Abstract
Although climate conditions primarily determine the distribution and functioning of vegetation, vegetation also influences climate via biophysical and biogeochemical features such as evapotranspiration, albedo, carbon cycling, trace gas emissions and the roughness of the land surface. Forecasts of rapid climate change during the next 100~200 years, fueled by an increase in greenhouse gases, have motivated the development of land surface models (LSMs) that predict changes in vegetation functions. Here, we review how these models have been developed and used to simulate interactive processes between climate and the land surface. Current limitations and future perspectives of the LSMs are also presented.
1. INTRODUCTION
The climatic environment (e.g., radiation, temperature, precipitation) is a major determinant of the types of vegetation that develop in particular global regions (Holdridge Citation1947). For example, tropical rain forests develop in areas with mild and humid climates throughout the year. In warm and arid zones, the pattern and degree of dryness determine the expansion of tropical seasonal forests, steppes and deserts. At the same time, vegetation influences climatic environments by controlling the land-surface water and radiation balance as well as atmospheric carbon dioxide (CO2) concentration (Foley et al. Citation2003; Pitman Citation2003) (). Between 80 and 90% of the total evapotranspiration from the land surface is caused by transpiration, and the process consumes almost half of the solar energy absorbed by the land surface (Jasechko et al. Citation2013). Increases in greenhouse gases (GHGs) such as CO2 and methane (CH4) in the atmosphere can cause global warming, which can itself lead to further emissions of GHGs from the land surface, resulting in an acceleration of global warming. If the effects of a change to a system induce an increase in the magnitude of the change, the process is referred to as a positive feedback. According to the Intergovernmental Panel on Climate Change (IPCC)’s fifth assessment report (IPCC Citation2013), as of 2011, the estimated carbon (C) pool on land consisted of 275–565 Pg C in living organisms and 1500–2400 Pg C in the pedosphere near the Earth’s surface (excluding permafrost). By comparison, there was only approximately 830 Pg C in the atmosphere. Because 2.1–3.6 times more carbon is stored in the terrestrial ecosystem than in the atmosphere, changes in the amount of carbon stored in the terrestrial ecosystem significantly affect the concentration of GHGs (e.g., CO2 and CH4) in the atmosphere.
Figure 1 Schematic of feedback loops among atmosphere, plant and soil systems. Arrows indicate the direction of influence. CO2, carbon dioxide.
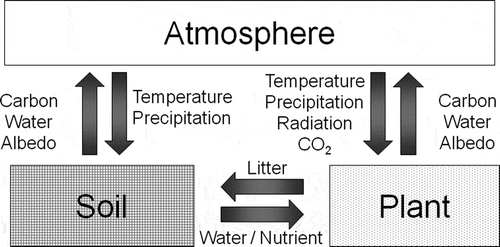
Therefore, vegetation is the most crucial component affecting water and energy cycles on the land surface. The impact of vegetation on the climatic environment is also evident in our everyday experience. For example, the temperatures of the land surface in urban areas covered in concrete and asphalt are typically higher than those in areas covered in vegetation (). The lower temperatures in vegetation-covered areas can be attributed to active transpiration, which removes heat from the land surface. In addition, sensible heat emission is also efficient in vegetation-covered areas, because leaves and branches expand over surface areas, where sensible heat emission occurs.
Figure 2 Geographical distribution of surface temperature observed at Sendai city during daytime on a mid-summer day. Observations were made in the air using a helicopter-based radiation thermometer (Observed by Hirofumi Sugawara).
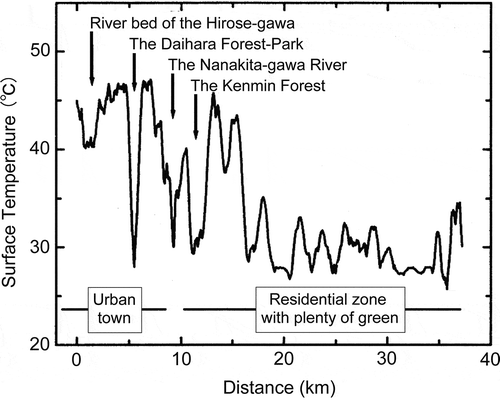
The opposite effect can be observed in high-latitude zones, where the mean annual temperature near the land surface in forest areas is generally higher than that on adjacent bare lands and tundra. One of the reasons for this difference is that the cooler air in high latitudes can hold a smaller amount of moisture, and thus the absolute transpiration rate is restricted. As a result, the cooling effect of forest cover due to transpiration is lower in high latitudes than in lower latitudes. Moreover, from autumn to spring, bare land and tundra in high-latitude zones have a high albedo mainly due to snow cover. In contrast, forests, which typically have lower albedo, absorb a larger proportion of the incident sunlight, resulting in increase of ambient temperatures near the land surface. Therefore, it is estimated that a large-scale removal of forest vegetation in subarctic zones would lower temperatures by 5–12°C for these zones (Bonan et al. Citation2003).
In this review, we present a brief explanation of the various land-atmosphere interactions and how such interactions have been modeled, and discuss what kind of limitations exist for modeling. The next section provides an overview of the structure of land surface models (LSMs). Subsequent sections consider some of the factors incorporated into LSMs including soil organic carbon (SOC), biogenic volatile organic compounds (BVOCs), plant migrations, and land-use changes. These factors were selected because intensive studies are currently underway to treat them in LSMs. Finally, we provide a brief introduction of the other challenges regarding the development of LSMs, and a current status of LSMs in terms of their uncertainty.
2. OVERVIEW OF THE STRUCTURE OF LSMs
As mentioned in the introduction, there are complicated interactions between the atmosphere and vegetation, which can significantly modify the spatiotemporal structure of local climates. Therefore, around middle of the 1980s, simulation models used for predicting long-term climate changes have embedded LSMs that consider such interactions (Pitman Citation2003). Initially, these models only treated the water and radiation balances on the land surface. Around the end of the 20th century, they gradually incorporated carbon balances to predict changes in atmospheric CO2 concentration. Subsequently, the changes in vegetation distribution caused by climate change were also taken into consideration.
LSMs simulate vegetation functions that can influence climatic environments (e.g., water and radiation balance) by inputting physical environmental factors such as air temperature and precipitation. LSMs that consider carbon cycles also output ecosystem structure and components such as biomass, soil organic matter and leaf area index (LAI). These simulations are enabled by the combination of a physical sub-model, which treats hydrological process and heat and energy exchanges between the atmosphere and land surfaces, and a plant physiology sub-model, which treats biological and biogeochemical processes such as photosynthesis, respiration, leaf phenology, the allocation of photosynthetic products, stomatal resistance and the rate of decomposition of soil organic matter. For example, rainfall and snow melt increase soil water content (in the physical sub-model), and soil-water content and climatic factors control stomatal resistance (in the physiology sub-model), and stomatal resistance and climatic factors determine the transpiration rate, which determines the soil water content (in the physical sub-model). In a land surface model that considers carbon balance, a higher stomatal resistance can decrease the rate of photosynthesis. Moreover, most LSMs consider the influence of changes in atmospheric CO2 concentration over plant physiology. Higher CO2 concentrations result in a higher photosynthetic rate and higher stomatal resistance. LSMs vary significantly in their complexity and simulation time-step for each elementary process. Adams et al. (Citation2004) summarized the plant physiology sub-models used in LSMs.
LSMs are often applied on global and continental scales, because atmospheric general circulation models (AGCMs) consider the atmospheric transportation of water, heat and momentum that occur at those geographic scales. To consider large geographical scales, LSMs divide the simulation area into numerous grids, which are employed as the simulation unit. The size of these grids is generally coarse (50–300 km) due to computation limitations for AGCMs. Early LSMs generally assumed that each grid was covered by a “big leaf,” a foliage-layer homogeneously spread over the grid. Because it is not feasible to treat plant species at large geographical scales, these models classify plant species into a small number of plant functional types (PFTs) such as boreal evergreen needle-leaf trees, temperate broad-leaf deciduous trees, and C4 and C3 grasses. PFT is a classification of plant species that is based on their ecological functions or their morphological, physiological or demographic characteristics (Lavorel et al. Citation2007).
3. SOIL ORGANIC CARBON
Soil organic matter contains several times more carbon than living organisms, and hence decomposition of a small portion can significantly impact the Earth’s climate. Yet our understanding of SOC dynamics at large geographic scales is still primitive, leading to uncertainties in the prediction of climate change. One reason for this lack of understanding is that, unlike carbon contained in the atmosphere or the ocean, SOC is unevenly distributed, making it difficult to project at large geographical scales.
The size of the SOC pool is determined by the relative rates of carbon input to and release from the soil. SOC is basically supplemented by litter fall (i.e., plant biomass, including dead branches, leaves and roots that are added to the soil) originating from plants, which are the producer of the terrestrial ecosystem. SOC is basically consumed by decomposition, which is performed by microorganisms such as fungi and bacteria. When this decomposition is aerobically conducted, carbon release from SOC occurs in the form of CO2 (). The rates of carbon input and release both depend on environmental conditions, but in different ways. The input rate is largely controlled by plant production, which depends on environmental factors such as the amount of sunlight, temperature, soil moisture and soil nutrient levels. Meanwhile, the release rate is largely controlled by the metabolic activity of microorganisms, which primarily depends on the soil temperature and moisture content (). For example, in a warm, moderately humid climate, SOC decomposes quickly and accumulation is scant. In contrast, accumulation is heavy in a cool, highly humid climate (e.g., high-latitude peatlands) because SOC decomposes slowly. Although SOC can also be released by fire or into river water, these routes are not discussed in this report.
Figure 3 (a) The typical relationship between soil temperature and soil microorganism activity. (b) The typical relationship between soil wetness and soil microorganism activity. The vertical axes of both graphs give a relative value, in which 1.0 is the optimum maximum. PPT/PET in (b) is the fraction of precipitation to potential evapotranspiration, and is used here as an environmental wetness index. Decreases in soil microorganism activity under low and high PPT/PET are due to shortages of water and oxygen, respectively.
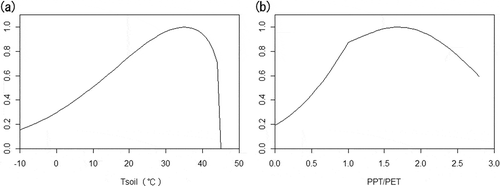
Because the influence of environmental factors on the input and release rates of SOC varies spatially and temporally, it is important to consider the effects of ongoing climate change on the soil carbon balance. A rise in temperature will increase the activity of soil microorganisms and accelerate the decomposition of SOC, but will also enhance plant production, increasing the amount of carbon stored in the soil. Hence, a quantitative and comprehensive understanding of changes in both release and uptake is crucial to determine whether the overall impact is a net input or release of soil carbon. If climate change causes a net release of SOC, then the resulting increase in the atmospheric concentration of GHGs will generate a cycle of positive feedback (). Because this positive feedback could dramatically accelerate climate change, there is now a heightened interest to determine if it will occur. Bond-Lamberty and Thomson (Citation2010) discovered that the soil respiration rate has been increasing by 0.1% every year since 1989, and concluded that one of the most likely causes is the rise in temperature. These findings may indicate that a positive feedback cycle has already begun.
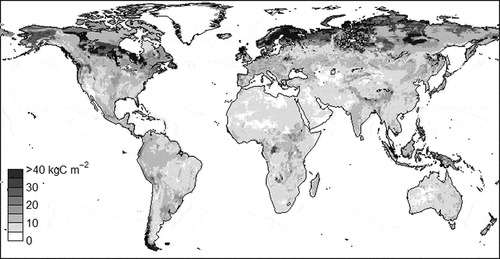
The massive amount of SOC stored in high-latitude regions forms a significant carbon reservoir at the global scale (). This large accumulation is thought to be due to the characteristic physical soil conditions of the region. In cold temperatures, the decomposition rate of soil organic matter is very slow. In areas with permafrost, the decomposition rate is so low that SOC is stored for long periods of time under stable conditions. Moreover, in regions with vast peatland coverage (e.g., Canada and Alaska), the soil is poorly drained and thus the groundwater level is high. These conditions inhibit the activity of aerobic microorganisms with high metabolic rates, leading to conditions favorable for the accumulation of SOC.
Figure 4 The estimated global distribution (in terms of density) of organic carbon (C) in the soil. One of the reasons for the high values at higher latitudes is the lower decomposition rate in a cold environment. Another reason is a lower decomposition rate due to oxygen deficiencies in peatlands, which are frequently distributed in wetlands at high latitudes.
It has been projected that global warming would thaw 37–81% of the permafrost near the Earth’s surface by the end of this century (IPCC Citation2013). Once the permafrost melts, the decomposition of SOC will accelerate rapidly because the rise in soil temperature will increase the overall activity of microorganisms and the improved drainage will lower the groundwater level, increasing the activity of aerobic microorganisms (Ise et al. Citation2008). This increased microorganism activity will release more CO2 and CH4 into the atmosphere, creating a strong positive feedback of global warming.
As mentioned above, the groundwater level plays an important role in SOC dynamics because it is the dividing line between aerobic (oxygenated) and anaerobic (deoxygenated) environments, in which the decomposition rate of SOC differs markedly. Thus, an important question is: how does climate change affect the groundwater level? Unfortunately, there is no simple answer that can be universally applied to all northern peatlands, because various factors need to be considered (e.g., location, actual temperature rise and transient changes). For example, one mechanism, which was reported to occur in central Canadian peatlands, wets the soil because the melting permafrost causes ground sinking due to the enhanced decomposition of SOC (Camill and Clark Citation2000). In contrast, another mechanism drains and dries the soil because the melting permafrost facilitates water percolation (Horiguchi and Miller Citation1980).
Further studies of the physical, chemical and biological features of the environmental responses of SOC are required. For example, Davidson and Janssens (Citation2006) provided an effective framework for understanding the temperature dependence of SOC decomposition, which is anticipated to play a critical role in future climate-carbon cycle feedbacks (Cox et al. Citation2000). Wagai et al. (Citation2013) explored the effect of the structural and biochemical properties of substrates on the temperature sensitivity of soil decomposition. There remain large uncertainties in our understanding of CH4 production and nitrogenous processes such as nitrification and denitrification (Blagodatsky and Smith Citation2012).
4. BVOCS FROM VEGETATED LAND, AND FEEDBACK
A variety of chemical species of BVOCs are synthesized via metabolic pathways in plants for adaptive purposes and are mainly released from terrestrial vegetation into the atmosphere (Laothawornkitkul et al. Citation2009). BVOCs in the atmosphere affect the climatic system in a variety of ways (Peñuelas and Llusià Citation2003). First, they generate large quantities of organic aerosols that could significantly affect the climate by directly scattering solar radiation and indirectly acting as cloud condensation nuclei. As a result, there is a net cooling of the Earth’s surface during the day because of radiation interception. BVOCs themselves act as greenhouse gases and thus can affect local radiative balance at high concentration (Fuentes et al. Citation2001). BVOCs also contribute indirectly to the greenhouse effect. This indirect contribution occurs because BVOCs increase the atmospheric lifetime of CH4 and the chemical production of ozone (O3), and thus enhance the atmospheric concentrations of GHGs.
One global emission model has suggested that a significant fraction of the total BVOCs (1000 Tg C yr−1) is emitted in the form of isoprene (472 Tg C yr−1) and monoterpenes (145 Tg C yr−1) (see Table 6 of Guenther et al. Citation2012). Emission models typically specify the emission capacity for vegetation functional units (e.g., PFTs) under standard environmental conditions. Then the observed responses of leaf emissions to varying conditions (e.g., light, temperature, leaf age, soil moisture, LAI and CO2 concentration) are used to simulate the responses of emissions to weather and other climatic changes.
Isoprene emissions from leaves exponentially increase with increasing leaf temperature to a temperature optimum of about 40°C (Guenther et al. Citation1999). The past temperature environment reflects long-term responses of physiological acclimation (Niinemets and Monson et al. Citation2010). Increases in temperature are the dominant meteorological driver of increases in isoprene emissions, which are reflected by the larger estimates in future scenarios shown in . When long-term changes in vegetation are accounted for, there is considerably larger uncertainty in the projected emissions due to climate change, land-use change and increases in atmospheric CO2 concentration (Heald et al. Citation2009). Projected changes in vegetation due to climate change include longer growing seasons, increased LAI, changes in water stress and changes in vegetation distribution, including an expansion of boreal and temperate forests (Lathière et al. Citation2005). The replacement of forest ecosystems with croplands and pastures generally leads to a decline in isoprene emissions, but the widespread adoption of biofuel plantations can result in an increase in isoprene emissions depending on the crop species (Wiedinmyer et al. Citation2006). The fertilization effect of CO2 on plant growth increases BVOCs emission rates, but leaf-level emissions of isoprene are suppressed at higher CO2 concentrations due to a direct CO2 effect on isoprene emissions (Arneth et al. Citation2011). Although the cellular mechanism behind the isoprene inhibition is not yet fully understood, intercellular metabolic competition for carbon substrate has been proposed as a mechanistic explanation for the CO2 inhibition effect (Rosenstiel et al. Citation2003; Wilkinson et al. Citation2009). Consequently, the projected increases in isoprene emissions due to global warming are largely offset mainly due to the counteracting effects of CO2 inhibition on isoprene emissions, which are reflected in smaller estimates in the future scenarios shown in . The acclimation processes and nutrient [nitrogen (N) and phosphorus (P)] limitations, which are not explicitly accounted for in many studies, limit plant growth, making future increases in isoprene emissions more modest (Niinemets and Arneth et al. Citation2010). Future modeling studies need to consider these counteracting effects, which should be analyzed separately to simulate the response of BVOC emissions to changes in climate, land use and atmospheric composition (e.g., CO2, O3, and aerosols).
Table 1 Present-day and future isoprene emissions [Tg carbon (C) yr−1] in global modeling studies
5. PLANT MIGRATION
Early LSMs assumed that the geographical distribution of vegetation does not change. However, this assumption gradually appeared to be inadequate, because it is widely recognized that the climatic changes in the next few hundred years could be very rapid. As a result, LSMs started to incorporate a mechanism for changing the vegetation distribution with climate change. Such models are known as dynamic global vegetation models (DGVMs).
The most challenging issue for simulating changes in the distribution of vegetation is how to model the time lags between climate change and changes in the structure and distribution of vegetation. These time lags can be very long (i.e., decades to millennia), because the adjustment of vegetation to new climatic conditions requires a series of processes related to plant population dynamics: seed dispersal, establishment, competition against existing plants and reproduction. A simple simulation study has demonstrated that a period of several thousand years can be required for the composition of woody species in a forest to reach equilibrium under new climatic conditions (Kohyama and Shigesada Citation1995), because woody plants generally have a long lifetime and require a long period from establishment to reproduction. An analysis of fossil pollen records has revealed that several hundred to 2000 years were required for a forest to expand in eastern England after the last glacial period (Adams Citation2010). The study also showed that woody plant species appeared at various periods, and the pollen number doubled every 31–158 years until equilibrium was reached. In simulations for periods of less than 10 years, it would be reasonable to assume that the vegetation distribution does not change. In simulations of periods of more than 1000 years, it would be reasonable to assume that the vegetation distribution would follow climatic change with a negligible time lag. However, predictions of climatic change generally consider a time scale of several dozen to several hundreds of years, and thus both of the above assumptions are inadequate.
To control the time lag between climate change and vegetation change, DGVMs consider the dynamic processes of plant populations. First-generation DGVMs divided grid cells into mosaics, each of which was assumed to be monopolized by one PFT (however, most DGVMs allow the coexistence of a woody PFT and a grass PFT by separating overstory and understory). The fraction of coverage of each mosaic of a PFT is adjusted once in a year based on a population growth rate index for the PFT, such as annual net primary production per unit area (e.g., Cox Citation2001). Such approaches for considering changes in vegetation coverage approximate complicated processes with a simple function, which does not reflect the actual mechanisms. Such simplification is called parameterization. Parameterization is an efficient way to treat phenomena with large amounts of observation data such as cloud formation processes, and hence is not a suitable way to treat the time lag between climate change and vegetation change.
Therefore, Friend et al. (Citation1997) developed a DGVM known as Hybrid3, which introduced a more mechanistic way to treat plant population dynamics in a forest. The simulation unit of the Hybrid3 is a forest stand whose size is about the same size as a dominant tree canopy in the forest. The model produces 10 independent simulations for one site and calculates the average to obtain a representative value for the entire site. In each forest stand, individual trees become established and compete with each other to receive more sunlight. This competition is calculated using a one-dimensional model. Although the leaves of the higher layers reduce the amount of sunlight received by those of the lower layers, the leaves of the lower layers do not affect the amount of sunlight acquired by those of the higher layers (). Moreover, there are no interactions among the 10 forest stands. Therefore, Hybrid3 explicitly considers one-directional and local competition for sunlight among trees, which causes gap dynamics (i.e., the cyclic pattern of forest regeneration and succession after the creation of a forest gap). Note that models treating gap dynamics were first introduced to explain forest structure and dynamics (Shugart et al. Citation1973; Bugmann Citation2001), which differs from the reasons for developing LSMs including DGVMs.
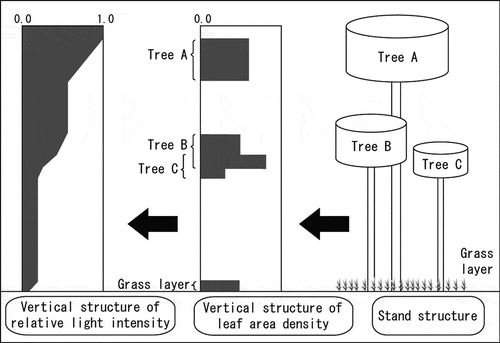
Figure 5 A light-competition model among trees in the Hybrid3 dynamic global vegetation model (DGVM). The simulation unit of this model is an individual tree, which competes with other trees for sunlight. Only the vertical one-directional distribution of leaves is considered, as follows: light penetrates forest stands from the top to the bottom, becoming weaker as it impacts leaves. In this way, the absorbed sunlight is distributed among individual trees according to the vertical position of their foliage.
Gap dynamics are expected to play a central role in regulating the time lags between climatic change and vegetation change, especially when forest types are switched (). Therefore, DGVMs that include gap dynamics, such as Hybrid3, are expected to output the time lag more reasonably. Several more recently developed DGVMs also consider gap dynamics, although the specific approaches differ significantly (Moorcroft et al. Citation2001; Sato et al. Citation2007; Scheiter and Higgins Citation2009). For example, in the Ecosystem Demography Model (EDM), gap dynamics are approximated by competition among cohorts that are classified by size, age and tree species (Moorcroft et al. Citation2001). Alternatively, in the Spatially Explicit Individual-Based DGVM (SEIB-DGVM), local interactions among individual trees are simulated within a spatially explicit virtual forest. Growth, competition and decay of each individual tree are calculated by considering the environmental conditions for that tree in relation to the trees that surround it (Sato et al. Citation2007). One common shortcoming of these approaches is that they require knowledge of the dynamics of every plant including its establishment, competition and mortality across large geographical scales, and such knowledge is far from complete.
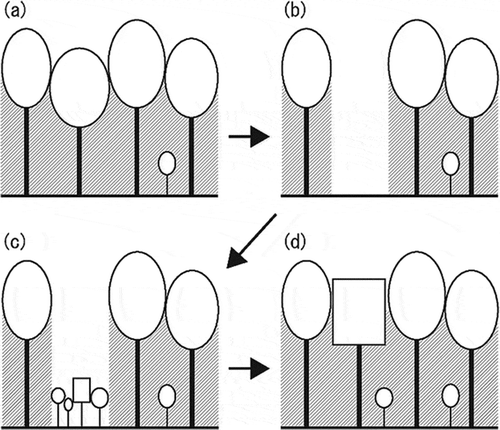
Figure 6 A model of the changes in tree composition in forests with climatic change. (a) In a closed forest, low levels of light intensity on the forest floor inhibit the growth of young trees. (b) When a large tree dies, a bright gap appears. (c) In this gap, young trees grow rapidly, competing with each other over light and space. (d) The tree species (or plant functional type, PFT) that best adapts to the new climate is most likely to occupy the cleared gap. Even if an existing tree species could successfully grow in a new climatic environment, tree composition in the forest gradually changes through repetition of this process from (a) to (d).
6. LAND-USE CHANGES
Human activities have intensively modified Earth’s systems, including the atmosphere, hydrosphere, lithosphere and biosphere systems. Modifications of the land surface have been particularly remarkable. During the last 10,000 years, a third to nearly half of all natural terrestrial ecosystems have been transformed into land for human use and management, such as cropland, pasture and urban areas (Klein Goldewijk et al. Citation2011). As demands for food and energy are still increasing, such land modifications will probably continue in the future (Bruinsma Citation2009).
Human-induced changes in land cover and land use have a huge impact on Earth’s systems; these include biogeophysical and biogeochemical effects on the atmosphere (Claussen et al. Citation2001; Pongratz et al. Citation2010). Biogeophysical effects include changes in albedo, surface roughness and evapotranspiration rate. Rising urban surface temperatures and decreasing temperatures in cutover areas of high-latitude regions, as mentioned in the introduction, are examples of regional climate changes induced by changes in biogeophysical factors. These localized temperature changes due to biogeophysical effects can also occur from the irrigation and soil management of croplands (Lobell et al. Citation2006). With regard to the biogeochemical effects of land-use changes, the most notable example is the net release of CO2 due to a rapid decomposition of the carbon stock remaining in the terrestrial ecosystem. The contribution of land-use changes to anthropogenic carbon emissions was estimated to be about 33% of total emissions over the last 150 yr (Houghton et al. Citation1999). In addition, applying nitrogen fertilizers to croplands has been shown to increase the emission of nitrous oxide (N2O), which is also a GHG (Bouwman et al. Citation2005; Bodirsky et al. Citation2012).
Simulation experiments for estimating the effects of past land-use changes on the climate have often used coupled models that combine the Earth-system Model of Intermediate Complexity (EMIC, Brovkin et al. Citation2006) or an AGCM (Pitman et al. Citation2009) with a LSM that considers land-use changes. In such coupled models, land-use changes are treated as follows. First, PFTs that correspond to croplands and pastures are assigned parameters of phenology, photosynthesis and so on. Then, during the simulation, the fractional coverage of the PFTs within each grid cell (a 0.5° × 0.5° resolution is commonly used) is adjusted to match the annual changes in the fraction of land use. To account for the changes in the carbon balance that accompany land-use changes, when vegetation destruction (e.g., through clear-cutting) occurs, the removed biomass is allocated to several product pools with different decomposition rates (McGuire et al. Citation2001). In addition, the accumulation of biomass through regrowth in secondary vegetation on abandoned land is considered for each grid cell (Shevliakova et al. Citation2009).
Pongratz et al. (Citation2010) conducted an AGCM experiment and reported that land-use changes in the 20th century resulted in a net increase of 0.13–0.15°C in the average global temperature, which was composed of a 0.03°C decrease through biogeophysical effects and a 0.16–0.18°C increase through biogeochemical effects. Many other simulation experiments have also shown that biogeophysical effects have a significantly weaker impact on the global climate than biogeochemical effects (Pitman et al. Citation2009).
However, quantitative estimates of the effects of land-use changes on the interactions between the land surface and atmosphere at the global scale have varied considerably. The latest estimate of carbon emissions due to global land-use changes is 0.9 ± 0.5 Pg C yr−1 (Le Quéré et al. Citation2013). This large standard deviation is considered to be due to inconsistencies among land-use datasets as well as variation in the implementation of the model. Land-use datasets differ in their definitions of classifications such as cropland and pasture, how to allocate regional data among grid cells, and whether they contain a transition matrix for land-use classifications (Jain and Yang Citation2005; Meiyappan and Jain Citation2012). Different models handle land-use data in different ways, and they also differ in whether or not they consider emissions due to shifting cultivation and wood harvesting. A standard protocol of how to handle the numerous elements in a model does not currently exist. When carbon emissions due to land-use changes between 1960 and 2009 are estimated by inputting the same dataset into four different models, the standard deviation is 0.42 Pg C yr−1 (Le Quéré et al. Citation2013). Similarly, if several different land-use datasets are inputted into a single model, the standard deviation is 0.27 Pg C yr−1 (Le Quéré et al. Citation2013). These results indicate that large uncertainties arise due to differences in both the model structure and the land-use data.
In the fifth phase of the Coupled Model Inter-Comparison Project (CMIP5), which provides a framework for the fifth assessment report of the IPCC, simulation experiments were conducted to predict climate change by considering the effects of land-use (IPCC Citation2013). The land-use dataset that was input into the Earth system models (ESMs) of the participating teams was developed so that past estimated land-use changes would be harmonized with the projected land-use change scenario until 2100 (Hurtt et al. Citation2011). This dataset was built using past data for croplands and pasture from the History Database of the Global Environment (HYDE), which was reconstructed by the Netherlands Environmental Assessment Agency (Klein Goldewijk et al. Citation2010, Citation2011), and data for recent wood harvesting from the Food and Agriculture Organization statistics. Then, the projected land-use change scenario until 2100 was appended. In addition, a transition matrix for land-use changes between different categories (e.g., land previously undisturbed by human activities, land previously disturbed by human activities and recovering, cropland, pasture, urban areas) and the amount of wood harvested was estimated both by year and for each 0.5° grid cell.
One of the key challenges to incorporating land-use changes into ESMs is accounting for the effects on the nitrogen cycle. Since the middle of the 20th century, application of nitrogen fertilizers on croplands has increased atmospheric N2O and CH4, both of which are major GHGs. However, models used in the CMIP5 experiment did not consider its effect due to the complexity of biochemical cycles of nitrogen within the soil. If these effects are considered, the estimated global temperature is forecasted to rise by an additional 0.4–0.5°C by 2300 (under the GHG concentrations of the high-emission/business-as-usual scenario) (Stocker et al. Citation2013). Nitrogen availability also has a significant impact on the rate of photosynthesis, which strongly controls the CO2 fertilization effect. The magnitude of the CO2 fertilization effect in different models varies depending on whether a model considers nitrogen limitation or not (Jain et al. Citation2013). In next-generation ESMs, incorporating the effects of nitrogen limitation and distribution is just as important as refining the land-use types in the land-use change data and the experimental protocols.
7. OTHER GAPS AND OPPORTUNITIES IN ATMOSPHERE-PLANT-SOIL LINKAGE STUDIES
As explained earlier, large uncertainty remains in our understanding and modeling of the interaction between atmospheric and terrestrial ecosystems due to the complexity of mechanisms at the land surface. This means there are many research opportunities to contribute to climate prediction and the mitigation or avoidance of devastating environmental deterioration. Here, we briefly discuss other gaps and opportunities in the research field. We also try to add visions of how to promote collaborative studies among modeling scientists and field scientists.
In terms of global warming, it is critically important to evaluate the total greenhouse effect of the three major GHGs (i.e., the sum of the effects of CO2, CH4 and N2O weighted by the global warming potential) on ecosystems. Although many observational and modeling studies have focused on the net budget of individual gases, only a few studies have evaluated the combined budget of the three GHGs (Dalal and Allen Citation2008; Hashimoto Citation2012). The mismatch is largely attributable to practical difficulties in the measurement of multiple trace gases, which differ in their chemical properties and flux magnitude. Similarly, a limited number of models simulating the three GHGs have been developed; these include Carnegie-Ames-Stanford Approach (CASA) (Potter Citation1997; Potter and Klooster Citation1998), Vegetation Integrative SImulator for Trace gase (VISIT) (Inatomi et al. Citation2010), Dynamic Land Ecosystem Model (DLEM) (Tian et al. Citation2011), and Land Processes and eXchanges Dynamic Global Vegetation Model (LPX-DGVM) (Stocker et al. Citation2013). Because different biogeochemical processes regulate the three GHGs, developing integrated models that include key carbon and nitrogen cycling processes is critically important and can be achieved through collaborations between interdisciplinary researchers.
As stated previously, LSMs usually classify plant species into PFTs, within which all parameters are identical. This abstraction is necessary for simulating large geographical scales. However, current LSMs have only about 3~12 PFTs, and hence they typically ignore most biodiversity within a simulation grid. This over-simplification can lead to LSMs overestimating the strength of some climate responses. This is because even if negative effects on vegetation occur due to climate change, they can be mitigated by increases in those species best adapted to the new conditions (Purves and Pacala Citation2008). To better address biodiversity in LSMs, the most promising method would be to divide a woody PFT into a few new PFTs according to life history tradeoffs such as the shade-tolerance spectrum from fast-growing, short-lived pioneers to slow-glowing, long-lived species (Gilbert et al. Citation2006). Life history tradeoffs are known to be strong and general. They are also tightly linked to the physiological and physical characteristics of the leaves (Reich et al. Citation1997; Wright et al. Citation2004). To improve the quantification and scale of global plant trait diversity, plant scientists formed the TRY network (www.try-db.org). By the year 2014, the TRY network has gathered 3 million trait records for about 69,000 plant species from 591 participants in 207 scientific institutes worldwide. Such efforts definitely support the design of a new generation of LSMs.
LSMs operate on large geographical scales to simulate interactions between the climate and land surface, such as exchanges of CO2, water vapor and energy. However, validation data for these fluxes can be only obtained as station data from flux tower sites. To fill this geographical gap, FLUXNET (daac.ornl.gov/FLUXNET/fluxnet.shtml) coordinates regional and global analyses of observations from micrometeorological tower sites, which use eddy covariance methods to measure fluxes. Observation networks such as FLUXNET enable intercomparisons among sites, including spatial comparisons across environmental gradients and biomes. They thereby allow validation of LSMs on large geographical scales.
Few LSMs include the effect of microbes, invertebrates and small animals in an explicit manner. These organisms can play fundamental roles in biogeochemical cycling as “ecosystem engineers” (e.g., Jones et al. Citation1997). For example, earthworms contribute to the decomposition of raw dead biomass and help mix minerals in soils, accelerating soil mineral cycling and the formation of aggregate structures. Most models assume these effects of soil microorganisms only in an implicit manner (i.e., by overall soil turnover rates) and cannot estimate the climatic impact of changes in soil biological community composition and diversity.
In broad-scale studies, mapped data for key ecosystem properties are required to conduct a reliable evaluation. The development of high-precision land surface data is still under development. Recently, a global 1-km mesh dataset of representative soil properties was produced, the Harmonized World Soil Database (HWSD), by the International Institute for Applied System Analysis (IIASA). However, this dataset was compiled from multiple independent data sources, and hence its accuracy differs among regions (Liu et al. Citation2013). A spatial resolution of 1 km may also not be sufficient to harvest spatial heterogeneity caused by topography and microclimate. The development of high-accuracy, fine-resolution and standardized datasets of terrestrial properties is important for both the interpretation of observational data and for model simulations.
Despite such deficiencies in data and knowledge, we should be able to cope with the overload of information. We can access terabytes of data provided by observations and model simulations, but extracting useful knowledge from this extensive and heterogeneous information is not easy. An actively growing area of data usage is data assimilation, in which observational data are sequentially incorporated into numerical models (e.g., Luo et al. Citation2011). Several studies have applied data assimilation methodologies to terrestrial models and have obtained remarkable findings. For example, Sakurai et al. (Citation2012) estimated the temperature dependence of soil decomposition by optimizing model parameters using long-term observational data. These techniques may contribute greatly to the reduction of estimation uncertainties from models and generate useful knowledge from large amounts of observational data.
8. MODEL INTERCOMPARISON FOR ASSESSING UNCERTAINTY IN LSMs
Model intercomparisons allow the behavior of models to be studied, along with the range of uncertainty in their predictions and areas that require further improvement. For example, Sitch et al. (Citation2008) coupled five DGVMs to a computationally efficient “climate analogue model” based on a GCM and ran simulations for varieties of scenarios for anthropogenic carbon emission. Although all five DGVMs have similar productivity responses to elevated atmospheric CO2, they are in less agreement with regard to their responses to changing climate. In particular, there is 494 Pg C difference in cumulative land uptake over the 21st century under the most extreme A1FI Special Report Emission Scenarios (SRES) for carbon emission. This uncertainty, which is equivalent to more than 50 years of anthropogenic emissions at current levels, is strongly linked to the response of tropical vegetation to drought and elevated temperatures. Another intercomparison study for coupled climate-carbon-system models also suggests that tropical forest dieback is a potential high-impact tipping element that could cause an abrupt change in Earth’s climate (Cox et al. Citation2013). These model intercomparison studies suggest that improving confidence regarding how tropical forests respond to hot and dry environment should be a higher-priority task for current LSMs.
9. CONCLUSION
LSMs used in long-term climate simulation research have become increasingly complex, and this trend is expected to continue for the foreseeable future. However, increased complexity does not necessarily equate to an improvement in precision. Building a model is about creating a conceptual and mathematical representation of a complex process in a sophisticated manner. A model should be constructed simply from only the essential components related to the phenomenon of concern, and one should be able to clearly understand the behavior of the components. Because there is rarely sufficient data or information to guarantee high simulation accuracy for all of the physical, physiological and ecological processes that comprise LSMs in all vegetation zones, many of these processes are treated in a conventional manner so that the results do not substantially conflict with common perceptions.
However, the estimated rate of change in the global vegetation distribution within the next 200–500 years is a factor of two to five times higher than the maximum rate within the last 18,000 years. Thus, we do not have empirical knowledge to directly determine how the terrestrial ecosystem will respond and provide climatic feedback under such rapid environmental changes. Therefore, it is reasonable to build a mechanistic model that includes all processes that may potentially have a significant impact on the system of interest.
Developing such a comprehensive model may lead to some secondary effects. For example, it may enhance collaborations between researchers in fields that traditionally do not have much in common (e.g., hydrology, microclimatology, plant physiology and ecology, and plant population ecology). In addition, advanced LSMs have the potential to be used as tools to rationally manage terrestrial ecosystems undergoing environmental change. Actively taking such “detours” as we respond to the demands of academic climate research would lead to more situations where ecologists can play a vital role.
ACKNOWLEDGMENTS
This work was financially supported by the following grants: (1) Program for Risk Information on Climate Change (SOUSEI Program) supported by the Ministry of Education, Culture, Sports, Science, and Technology-Japan (MEXT), (2) Nagoya University Global Center of Excellence (COE) Program “From Earth System Science to Basic and Clinical Environmental Studies” (GCOE-BCES) of the MEXT, (3) MEXT/JSPS KAKENHI (Grant No. 25281003) and (4) the Environment Research and Technology Development Fund (S-10) of the Ministry of Environment of Japan.
REFERENCES
- Adams J 2010: Plants on the move. Vegetation-Climate Interaction - How Plants Make the Global Environment -, pp. 67–96. Springer, Published in association with Praxis Publishing Ltd, Chichester, UK.
- Adams B, White A, Lenton TM 2004: An analysis of some diverse approaches to modelling terrestrial net primary productivity. Ecol. Model., 177, 353–391. doi:10.1016/j.ecolmodel.2004.03.014
- Arneth A, Miller PA, Scholze M, Hickler T, Schurgers G, Smith B, Prentice IC 2007: CO2 inhibition of global terrestrial isoprene emissions: potential implications for atmospheric chemistry. Geophys. Res. Lett., 34, L18813. doi:10.1029/2007GL030615
- Arneth A, Schurgers G, Lathiere J, Duhl T, Beerling DJ, Hewitt CN, Martin M, Guenther A 2011: Global terrestrial isoprene emission models: sensitivity to variability in climate and vegetation. Atmos. Chem. Phys., 11, 8037–8052. doi:10.5194/acp-11-8037-2011
- Blagodatsky S, Smith P 2012: Soil physics meets soil biology: towards better mechanistic prediction of greenhouse gas emissions from soil. Soil Biol. Biochem., 47, 78–92. doi:10.1016/j.soilbio.2011.12.015
- Bodirsky BL, Popp A, Weindl I, Dietrich JP, Rolinski S, Scheiffele L, Schmitz C, Lotze-Campen H 2012: N2O emissions from the global agricultural nitrogen cycle – current state and future scenarios. Biogeosciences, 9, 4169–4197. doi:10.5194/bg-9-4169-2012
- Bonan GB, Levis S, Sitch S, Vertenstein M, Oleson KW 2003: A dynamic global vegetation model for use with climate models: concepts and description of simulated vegetation dynamics. Global Change Biol., 9, 1543–1566. doi:10.1046/j.1365-2486.2003.00681.x
- Bond-Lamberty B, Thomson A 2010: Temperature-associated increases in the global soil respiration record. Nature, 464, 579–582. doi:10.1038/nature08930
- Bouwman AF, Van Drecht G, Van der Hoek KW 2005: Nitrogen surface balances in intensive agricultural production systems in different world regions for the period 1970–2030. Pedosphere, 15, 137–155.
- Brovkin V, Claussen M, Driesschaert E, Fichefet T, Kicklighter D, Loutre MF, Matthews HD, Ramankutty N, Schaeffer M, Sokolov A 2006: Biogeophysical effects of historical land cover changes simulated by six Earth system models of intermediate complexity. Clim. Dyn., 26, 587–600. doi:10.1007/s00382-005-0092-6
- Bruinsma J 2009: The resource outlook to 2050: by how much do land, water, and crop yields need to increase by 2050? FAO Expert Meeting on ‘How to feed the world in 2050’. 24–26. June 2009, Rome: FAO.
- Bugmann H 2001: A review of forest gap models. Clim. Change, 51, 259–305. doi:10.1023/A:1012525626267
- Camill P, Clark JS 2000: Long-term perspectives on lagged ecosystem responses to climate change: permafrost in boreal peatlands and the Grassland/Woodland boundary. Ecosystems, 3, 534–544. doi:10.1007/s100210000047
- Claussen M, Brovkin V, Ganopolski A 2001: Biogeophysical versus biogeochemical feedbacks of large-scale land cover change. Geophys. Res. Lett., 28, 1011–1014. doi:10.1029/2000GL012471
- Cox PM 2001: Description of the “TRIFFID” Dynamic Global Vegetation Model. Centre Technical Note, 24, Hadley Centre, Met Office, UK.
- Cox PM, Betts RA, Jones CD, Spall SA, Totterdell IJ 2000: Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature, 408, 184–187. doi:10.1038/35041539
- Cox PM, Pearson D, Booth BB, Friedlingstein P, Huntingford C, Jones CD, Luke CM 2013: Sensitivity of tropical carbon to climate change constrained by carbon dioxide variability. Nature, 494, 341–344. doi:10.1038/nature11882
- Dalal RC, Allen DE 2008: TURNER REVIEW No. 18. Greenhouse gas fluxes from natural ecosystems. Aust. J. Bot., 56, 369–407. doi:10.1071/BT07128
- Davidson EA, Janssens IA 2006: Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature, 440, 165–173. doi:10.1038/nature04514
- Foley JA, Costa MH, Delire C, Ramankutty N, Snyder P 2003: Green surprise? How terrestrial ecosystems could affect earth’s climate. Front. Ecol. Environ., 1, 38–44.
- Friend AD, Stevens AK, Knox RG, Cannell MGR 1997: A process-based, terrestrial biosphere model of ecosystem dynamics (Hybrid v3.0). Ecol. Model., 95, 249–287. doi:10.1016/S0304-3800(96)00034-8
- Fuentes JD, Hayden BP, Garstang M, Lerdau M, Fitzjarrald D, Baldocchi DD, Monson R, Lamb B, Geron C 2001: New directions: VOCs and biosphere-atmosphere feedbacks. Atmos. Environ., 35, 189–191. doi:10.1016/S1352-2310(00)00365-4
- Ganzeveld L, Bouwman L, Stehfest E, van Vuuren DP, Eickhout B, Lelieveld J 2010: Impact of future land use and land cover changes on atmospheric chemistry‐climate interactions. J. Geophys. Res., 115, D23301. doi:10.1029/2010JD014041
- Gilbert B, Wright SJ, Muller-Landau HC, Kitajima K, Hernandéz A 2006: Life history trade-offs in tropical trees and lianas. Ecology, 87, 1281–1288. doi:10.1890/0012-9658(2006)87[1281:LHTITT]2.0.CO;2
- Global Soil Data Task Group. 2000. Global Gridded Surfaces of Selected Soil Characteristics (IGBP-DIS). [Global Gridded Surfaces of Selected Soil Characteristics (International Geosphere-Biosphere Programme - Data and Information System)]. Data set. Available on-line [http://www.daac.ornl.gov] from Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, U.S.A. doi:10.3334/ORNLDAAC/569.
- Guenther A, Baugh B, Brasseur G, Greenberg J, Harley P, Klinger L, Serça D, Vierling L 1999: Isoprene emission estimates and uncertainties for the central African EXPRESSO study domain. J. Geophys. Res., 104, 30625–30639. doi:10.1029/1999JD900391
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, Mckay WA et al. 1995: A global model of natural volatile organic compound emissions. J. Geophys. Res., 100, 8873–8892. doi:10.1029/94JD02950
- Guenther A, Karl T, Harley P, Wiedinmyer C, Palmer PI, Geron C 2006: Estimates of global terrestrial isoprene emissions using MEGAN (Model of Emissions of Gases and Aerosols from Nature). Atmos. Chem. Phys., 6, 3181–3210. doi:10.5194/acp-6-3181-2006
- Guenther AB, Jiang X, Heald CL, Sakulyanontvittaya T, Duhl T, Emmons LK, Wang X 2012: The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model. Dev., 5, 1471–1492. doi:10.5194/gmd-5-1471-2012
- Hashimoto S 2012: A new estimate of global soil greenhouse gas fluxes using a simple data-oriented model. Plos One, 7, e41962. doi:10.1371/journal.pone.0041962
- Heald CL, Henze DK, Horowitz LW, Feddema J, Lamarque JF, Guenther A, Hess PG, Vitt F, Seinfeld JH, Goldstein AH et al. 2008: Predicted change in global secondary organic aerosol concentrations in response to future climate, emissions, and land use change. J. Geophys. Res., 113, D05211. doi:10.1029/2007JD009092
- Heald CL, Wilkinson MJ, Monson RK, Alo CA, Wang GL, Guenther A 2009: Response of isoprene emission to ambient CO2 changes and implications for global budgets. Global Change Biol., 15, 1127–1140. doi:10.1111/j.1365-2486.2008.01802.x
- Holdridge LR 1947: Determination of world plant formations from simple climatic data. Science, 105, 367–368. doi:10.1126/science.105.2727.367
- Horiguchi K, Miller RD 1980: Experimental studies with frozen soil in an ice sandwich permeameter. Cold Reg. Sci. Technol., 3, 177–183. doi:10.1016/0165-232X(80)90023-3
- Houghton RA, Hackler JL, Lawrence KT 1999: The U.S. carbon budget: contributions from land-use change. Science, 285, 574–578. doi:10.1126/science.285.5427.574
- Hurtt GC, Chini LP, Frolking S, Betts RA, Feddema J, Fischer G, Fisk JP, Hibbard K, Houghton RA, Janetos A et al. 2011: Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Clim. Change, 109, 117–161. doi:10.1007/s10584-011-0153-2
- Inatomi M, Ito A, Ishijima K, Murayama S 2010: Greenhouse gas budget of a cool-temperate deciduous broad-leaved forest in Japan estimated using a process-based model. Ecosystems, 13, 472–483. doi:10.1007/s10021-010-9332-7
- IPCC 2013: Climate change 2013: the physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Eds. Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, 1535 pp. Cambridge University Press, Cambridge (UK) and New York, NY, (USA).
- Ise T, Dunn AL, Wofsy SC, Moorcroft PR 2008: High sensitivity of peat decomposition to climate change through water-table feedback. Nature Geosci., 1, 763–766. doi:10.1038/ngeo331
- Ise T, Moorcroft PR 2006: The global-scale temperature and moisture dependencies of soil organic carbon decomposition: an analysis using a mechanistic decomposition model. Biogeochemistry, 80, 217–231. doi:10.1007/s10533-006-9019-5
- Ito A, Sillman S, Penner JE 2009: Global chemical transport model study of ozone response to changes in chemical kinetics and biogenic volatile organic compounds emissions due to increasing temperatures: sensitivities to isoprene nitrate chemistry and grid resolution. J. Geophys. Res., 114, D09301.
- Jain AK, Meiyappan P, Song Y, House JI 2013: CO2 emissions from land-use change affected more by nitrogen cycle, than by the choice of land-cover data. Global Change Biol., 19, 2893–2906. doi:10.1111/gcb.12207
- Jain AK, Yang X 2005: Modeling the effects of two different land cover change data sets on the carbon stocks of plants and soils in concert with CO2 and climate change. Global Biogeochem. Cycles, 19, GB2015. doi:10.1029/2004GB002349
- Jasechko S, Sharp ZD, Gibson JJ, Birks SJ, Yi Y, Fawcett PJ 2013: Terrestrial water fluxes dominated by transpiration. Nature, 496, 347–350. doi:10.1038/nature11983.
- Jones CG, Lawton JH, Shachak M 1997: Positive and negative effects of organisms as physical ecosystem engineers. Ecology, 78, 1946–1957. doi:10.1890/0012-9658(1997)078[1946:PANEOO]2.0.CO;2
- Klein Goldewijk K, Beusen A, Janssen P 2010: Long-term dynamic modeling of global population and built-up area in a spatially explicit way: HYDE 3.1. The Holocene, 20, 565–573. doi:10.1177/0959683609356587
- Klein Goldewijk K, Beusen A, van Drecht G, de Vos M 2011: The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Global Ecol. Biogeogr., 20, 73–86. doi:10.1111/j.1466-8238.2010.00587.x
- Kohyama T, Shigesada N 1995: A size-distribution-based model of forest dynamics along a latitudinal environmental gradient. Vegetatio, 121, 117–126. doi:10.1007/BF00044677
- Kondo J 2000: Atmospheric Science near the Ground Surface, University of Tokyo Press, Tokyo, pp. 195, (in Japanese).
- Laothawornkitkul J, Taylor JE, Paul ND, Hewitt CN 2009: Biogenic volatile organic compounds in the Earth system. New Phytol., 184, 276–276.
- Lathière J, Hauglustaine DA, De Noblet-Ducoudré N, Krinner G, Folberth GA 2005: Past and future changes in biogenic volatile organic compound emissions simulated with a global dynamic vegetation model. Geophys. Res. Lett., 32, L20818. doi:10.1029/2005GL024164
- Lathière J, Hewitt CN, Beerling DJ 2010: Sensitivity of isoprene emissions from the terrestrial biosphere to 20th century changes in atmospheric CO2 concentration, climate, and land use. Glob. Biogeochem. Cycles, 24, GB1004. doi:10.1029/2009GB003548
- Lavorel S, Díaz S, Cornelissen H, Garnier E, Harrison SP, McIntyre S, Pausas JG, Pérez-Harguindeguy N, Urcelay C 2007: Plant functional types: are we getting any closer to the Holy Grail? In Terrestrial Ecosystems in a Changing World, Eds. Canadell JG, Pataki DE,Pitelda LF, pp. 171–186. Springe-Verlag, Heidelberg.
- Le Quéré C, Andres RJ, Boden T, Conway T, Houghton RA, House JI, Marland G, Peters GP, van der Werf GR, Ahlström A et al. 2013: The global carbon budget 1959–2011. Earth Sys. Sci. Data, 5, 165–185. doi:10.5194/essd-5-165-2013
- Liao H, Chen WT, Seinfeld JH 2006: Role of climate change in global predictions of future tropospheric ozone, and aerosols. J. Geophys. Res., 111, D12304. doi:10.1029/2005JD006852
- Liu S, Wei Y, Post WM, Cook RB, Schaefer K, Thornton MM 2013: The Unified North American Soil Map and its implication on the soil organic carbon stock in North America. Biogeosciences, 10, 2915–2930. doi:10.5194/bg-10-2915-2013
- Lobell DB, Bala G, Duffy PB 2006: Biogeophysical impacts of cropland management changes on climate. Geophys. Res. Lett., 33, L06708. doi:10.1029/2005GL025492
- Luo Y, Ogle K, Tucker C, Fei SF, Gao C, LaDeau S, Clark JS, Schimel DS 2011: Ecological forecasting and data assimilation in a data-rich era. Ecol. Appl., 21, 1429–1442. doi:10.1890/09-1275.1
- McGuire AD, Sitch S, Clein JS, Dargaville R, Esser G, Foley J, Heimann M, Joos F, Kaplan J, Kicklighter DW et al. 2001: Carbon balance of the terrestrial biosphere in the twentieth century: analyses of CO2, climate and land use effects with four process-based ecosystem models. Global Biogeochem. Cycles, 15, 183–206. doi:10.1029/2000GB001298
- Meiyappan P, Jain AK 2012: Three distinct global estimates of historical land-cover change and land-use conversions for over 200 years. Front. Earth Sci., 6, 122–139. doi:10.1007/s11707-012-0314-2
- Moorcroft PR, Hurtt GC, Pacala SW 2001: A method for scaling vegetation dynamics: the ecosystem demography model (ED). Ecol. Monogr., 71, 557–586. doi:10.1890/0012-9615(2001)071[0557:AMFSVD]2.0.CO;2.
- Niinemets Ü, Arneth A, Kuhn U, Monson RK, Peñuelas J, Staudt M 2010: The emission factor of volatile isoprenoids: stress, acclimation, and developmental responses. Biogeosciences, 7, 2203–2223. doi:10.5194/bg-7-2203-2010
- Niinemets Ü, Monson RK, Arneth A, Ciccioli P, Kesselmeier J, Kuhn U, Noe SM, Peñuelas J, Staudt M 2010: The leaf level emission factor of volatile isoprenoids: caveats, model algorithms, response shapes and scaling. Biogeosciences, 7, 1809–1832. doi:10.5194/bg-7-1809-2010
- Pacifico F, Folberth GA, Jones CD, Harrison SP, Collins WJ 2012: Sensitivity of biogenic isoprene emissions to past, present, and future environmental conditions and implications for atmospheric chemistry. J. Geophys. Res., 117, D22302.
- Peñuelas J, Llusià J 2003: BVOCs: plant defense against climate warming? TRENDS Plant Sci., 8, 105–109. doi:10.1016/S1360-1385(03)00008-6
- Pitman AJ 2003: The evolution of, and revolution in, land surface schemes designed for climate models. Int. J. Climatol., 23, 479–510. doi:10.1002/joc.893
- Pitman AJ, de Noblet-Ducoudré N, Cruz FT, Davin EL, Bonan GB, Brovkin V, Claussen M, Delire C, Ganzeveld L, Gayler V et al. 2009: Uncertainties in climate responses to past land cover change: first results from the LUCID intercomparison study. Geophys. Res. Lett., 36, L14814. doi:10.1029/2009GL039076
- Pongratz J, Reick CH, Raddatz T, Claussen M 2010: Biogeophysical versus biogeochemical climate response to historical anthropogenic land cover change. Geophys. Res. Lett., 37, L08702. doi:10.1029/2010GL043010
- Potter CS 1997: An ecosystem simulation model for methane production and emission from wetlands. Global Biogeochem. Cycles, 11, 495–506. doi:10.1029/97GB02302
- Potter CS, Klooster SA 1998: Interannual variability in soil trace gas (CO2, N2O, NO) fluxes and analysis of controllers on regional to global scales. Global Biogeochem. Cycles, 12, 621–635. doi:10.1029/98GB02425
- Purves D, Pacala S 2008: Predictive models of forest dynamics. Science, 320, 1452–1453. doi:10.1126/science.1155359
- Reich PB, Walters MB, Ellsworth DS 1997: From tropics to tundra, Global convergence in plant functioning. Proc. Natl. Acad. Sci. U.S.A, 94, 13730–13734. doi:10.1073/pnas.94.25.13730
- Rosenstiel TN, Potosnak MJ, Griffin KL, Fall R, Monson RK 2003: Increased CO2 uncouples growth from isoprene emission in an agriforest ecosystem. Nature, 421, 256–259. doi:10.1038/nature01312
- Sakurai G, Jomura M, Yonemura S, Iizumi T, Shirato Y, Yokozawa M 2012: Inversely estimating temperature sensitivity of soil carbon decomposition by assimilating a turnover model and long-term field data. Soil Biol. Biochem., 46, 191–199. doi:10.1016/j.soilbio.2011.11.005
- Sanderson MG, Jones CD, Collins WJ, Johnson CE, Derwent RG 2003: Effect of climate change on isoprene emissions and surface ozone levels. Geophys. Res. Lett., 30, 1936. doi:10.1029/2003GL017642
- Sato H 2008: Current status and future direction of biogeochemical models, a review. Jpn. J. Ecol., 58, 11–21. (in Japanese).
- Sato H 2014: Dynamic global vegetation models, and interactions between vegetation and atmosphere. In Ecology of global environmental change, Eds. Hara T, Kyoritsu Shuppan Press, Tokyo. (in Japanese).
- Sato H, Itoh A, Kohyama T 2007: SEIB-DGVM: a new dynamic global vegetation model using a spatially explicit individual-based approach. Ecol. Model., 200, 279–307. doi:10.1016/j.ecolmodel.2006.09.006
- Scheiter S, Higgins SI 2009: Impacts of climate change on the vegetation of Africa: an adaptive dynamic vegetation modelling approach. Global Change Biol., 15, 2224–2246. doi:10.1111/j.1365-2486.2008.01838.x
- Shevliakova E, Pacala SW, Malyshev S, Hurtt GC, Milly PCD, Caspersen JP, Sentman LT, Fisk JP, Wirth C, Crevoisier C 2009: Carbon cycling under 300 years of land use change: importance of the secondary vegetation sink. Global Biogeochem. Cycles, 23, GB2022. doi:10.1029/2007GB003176
- Shugart HH, Crow TR, Hett JM 1973: Forest succession models - rationale and methodology for modeling forest succession over large regions. For. Sci., 19, 203–212.
- Sitch S, Huntingford C, Gedney N, Levy PE, Lomas M, Piao SL, Betts R, Ciais P, Cox P, Friedlingstein P et al. 2008: Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five dynamic global vegetation models (DGVMs). Global Change Biol., 14, 2015–2039. doi:10.1111/j.1365-2486.2008.01626.x
- Stocker BD, Roth R, Joos F, Spahni R, Steinacher M, Zaehle S, Bouwman L, Ri Xu, Prentice IC 2013: Multiple greenhouse-gas feedbacks from the land biosphere under future climate change scenarios. Nat. Clim. Change, 3, 666–672. doi:10.1038/nclimate1864
- Tai APK, Mickley LJ, Heald CL, Wu S 2013: Effect of CO2 inhibition on biogenic isoprene emission: implications for air quality under 2000 to 2050 changes in climate, vegetation, and land use. Geophys. Res. Lett., 40, 3479–3483. doi:10.1002/grl.50650
- Tian H, Xu X, Lu C, Liu ML, Ren W, Chen GS, Melillo J, Liu JY 2011: Net exchanges of CO2, CH4, and N2O between China’s terrestrial ecosystems and the atmosphere and their contributions to global climate warming. J. Geophys. Res., 116, G02011. doi:10.1029/2010JG001393
- Turner DP, Baglio JV, Wones AG, Pross D, Vong R, Mcveety BD, Phillips DL 1991: Climate change and Isoprene emissions from vegetation. Chemosphere, 23, 37–56. doi:10.1016/0045-6535(91)90115-T
- Wagai R, Kishimoto-Mo A, Yonemura S, Shirato Y, Hiradate S, Yagasaki Y 2013: Linking temperature sensitivity of soil organic matter decomposition to its molecular structure, accessibility, and microbial physiology. Global Change Biol., 19, 1114–1125. doi:10.1111/gcb.12112
- Wiedinmyer C, Tie X, Guenther A, Neilson R, Granier, C 2006: Future changes in biogenic isoprene emissions: how might they affect regional and global atmospheric chemistry? Earth Interact., 10, 1–19. doi:10.1175/EI174.1
- Wilkinson M, Monson RK, Trahan N, Lee S, Brown E, Jackson RB, Polley HW, Fay PA, Fall R 2009: Leaf isoprene emission rate as a function of atmospheric CO2 concentration. Glob. Change Biol., 15, 1189–1200. doi:10.1111/j.1365-2486.2008.01803.x
- Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M et al. 2004: The worldwide leaf economics spectrum. Nature, 428, 821–827. doi:10.1038/nature02403
- Wu S, Mickley LJ, Jacob DJ, Rind D, Streets DG 2008: Effects of 2000–2050 changes in climate and emissions on global tropospheric ozone and the policy-relevant background surface ozone in the United States. J. Geophys. Res., 113, D18312. doi:10.1029/2007JD009639
- Wu S, Mickley LJ, Kaplan JO, Jacob DJ 2012: Impacts of changes in land use and land cover on atmospheric chemistry and air quality over the 21st century. Atmos. Chem. Phys., 12, 1597–1609. doi:10.5194/acp-12-1597-2012
- Young PJ, Arneth A, Schurgers G, Zeng G, Pyle JA 2009: The CO2 inhibition of terrestrial isoprene emission significantly affects future ozone projections. Atmos. Chem. Phys., 9, 2793–2803. doi:10.5194/acp-9-2793-2009
