringe into a 10-mL vacuum vial (SVF-10; Nichiden-Rika, Kobe, Japan), and the air volume inside of the plastic bag was measured. Using this volume of air content in ice, taking the air pressure in the bubbles as 1 atm and the density of CH4 (0.717 kg m−3), the total amount of stored CH4 in the ice block was calculated for each sample. Then, CH4 storage in ice per area was calculated by taking ice thickness into account.
Summer ebullition was measured during 7 d in August 2009. Cumulative CH4 emission via ebullition during the warmest three months (June, July and August; 90 days) was estimated by extrapolating the obtained data for all 90 days. Circular floating bubble traps (plastic hoop and polyethylene) with an area of 0.64 m2 (0.9 m in diameter) were installed at the same locations as those for winter measurement (edge, middle and center). Traps were fixed using hard wire at a distance of 50–70 cm from the anchor pole to prevent the collection of bubbles released by movements of the anchor in windy weather (). Seven days after the trap setting, air volume of summer ebullition samples was measured using a 50-mL syringe, and 20 mL of gas sample was taken into a 10 mL vacuum vial. Concentration of CH4 was estimated by the same method of analysis with the winter ebullition. Then, CH4 emission via ebullition per area was calculated by multiplying area of trap, averaged air volume and CH4 concentration.
In total, those two types of measurements covered 10 months of the year. CH4 emission during the remaining two months (May and September) was estimated by using the averaged summer and winter daily ebullition rate.
Estimation of CH4 emission from the whole alas ecosystem
To estimate the total CH4 emission from the studied alas ecosystem, measurements of diffusive flux, ebullition and CH4 transport via plant bodies were combined. Each CH4 emission rate was multiplied by its biome area obtained from four years (2006–2009) of a theodolite survey. For estimation of the whole emission, the area of “wet grassland, via plant body” was included in the area of “wet grassland”. Similarly, the areas of “lake, via plant body” and “lake, ebullition” were included in the area of “lake”. CH4 emissions via plant bodies in wet grassland and lake vegetation area in 2006 were estimated using the percentage rate of three measured years’ (2007–2009) average of CH4 emission via plant bodies to diffusive CH4 emission measured in 2006. Since ebullition measurement was carried out only in 2009, the ebullition for 2006, 2007 and 2008 was estimated by multiplying the ratio of ebullition to diffusive CH4 emission measured in 2009 by diffusive CH4 emissions of each year.
Statistical analysis
The differences in CH4 fluxes between measurement periods and among ecosystems of different thermokarst depressions were compared by a two-way analysis of variance (ANOVA) using SPSS 13.0 for Windows 2004 (SPSS Inc.).
RESULTS
Cumulative CH4 emission by diffusion
Cumulative CH4 fluxes (during the growing period) in forest, grasslands and lake showed significant differences among locations and years depending on climatic factors.
In the forest location, all years (2006–2009) showed a negative value of cumulative CH4 fluxes, indicating net CH4 uptake from the atmosphere to the soil. During measurement time, the lowest CH4 uptake was observed in 2007 (–0.34 ± 0.14 kg C ha−1). The maximum CH4 uptake occurred in 2008 and 2009 it was –1.04 ± 0.18 and –0.88 ± 0.21 kg C ha−1, respectively ().
Table 3 Cumulative values of diffusion methane (CH4) flux from different locations and measurement years during the measurement period (April–October) [mean ± standard deviation (SD) of different months]
In dry grassland, both negative and positive cumulative CH4 flux was observed, but values were low. In 2008 and 2009, net CH4 emission was observed; it was 0.26 ± 0.31 and 0.15 ± 0.12 kg C ha−1, respectively. he maximum CH4 uptake was recorded in 2007 (–0.11 ± 0.11 kg C ha−1), whereas in 2006, with highest precipitation, uptake was –0.07 ± 0.07 kg C ha−1 ().
In wet grassland, cumulative CH4 flux in all years was positive, indicating net CH4 emission. The lowest emission was observed in 2006–2007 when this grassland started to flood (1.72 ± 1.31 and 2.99 ± 0.61 kg C ha−1 respectively). Cumulative CH4 flux in 2008 (the second year of flooding) abruptly increased to 397 ± 240 kg C ha−1, but in 2009 decreased to 4.89 ± 0.81 kg C ha−1 ().
In the lake location, the cumulative CH4 emission was stably high among measured locations during the four studied years. However, the maximum value was lower than in wet grassland (in 2008). But, in other years, CH4 emission was considerably higher than that in wet grassland. During four years, the lowest cumulative CH4 flux was observed in 2007 (56.8 ± 44.9 kg C ha−1) (). After the lake expanded and occupied wet grassland territory, high CH4 emissions were observed in the second and third years of flooding, 2008–2009 (284 ± 169 and 250 ± 195 kg C ha−1, respectively).
CH4 emission via plants
The whole ecosystem cumulative CH4 flux of wet grassland shown in was much higher than the CH4 emission from soil, except for in 2008. Cumulative CH4 emission via plant bodies in 2007 accounted for 82.8% of the whole ecosystem cumulative flux. But in 2008, it became negative (–84.3 kg C ha−1), probably because of the late response of organic matter decomposition to flooding. Also, the standard deviation (SD) value of soil and CH4 emission from the whole ecosystem was considerably high, and this negative value of CH4 emission via plant bodies specifically does not indicate uptake because it is within the error range. In 2009, emission via plant bodies accounted for 85.4% (28.59 kg C ha−1) of the whole ecosystem emission ().
Table 4 Cumulative methane (CH4) flux via plant body and its contribution to the whole ecosystem CH4 emission [mean ± standard deviation (SD)]
At water locations, the cumulative flux of the whole ecosystem was much higher than that in wet grassland (). The lowest (143 ± 66 kg C ha−1) and the highest (2406 ± 1018 kg C ha−1) CH4 emissions were observed in 2007 and 2009, respectively. In 2008, the cumulative flux of the whole ecosystem was 458 ± 500 kg C ha−1.
Cumulative CH4 emission via plant bodies ranged from 86.5 (2007) to 2155.2 kg C ha−1 (2009). The contribution of emission via plant bodies to the whole ecosystem flux, ranged from 38.1% to 88.4% ().
Emission of CH4 via plant bodies was significant in both stages. In total, during the considered years (2007–2009), wet grassland emission via plant bodies ranged from 82.8% up to 85.4% of the whole ecosystem flux (–27.0% of 2008 was not considered because it was not uptake) (). In lake water locations, the contribution to CH4 emission via plant bodies ranged from 38.1% to 88.4% of the whole ecosystem for the observed years.
CH4 ebullition
The concentration of CH4 in bubbles was similar during winter and summer and ranged between 3.7 ± 2.3 and 14.6 ± 5.3 g C m−3. In winter, CH4 concentration was more stable than in summer and there was no significant difference among the locations. The summer ebullition rate was averaged as 282.9 ± 2.2, 282.9 ± 2.1 and 237.1 ± 2.4 mL m−2 day of bubbles in the traps for edge, middle and center locations, respectively (). In winter, the bubble content in the traps of the center location was averaged as 7.5 ± 2.6 mL m−2 day. At the edge and middle locations, bubbles in ice inside the traps occupied 30–40% of total ice volume and ebullition rates were averaged as 20.9 ± 0.1 and 13.6 ± 0.1 mL m−2 day ().
Table 5 Averaged methane (CH4) concentration in bubble and air volume in traps during winter and summer times of measurement and emission from lake water surface via ebullition [mean ± standard deviation (SD)]
CH4 ebullition from the studied lake was calculated using CH4 content in one trap and trap area. The average of the edge, middle and center locations from this thermokarst lake was 4.1 ± 1.1 kg C ha−1, during the summer period (three months) ().
Winter ebullition at the center of lake, which was not influenced by freezing disturbance, did not show significant difference among the different traps (). The average of ebullition at the center of lake in winter was 3.24 ± 2.6 kg C ha−1 for seven months. Winter CH4 emission at the edge and middle of the lake was 5.11 kg C ha−1 on average. The average of the three locations is 4.5 ± 1.1 kg C ha−1. For the remaining two months, the average was 1.3 kg C ha−1. It was calculated as the monthly average value of measured and extrapolated data. The cumulative annual C emission through bubbles during 365 days amounted to 9.9 ± 1.6 kg C ha−1 ().
Total CH4 emission from the whole alas ecosystem
Using the spatial structure of this thermokarst depression (dry and wet grasslands, lake and lake vegetation areas), emission and uptake of CH4 in the ecosystems of the depression, the CH4 budget was calculated and the results are shown in . Although the dry grassland area during the four years was reducing, the cumulative flux from uptake in 2006 and 2007 turned to emission in 2008 and 2009. High CH4 emissions from the wet grasslands or from the lake surface were observed compared with the emissions from the dry grassland. The wet grassland area showed an abrupt decrease from 2006 to 2007 (). This was the main reason for total CH4 emission dropping from 0.0415 to 0.0098 Mg C between 2006 and 2007. There was extremely high emission in the wet grassland in 2008 (2.444 Mg C), whereas the area increased only to 6.2 ha. In 2009, the area of wet grassland increased to 8.7 ha and CH4 emission decreased to 0.0423 Mg C.
Table 6 Emission of methane (CH4) from thermokarst depression during 2006–2009. Different location values given in Mg carbon (C) area−1 (%: contribution of total emission)
The amount of CH4 emission from the lake increased from 2.85 and 2.46 Mg C in 2006 and 2007 to 13.17 and 11.11 Mg C in 2008 and 2009, respectively. A slight decrease of CH4 emission from the lake in 2009 occurred owing to the decrease in lake area. CH4 emission via plant bodies was measured from 2007 to 2009. Taking into account the lake and vegetation area, CH4 emission via plant bodies increased from 2.25 to 4.06 and 38.25 Mg C from 2007 to 2009. The average ebullition measured in this lake in 2009 (0.0098 Mg C ha−1) was used for the estimation of total CH4 emission from this thermokarst depression during the four considered years (2006–2009). The amount of CH4 ebullition was 0.199 Mg C in 2006, then, with the increase of lake area, it increased to 0.422, 0.453 and 0.433 Mg C in 2007, 2008 and 2009 respectively (). Total CH4 emission (including grasslands and ebullition) from this depression was 5.7, 5.2, 20.1 and 50.1 Mg C in 2006–2009, and its difference during the year reached about 10 times.
DISCUSSION
CH4 flux
CH4 flux in the studied region is considerably different between measured biomes (), and each biome has its own reaction to environmental factors such as temperature, moisture etc.
Inside the thermokarst depression, soil moisture was the lowest in the dry grassland location at a relatively elevated place, owing to the drainage of soil water towards the lower parts, and evaporation. Within the four years of observation, the maximum values were observed in May (0.33 m3 m−3) just after snow melting. Then, during the summer period depending on monthly precipitation, it ranged from 0.02 m3 m−3 in dry months of 2006 up to 0.21 m3 m−3 in months exceeding the long-term average of the following years. On average, during the measurement period, the soil moisture of the dry grassland was 0.13 m3 m−3. Wet grassland soil moisture was considerably higher and ranged from 0.14–0.41 m3 m−3 with an average of 0.34 m3 m−3. Consequently, CH4 consumption exceeded CH4 production under aerobic conditions on the dry grassland.
In the lake location, the area of the lake was abruptly increased in 2007 due to high precipitation in 2006 and the area was maintained until 2009 despite the decreased annual precipitation after 2006 (). This flooding might promote a large supply of fresh organic matter for CH4 production in the flooded wet grassland under anaerobic conditions.
Emission of CH4 by flooded aquatic vegetation goes to the atmosphere through the plant’s body. Plant-mediated transportation of CH4 is faster than diffusion through the water column and, therefore, the oxidation of CH4 is much lower (Dacey and Klug Citation1982; Harris and Bartlett Citation1983) than non-plant-mediated transportation of CH4 in water. Temperature is one of the most important factors affecting CH4 emissions (Kankaala and Bergstrom Citation2004; Kankaala et al. Citation2005; Xing et al. Citation2005). Increasing temperature and consequent increase of microbial activity can enhance CH4 transport from peatlands by promoting ebullition through an increase in bubble volume and repartition of CH4 to the gaseous phase (Fechner-Levy and Hemond Citation1996), as well as promoting plant-mediated transport through greater pressurized ventilation and consequently CH4 bulk flow in the aerenchyma (Grosse Citation1996). Therefore, the highest CH4 transportation via plant bodies from the wet grassland vegetation was apparently observed in July–August (Desyatkin et al. Citation2009). For boreal regions, the second important factor is vegetation growing time, due to the extremely short summer period. For example, vegetation in observed locations might not grow enough in June, because of oppressed conditions after flooding by snowmelt water, and the cold water temperature. Among three measured months (June, July and August), CH4 emission via plant bodies was highest in August, because the maximum biomass of aquatic vegetation grows at this time (Desyatkin et al. Citation2009). It is reported that in dense vegetation stands in lake areas from tropic to boreal regions, about 90% of the CH4 released from the sediment is transported through aerenchymal tissues of emergent and floating-leaved plants (Chanton and Dacey Citation1991; van der Nat et al. Citation1998; Kankaala et al. Citation2004; Gauci et al. Citation2010). In this study, we observed lower emission via plant bodies because of the short vegetative period and comparatively low vegetation coverage than in warmer temperate and south boreal lakes. The maximum transportation of CH4 via plant bodies from the lake of studied alas accounted for 88% of the whole emission in 2009 (). Previous years had lower contributions: 60% and 38% for 2007 and 2008, respectively. The second year of flooding is the year of vegetation species change, so flooded hygrophytes turn to hydrophytes. It can be explained that after growing of hydrophytes, the CH4 transport via plant bodies inside of the lake was increased in 2009. Global climate change scenarios are predicting an increase of annual mean temperatures (IPCC-ACIA models Citation2007); this increase will be higher in spring and autumn than in summer (IPCC-ACIA models Citation2007). Consequently, in this region, the growing period will be prolonged, and this can increase CH4 emission significantly. The warming climatic conditions can also promote the expansion of lakes and prolong the flooding period, which will accelerate CH4 production and change vegetation types that contribute greatly to CH4 emission of the whole ecosystem.
Ebullition
When CH4 was produced at high rates in the sediments at the bottom of lake, ebullition was an important mechanism transporting CH4 from the sediments. Among factors affecting the intensity of CH4 ebullition from the sediments, there is a reduction of hydrostatic pressure due to decreasing water level and air pressure (Mattson and Likens Citation1987; Jakobsen et al. Citation1997; Dove et al. Citation1999). These factors can be significant in controlling the CH4 ebullition due to large fluctuations of lake depth and size in thermokarst lakes of Central Yakutia. The results of this study showed that the highest emission of bubbles occurs at the shallow places of the studied lake. Some studies reported the same tendency (Bastviken et al. Citation2008; Flury et al. Citation2010). As the lake water was shallow at the lake edge, bubbles released from sediments were emitted easily through the shallow water to atmosphere. Walter et al. (Citation2006) reported that the annual CH4 flux from a lake of north east Siberia was 24.9 ± 2.3 g CH4 m−2 yr−1 (0.187 Mg C ha−1 yr−1), when background, point-source and hotspot ebullition fluxes were aggregated on a whole-lake basis. Zimov et al. (Citation1997) reported a CH4 ebullition of 5.4 g CH4 m−2 yr−1 (0.0027 Mg C ha−1 yr−1) from two Siberian lakes which were the same lakes as those observed by Walter et al. (Citation2006). In Switzerland, ebullition from littoral marsh on the eastern shore of Lake Hallwil is estimated to be 20.5 g C m−2 yr−1 (0.250 Mg C ha−1 yr−1) (Flury et al. Citation2010). The ebullition of three northern temperate lakes in WI, USA, ranged from 4.3 to 16.4 mg CH4 m−2d−1 (up to 0.0075 Mg C ha−1 period−1) during an 82-d summer period (Bastviken et al. Citation2008). In the northern boreal lakes of Finland, the CH4 ebullition rate was 36–46 mg CH4 m−2 day−1 (0.098–0.126 Mg C ha−1 yr−1) (Huttunen et al. Citation2001). In tropical artificial water reservoirs with 10 m depth, the ebullition rate was estimated as 0.0030–0.0070 Mg CH4 km−2 d−1 (0.008–0.0019 Mg C ha−1 yr−1) (Gunkel Citation2009). Compared to these previously reported values, the value obtained in this study (0.0075–0.00115 Mg C ha−1 yr−1) belongs to the lower range. Compared to other CH4 sources, CH4 emission via ebullition was considered an insignificant source in the studied thermokarst depression.
Another question is the source of CH4 in bubbles; Zimov et al. (Citation1997) reported that a large proportion of CH4 emission from thaw lakes in North Siberia was derived from Pleistocene-aged organic C stored in the permafrost, and the old C was supplied by erosion on the perimeter of the thaw lakes. In Central Yakutia, the mature thermokarst depressions (i.e., alas) have a very slow speed of erosion. Besides, the CH4 content in the permafrost of this region was very low as reported previously (Desyatkin et al. Citation2009), and makes no significant contribution to emission. Therefore, the CH4 emitted from this alas should be produced mainly from new organic C from the residual decomposition of vegetation that has recently died.
Fluctuation of thermokarst spatial structure and its effect on CH4 emission
The total area of studied thermokarst depression is 63.7 ha including dry and wet grasslands and lake area. The lake area inside the depression controlled the factor for allocation of two grasslands. The lake area in this thermokarst depression greatly increased in 2007 and was continuously flooded in 2008 and 2009. Consequently, the area of dry and wet grasslands significantly decreased, especially in 2007. Inside the lake, growing grassy vegetation contributed a great share of CH4 emission via stems. Their area greatly increased in 2007, but slightly decreased in 2008 and 2009 due to the reduction of hygrophyte vegetation ().
In thermokarst depressions of Central Yakutia, flooding is an important factor in controlling CH4 emission (Morishita et al. Citation2003; Takakai et al. Citation2008). The total CH4 emission from the whole alas ecosystem in 2007 (first year of flooding) was not as high as that in 2006, while the emission increased abruptly in 2008 and 2009 (second and third year of flooding) owing to the high CH4 emission rate in the wet grassland and lake locations. Those high CH4 emissions in 2008 and 2009 might be due to the change of vegetation type, which provided larger organic C input. However, the change of vegetation and flooding in the wet grassland did not cause large CH4 emission during the first year just after flooding in 2007. According to our assumptions, that happened because the plant residues did not decompose due to the low temperature in winter. Then, in the second year of flooding (2008), the accumulated plant residues must have decomposed anaerobically in summer. In addition, summer (June and July) air temperatures in 2008 and 2009 were higher than those in 2006 and 2007. The high temperature also could enhance organic matter decomposition under the flooding condition in 2008 and 2009. Besides, the development of soil anaerobic conditions by continuation of flooding could be also an important factor in the large CH4 emission in 2008 and 2009. It was reported that in a cool temperate region in Japan, CH4 emission from a paddy field which was converted from upland field was low in the first year after conversion and increased in the second and third year due to the development of anaerobic conditions by continuous flooding (Kumagai and Konno Citation1998). A similar phenomenon could occur in the newly flooded area by lake expansion in this study. The decrease in CH4 emission from the wet grassland in 2009 might be caused by a decrease in soil moisture after flood water recession at the measurement plot.
As mentioned above, the extreme increase of CH4 emission occurred in the second year of continuous flooding (2008), which is promoted by decomposition of previously accumulated organic C in wet grassland vegetation. Thus, the lake water ecosystem was the main source of CH4 in the thermokarst depression controlled by the duration of flooding. Under future global climate change, it is important to take into account that thermokarst depressions in Central Yakutia which occupy 440,000 ha and have the potential to expand.
CONCLUSION
The lake inside the thermokarst depression was the main source of high CH4 emission. Thermokarst lakes were affected by climatic conditions and had a significant fluctuation in area. The flooding of grasslands inside the depression leads to a considerable increase in CH4 emission. Climate warming scenarios predict increased air temperature and precipitation. According to these scenarios, thermokarst depressions in Central Yakutia may be continuously flooded under the increased air temperature. This will cause an increase in CH4 production and emission in huge amounts since higher temperature may result in increasing productivity of grassland vegetation. This will increase the supply of fresh organic C in the lake and in turn support CH4 emission. Thus, Central Yakutia is a potentially risky region for future climate warming by positive feedback.
ACKNOWLEDGMENTS
We are deeply thankful to Hokkaido University and the Institute for Biological Problems of Cryolithozone SB RAS (Siberian Branch of Russian Academy of Science) members for their field and scientific support. This study was funded by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Science and Technology of Japan (No. 18255014).
REFERENCES
- Bastviken D, Cole J, Pace M, Van de Bogert M 2008: Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. J. Geophys. Res., 113, G02024. doi:10.1029/2007JG000608.
- Bastviken D, Ejlertsson J, Tranvik L 2002: Measurement of methane oxidation in lakes - a comparison of methods. Environ. Sci. Technol., 36, 3354–3361.
- Bosikov NP 1991: Evolution of Alases in Central Yakutia. Permafrost Institute Press, Yakutsk. (in Russian).
- Chanton JP, Dacey JW 1991: Effects of vegetation on methane flux, reservoirs, and carbon isotopic composition. In Trace gas emission by plants, Ed. Rogers JE, Whitman WB, pp. 65–92. Academic Press Inc, New York.
- Conrad R 1989: Control of methane production in terrestrial ecosystems. In Exchange of trace gases between terrestrial ecosystems and the atmosphere, Ed. Andreae MO, Schimel DS, pp. 39–58. Dahlem Konferenzen, Wiley, Chichester.
- Dacey J, Klug M 1982: Ventilation by floating leaves in nuphar. Am. J. Bot., 69, 999–1003.
- Desyatkin RV 1993: Syngenetic soil salinization during thermokarst Alas formation. Eur. Soil Sci., 25, 38–46.
- Desyatkin AR, Takakai F, Fedorov PP, Desyatkin RV, Hatano R 2007: Effect of human activity on carbon balance in meadows in a thermokarst depression in Siberia. Eur. J. For. Res., 10, 89–96.
- Desyatkin AR, Takakai F, Fedorov PP, Nikolaeva MC, Desyatkin RV, Hatano R 2009: CH4 emission from different stages of thermokarst formation in Central Yakutia, East Siberia. Soil Sci. Plant Nutr., 55, 558–570.
- Dove A, Roulet N, Crill P, Chanton J, Bourbonniere R 1999: Methane dynamics of a northern boreal beaver pond. Ecoscience, 6, 577–586.
- Fechner-Levy EJ, Hemond HF 1996: Trapped methane volume and potential effects on methane ebullition in a northern peatland. Limnol Oceanogr., 41, (7) 1375–1383.
- Flury S, McGinnis DF, Gessner MO 2010: Methane emissions from a freshwater marsh in response to experimentally simulated global warming and nitrogen enrichment. J. Geophys. Res., 115, G01007. doi:10.1029/2009JG001079.
- Gauci V, Gowing DJG, Hornibrook ERC, Davis JM, Dise NB 2010: Woody stem methane emission in mature wetlands alder trees. Atmos. Environ., 44, (17) 2157–2160.
- Grosse W 1996: The mechanisms of thermal transpiration (= thermal smosis). Aquat. Bot., 54, 101–110.
- Gunkel G 2009: Hydropower - a green energy? Tropical reservoirs and greenhouse gas emissions. Clean-Soil Air Water, 37, 726–734.
- Harris KB, Bartlett RC 1983: Review and assessment of methane emission from wetlands. Chemosphere, 26, 261–320.
- Huttunen JT, Lappalainenb KM, Saarijarvi E, Vaisanenc T, Martikainen PJ 2001: A novel sediment gas sampler and a subsurface gas collector used for measurement of the ebullition of methane and carbon dioxide from a eutrophied lake. Sci. Total Environ., 266, 153–158.
- IPCC-ACIA (Arctic Climate Impact Assessment) 2007: Impacts of a Warming Climate - Arctic Climate Impact Assessment 2007. p. 140. Cambridge University Press, Cambridge, UK.
- Ivanov MS 1984: Cryogenic Composition of Quaternary Deposits of Lena-Aldan Depression. Novosibirsk, Nauka. (in Russian).
- Jakobsen HA, Sannaes BH, Grevskott S, Svendsen HF 1997: Modeling of vertical bubble-driven flows. Ind. Eng. Chem. Res., 36, 4052–4074.
- Kankaala P, Bergstrom I 2004: Emission and oxidation of methane in Equisetum fluviatile stands. Biogeochemistry, 67, 21–37.
- Kankaala P, Kaki T, Makela S, Ojala A, Pajunen H, Arvola L 2005: Methane efflux in relation to plant biomass and sediment characteristics in stands of three common emergent macrophytes in boreal mesoeutrophic lakes. Glob. Chang. Biol., 11, 145–153.
- Kankaala P, Ojala A, Kaki T 2004: Temporal and spatial variation in methane emissions from a flooded transgression shore of a boreal lake. Biogeochemistry, 68, 297–311.
- Katamura F, Fukuda M, Bosikov NP, Desyatkin RV, Nakamura T, Moriizumi J 2006: Thermokarst formation and vegetation dynamics inferred from a palynological study in central Yakutia, eastern Siberia. Arct. Antarct. Alp. Res., 38, 561–570.
- Kumagai K, Konno Y 1998: Methane emission from rice paddy fields after upland farming. Jpn. J. Soil Sci. Plant Nutr., 69, 333–339. (In Japanese with English summary).
- Liikanen A, Huttunen JT, Valli K, Martikainen PJ 2002: Methane cycling in the sediment and water column of mid-boreal hyper-eutrophic Lake Kevaton, Finland. Archiv fur Hydrobiol., 154, 585–603.
- Mattson MD, Likens GE 1987: The effect of barometric pressure on methane ebullition rates. Eos, 68, 1691.
- Morishita T, Hatano R, Desyatkin RV 2003: CH4 flux in an Alas ecosystem formed by forest disturbance near Yakutsk, eastern Siberia, Russia. Soil Sci. Plant Nutr., 49, 369–377.
- Nakano T, Kuniyoshi S, Fukuda M 2000: Temporal variation in methane emission from tundra wetlands in a permafrost area, northeastern Siberia. Atmos. Environ., 34, 1205–1213.
- Nikolaeva MCH, Desyatkin AR 2004: Change of spatial structure of Alas vegetative cover as a parameter of soil conditions change. Permafrost soils: ecology and protection. In Materials of the All-Russia Scientific Conference Devoted to the 4th Congress of the Dokuchaev Society of soil Scientists and the 100-anniversary of Professor Vasily Georgievich Zolnikov, 28–30 June. 114–119. Publishing House of Permafrost Institute SB RAS, Yakutsk.
- Suzuki R, Hiyama T, Strunin M, Ohata T, Koike T 2001: Airborne observation of land surface by video camera and spectrometers around Yakutsk. In Activity Report of GAME-Siberia 2000, Ed. Jamstec TO, pp. 61–64. GAME Publication 26. GAME.
- Takakai F, Desyatkin AR, Lopez CML, Fedorov AN, Desyatkin RV, Hatano R 2008: CH4 and N2O emissions from a forest-alas ecosystem in the permafrost taiga forest region, eastern Siberia, Russia. J. Geophys. Res-Biogeo., 113, G02002. doi:10.1029/2007JG000521.
- Terazawa K, Ishizuka S, Sakata T, Yamada K, Takahashi M 2007: Methane emissions from stems of Fraxinus mandshurica var. japonica trees in a floodplain forest. Soil Biol. Biochem., 39, 2689–2692.
- Thomas K, Benstead J, Davies KL, Lloyd D 1996: Role of wetland plants in the diurnal control of CH4 and CO2 fluxes in peat. Soil Biol. Biochem., 28, 17–23.
- van der Nat FJWA, Middelburg JJ, van Meteren D, Wielemaker A 1998: The methane emission patterns from Scirpus lacustris and Phragmites australis. Biogeochemistry, 41, 1–22.
- Walter KM, Zimov SA, Chanton JP, Verbyla D, Chapin IIIFS 2006: Methane bubbling from Siberian thaw lakes as a positive feedback to climate warming. Nature, 443, 71–75.
- Xing Y, Xie P, Yang H, Ni L, Wang Y, Rong K 2005: Methane and carbon dioxide fluxes from a shallow hypereutrofic subtropical Lake in China. Atmos. Environ., 39, 5532–5540.
- Zimov SA, Voropaev YV, Semiletov IP, Davidov SP, Prosiannikov SF, Chapin IIIFS, Chapin MC, Trumbore S, Tyler S 1997: North Siberian lakes: A methane source fuelled by Pleistocene carbon. Science, 277, 800–802.