Abstract
Graminaceous plants can take up iron-phytosiderophore complexes, whereas non-graminaceous plants absorb ferrous ions after the reduction of ferric compounds at the root cell membranes. The iron (Fe) in the roots may be transported to the aerial plant parts through the xylem. We compared the chemical forms in xylem sap collected from the cut stems of three graminaceous plants (rice [Oryza sativa L.], maize [Zea mays L.], barley [Hordenum vulgare L.]) and three non-graminaceous plants (tomato [Lycopersicon esculentum Mill.], soybean [Glycine max Merr.], castor bean [Ricinus communis L.]) grown in composite soils for the concentrations of iron and iron-chelating compounds (nicotianamine, phytosiderophores, citrate). We also fractionated the xylem saps by size-exclusion chromatography to gain insight into the chemical forms of iron. The Fe concentrations in the xylem sap ranged from 9 to 40 μM. Nicotianamine was found in the xylem sap from all the plants examined, with higher concentrations in the non-graminaceous plants. In contrast, phytosiderophores (2’-deoxymugineic acid and mugineic acid) were predominantly detected in the graminaceous plants. The concentrations of free citrate varied greatly (from 4 to 2200 μM) among the six plant species. The xylem sap iron in non-graminaceous plants may form two types of Fe-citrate, whereas in graminaceous plants, the bound Fe forms may be largely two types of Fe-citrate with various Fe-phytosiderophores.
INTRODUCTION
Iron (Fe) is essential for higher plants in the synthesis of redox-related proteins such as ferredoxins, cytochromes and superoxide dismutase. Iron is also involved in the synthesis of green pigments of leaves (chlorophylls). The higher plants’ need for iron in the shoots is satisfied by the continuous acquisition of iron from the soils by transporters and by symplasmic radial transfer through root cells and loading into the xylem, which delivers iron to the aerial parts of plants.
In fact, irons in soils, in particular calcareous soils, bind to mineral crystals and organic compounds so strongly that such soil irons are hardly available to plants. It had been thought that all plants absorb Fe2+ after the reduction (obligatory reduction, Chaney et al. Citation1972) of Fe(III)-containing compounds that are present in the soil solution and culture medium; this system was named “Strategy I” (Romheld and Marschner Citation1986). In the 1970s, monocotyledonous species were thought to be less iron-efficient than dicotyledonous species (Brown Citation1978). This belief was challenged by a report by Takagi (Citation1976) and subsequently by the clear statement of Takagi et al. (Citation1984) that graminaceous plants may develop a specific system for iron uptake: excretion of phytosiderophores (PSs) such as 2’-deoxymugineic acid (DMA) from the roots to the rhizosphere to form soluble Fe(III)-PS complexes, and the Fe(III)-PS may be taken up directly without the reduction of Fe(III). This system in graminaceous plants is called “Strategy II” (Romheld and Marschner Citation1986). Iron uptake transporters with genes for Fe2+ absorption in Strategy I (Arabidopsis, Eide et al. Citation1996) and for Fe(III)-DMA uptake in Strategy II (maize [Zea mays L.], Curie et al. Citation2001) were identified.
As described above, iron is transported via xylem vessels to be utilized in aerial parts. The chemical forms of iron in the xylem sap, which is slightly acidic, have been investigated. The involvement of citrate has been frequently suggested since the early reports by Brown and Tiffin (Citation1965) and Tiffin (Citation1966a, Citation1966b). In addition, iron deficiency in tomato (Lycopersicon esculentum Mill., Pich et al. Citation1994), soybean (Glycine max Merr., Brown and Tiffin Citation1965), and fava bean (Vicia faba L., Nikolic and Römheld Citation1999) caused the increase of citrate concentrations in xylem saps. Phytosiderophores, which may bind to iron, were present in the xylem sap of barley (Hordeum vulgare L., Alam et al. Citation2001) and rice (Oryza sativa L., Ando et al. Citation2013). Ishimaru et al. (Citation2006) reported that 59Fe-DMA added to the culture medium was absorbed by rice plants and transported to the shoots, probably in the form of 59Fe-DMA via the xylem.
The chemical forms of iron in the phloem sap, which delivers nutrients to the developing organs, have also been investigated. The iron-chelating compounds in castor bean (Ricinus communis L.) phloem sap were suggested to be nicotianamine (NA), cysteine and histidine (Schmidke et al. Citation1999), and a 17-kDa iron-containing protein called iron transport protein (ITP) was identified (Krüger et al. Citation2002). The major low-molecular-weight chemical forms of iron in rice phloem sap are Fe-DMA (Nishiyama et al. Citation2012) and Fe-citrate (Kato et al. Citation2010).
Over the last 10 years, we have focused on the chemical forms of metals (cadmium [Cd], Fe, zinc [Zn], copper [Cu]) in xylem and phloem saps from rice. In the present study, we identified concentrations of Fe and Fe-chelating compounds (NA, PSs, citrate) in xylem sap from soil-grown graminaceous (rice, maize, barley) and non-graminaceous (tomato, soybean, castor bean) plants. We estimated chemical forms of iron in a fractionation analysis by size-exclusion chromatography (SEC).
MATERIALS AND METHODS
Culture of plants on soils
Oryza sativa L. cv. Nipponbare) were carried out in wet towels for 2 d, and the germinated seeds were placed on pots with 650-mL of synthetic composite soil (Izeki-Kanto Co., Tokyo). The potted soil was flooded with tap water at the level of the soil surface. The rice plants were grown for 4 weeks under natural sunshine during May and July.
The seeds of maize (Zea mays L. cv. Peter-corn), barley (Hordenum vulgare cv. Amagi-Nijo), castor bean (Ricinus communis cv. Mizuma), soybean (Glycine max Merr. cv. Fukuyutaka) and tomato (Lycopersicon esculentum Mill. cv. Momotaro) were germinated by seeding at 1 cm depth in wet vermiculite for 7 d in the dark at room temperature. The young seedlings were transferred to the seeding soils (Takii-Shubyo Co., Tokyo), and grown for 4 weeks under sunshine during April and August.
Collection of xylem sap
The stems of 4-week-old plants on soils were cut at 2 cm above the root/shoot interfaces. The exudates (except the first 1-min drops, which were discarded) were collected for 10 min during 10:00 a.m. and 2:00 p.m. by 10-μL microcapillary tubes (Drummond Scientific Company, Broomall, PA) into 1.5-mL test tubes on ice with three replicated samples. The collections of the xylem sap samples from barley, maize and rice were conducted on June 8, July 6 and July 11, respectively. The collections of the xylem sap samples from soybean, castor bean and tomato were conducted on May 17, June 11 and August 6, respectively. The exudate samples were stored at ‒20°C until analysis.
Fe analysis by GFAAS
Fe contents in the xylem exudates after appropriate dilutions were determined by graphite furnace atomic absorption spectrometry (GFAAS, Z-8100 type, Hitachi Co., Tokyo).
CE-MS of Fe-binding compounds
To 10 μL of each xylem sap sample, 10 μL of 50% [volume/volume (v/v)] methanol containing 100 μM methionine sulfone (MES) and/or 100 μM piperazine-1,4-bis(2-ethane-sulfonic acid) (PIPES) were added and mixed. The mixtures were placed on 3-kDa cut filters (YM-3, Millipore, Bedford, MA) and centrifuged at 6000 rpm for 90 min at 4°C. The 10-μL filtrates were used for capillary electrophoresis-mass spectrometry (CE-MS).
The CE-MS analysis essentially followed the methods described by Kato et al. (Citation2010). The whole CE-MS system was controlled by ChemStation Software (Agilent Technologies, Waldbronn, Germany). The capillary electrophoresis (CE) of anionic compounds such as organic acids were conducted using 100-cm-long polyethylene-coated DB-WAX capillary columns (I.D. 0.050 mm, J&W Scientific, Folsom, CA) with elution buffer of 20 mM ammonium acetate (pH 6.8) at -24 v at 20°C. The sheath solution was a mixture of 2 mM ammonium acetate and 50% (v/v) methanol. Ion detection was by negative mode. Citric acid, as a standard chemical (Wako Chemicals, Tokyo), was dissolved in 20 mM ammonium acetate (pH 6.0), and we added MES/PIPES solution to produce 100 μM citric acid and 50 μM MES/PIPES.
The CE of cationic compounds like NA and PSs was carried out using a fused silica capillary column (I.D. 0.050 mm, GL Sciences, Torrance, CA) with the elution buffer of 1 M formic acid (pH 1.8) at +24 V at 20°C. The sheath solution was a mixture of 0.1-% (v/v) ethanol, and ion detection was by positive mode. As the standard chemicals, NA and DMA (provided by N. K. Nishizawa) were employed. NA and DMA were dissolved in 20 mM ammonium acetate (pH 6.0) and added to MES/PIPES solution to produce 100 μM NA and DMA and 50 μM MES/PIPES. The DMA and mugineic acid (MA) were detected by CE-MS, and their contents were simulated using DMA as the reference. It should be noted that the citrate bound to Fe was not included as “citrate”, but PSs bound to Fe were included as “PSs” in the present CE-MS analysis.
Fractionation by SE-HPLC
The xylem sap samples and the mixtures of ferrous sulfate (FeSO4) or ferric chloride (FeCl3) with possible Fe-liganding compounds (citrate, NA, DMA) were diluted with 10 mM ammonium acetate (pH 6.0) and fractionated by size-exclusion high-performance liquid chromatography (SE-HPLC, La Chrom type, Hitachi Co., Tokyo) using a Superdex 75 10/300 GL column (GE Healthcare Bioscience Co., Tokyo). Ten millimolar ammonium acetate solution (adjusted to pH 6.0 by formic acid) was used as the elution buffer. The flow rate was 0.5 mL per min, detected by a UV detector at 214 nm. Twenty microliters of xylem sap samples were injected by an autosampler and elutes were collected by a fractionation collector at 1-min intervals. The fractionated elutes were added with 4 M nitric acid (HNO3) to make a final concentration of 0.05 M HNO3.
In this analysis, the 30-min fraction, for example, signifies the elute after 30.0 min and before 31.0 min (500 μL).
Treatment with proteinaseK
To determine the presence/absence of iron-containing proteins, we prepared two types of rice xylem sap samples as the control sample without proteinaseK treatment: 20 μL of the xylem sap were mixed with 10 μL Milli-Q water. As the proteinaseK-treated sample, 20 μL of the xylem sap were mixed with 10 μL of 0.4 mg mL−1 proteinaseK (Takara-Bio Co., Shiga, Japan) solution and incubated at 37°C for 3 h prior to the SE-HPLC.
RESULTS
The concentrations of Fe, NA, PSs and citrate in the xylem saps
The Fe concentrations determined by GFAAS in the xylem saps from six plant species grown on soils for 4 weeks are shown in . The Fe concentrations in the graminaceous plants (rice, maize, barley) ranged from 9 to 25 μM, and those in non-graminaceous plants (tomato, soybean, castor bean) ranged from 9 to 38 μM, indicating no significant difference between graminaceous and non-graminaceous plants.
Table 1 The concentrations of potentially iron (Fe)-combining compounds and Fe fractions in xylem saps from graminaceous and non-graminaceous plants grown on soils
The concentrations of potentially Fe-chelating compounds, determined by CE-MS, are also shown in . The NA concentrations in the non-graminaceous plants (9–22 μM) were higher than those in the graminaceous plants (1–6 μM). The concentrations of total PSs (the sum of DMA and MA) in the graminaceous plants ranged from 4 to 18 μM, indicating an exceptionally large presence above those in the non-graminaceous plants. Among the PSs, DMA was present in large concentrations in the xylem sap from rice and maize. MA was contained predominantly in barley xylem sap. In the xylem saps from tomato and soybean plants, low levels of signals were detected at the m/z 305 and the retention time corresponding to [DMA + H]+ in the CE-MS analysis, although the identification of DMA in such non-graminaceous plants was not carried out.
The concentrations of citrate in the xylem saps varied greatly (from 4 to 2200 μM) and no difference between graminaceous and non-graminaceous plants was observed.
SE-HPLC fractionation of xylem saps
The SE-HPLC fractionation of xylem sap from the rice plants is shown in for three samples (a1, a2 and a3). The alc sample without ProteinaseK treatment showed Fe peaks at 23-min, 27-min and 30-min fractions with an Fe recovery of 68%. The a2c sample showed Fe peaks at 23-min, 27-min and 30-min fractions with an Fe recovery of 88%. The a3c sample showed Fe peaks at 23-min, 27-min and 30-min fractions with an Fe recovery of 52%. The highest Fe peak was found at the 23-min fraction, followed by the 27-min fraction. The peak at the 30-min fraction was the smallest. Since Fe2+ or Fe3+ ions administered as 50 μM FeSO4 or FeCl3, respectively, were not detected during the elution time of 10 and 60 min, the Fe recovered from rice xylem sap samples in this SE-HPLC analysis (69 ± 10%, n = 3) may be in bound forms, and the rest (31%) may be in the form of free Fe ions.
Figure 1 Size-exclusion high-performance liquid chromatography (SE-HPLC) analysis of iron (Fe) in three samples of xylem sap from soil-grown rice (Oryza sativa L.), treated with and without proteinaseK. a1c, a2c, and a3c were non-treated, and a1p, a2p, and a3p were treated with proteinaseK.
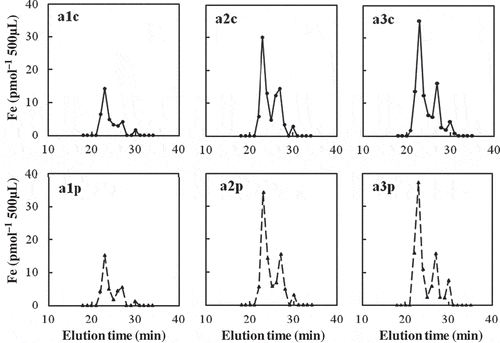
The xylem sap from rice was treated with ProteinaseK before the SE-HPLC analysis as described in Materials and methods (Kato et al. Citation2010). This treatment (a1p, a2p and a3p) did not cause any changes of Fe peaks compared to the non-treatment (control).
The SE-HPLC fractionation of three samples from the maize xylem sap is shown in (b1, b2 and b3). The Fe peaks were found at 24-min, 27-min and 30-min fractions with the highest at 27 min. The Fe recovery was 91 ± 5% (n = 3), indicating that a small fraction of xylem sap Fe (9%) may be free Fe ions.
Figure 2 Size-exclusion high-performance liquid chromatography (SE-HPLC) analysis of iron (Fe) in xylem sap from soil-grown maize (Zea mays L. cv. Peter-corn; b1–b3) and barley (Hordenum vulgare cv. Amagi-Nijo; c1–c3).
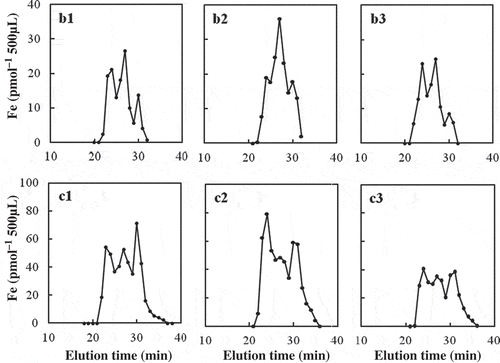
The SE-HPLC fractionation of three samples from barley xylem sap is also shown in (c1, c2 and c3). Fe peaks were found at 23–24-min, 27-min, and 30–31-min fractions with high Fe peaks at 23–24 min or 30 min. The Fe recovery was 94 ± 7% (n = 3). The free Fe ions may be low, about 6%.
The SE-HPLC fractionation of three samples from tomato xylem sap is shown in (d1, d2 and d3). Large Fe peaks were found at 24-min and 27-min fractions. The Fe recovery was 65 ± 2% (n = 3). A considerable fraction of tomato xylem sap Fe (35%) may be in the forms of free Fe ions.
Figure 3 Size-exclusion high-performance liquid chromatography (SE-HPLC) analysis of iron (Fe) in xylem saps from soil-grown tomato (Lycopersicon esculentum Mill. cv. Momotaro; d1–d3), soybean (Glycine max Merr. cv. Fukuyutaka; e1–e3), and castor bean (Ricinus communis cv. Mizuma; f1–f3).
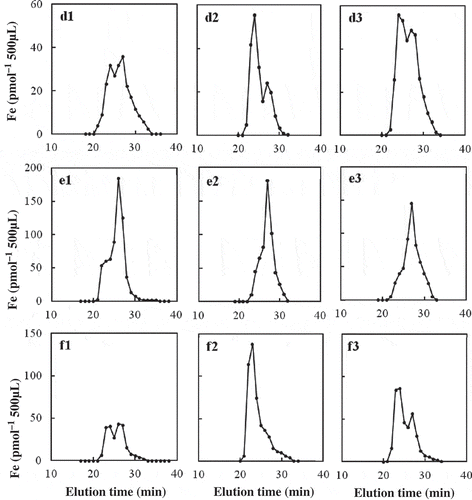
The SE-HPLC fractionation of three samples from soybean xylem sap is shown in (e1, e2 and e3). One major Fe peak was found at 26–27 min, with a shoulder at 23–24 min. The Fe recovery was 82 ± 11% (n = 3). About 18% of soybean xylem sap Fe may be free Fe ions.
The SE-HPLC fractionation of three samples from castor bean xylem sap is shown in (f1, f2 and f3). Fe peaks were found at the 23–24-min and 26–27-min fractions. The Fe recovery was 48 ± 6% (n = 3). A large fraction of xylem sap Fe (52%) in the xylem sap of castor bean may be free Fe ions.
Fractionation of Fe-chelates by SE-HPLC
The Fe contents in the 1.0-mL fractions separated at pH 6.0 by SE-HPLC for the mixture of 500 μM citrate and 18 μM FeCl3 showed the highest Fe peak at the 27-min fraction. The Fe recovery was 66%.
The Fe contents separated at pH 6.0 by SE-HPLC for the mixtures of 2.5 mM NA and 18 μM FeSO4 showed the highest Fe peak at the 34-min fraction with the recovery of 32%.
The Fe contents separated at pH 6.0 by SE-HPLC for the mixture of 2.5 mM DMA and 18 µM FeCl3 showed a single peak at the 30-min fraction with the Fe recovery of 39%.
These results suggest that the 27-min Fe peak of the xylem sap may be Fe-citrate, and the 30-min Fe peak may be ascribed to Fe-DMA. No peak for Fe-NA (at the 34-min fraction) was found in any xylem sap. The 23-min Fe peak in the xylem sap was not identified from retention times of Fe-chelate complexes under our present experimental conditions.
DISCUSSION
Fe-chelating compounds in xylem saps
Our CE-MS analysis of xylem saps from six plant species () showed that NA and citrate, which have been thought to be Fe-chelating compounds, were found in non-graminaceous plants (tomato, soybean and castor bean), and that NA, citrate and PSs (DMA, MA) were found in graminaceous plants (rice, maize and barley). The ratios of NA (μM) to Fe concentrations (μM) were between 1.1 (tomato) and 0.08 (maize). The ratios of PSs (μM) to Fe concentrations (μM) were between 0.49 (rice) and 0.71 (maize and barley) in the graminaceous plants, but negligible in the non-graminaceous plants. The ratios of free citrate (μM) to Fe concentrations (μM) were between 58.8 (soybean) and 7.8 (tomato), with the exception of a low ratio (0.1) in castor bean. As noted in the Materials and methods section, citrate bound to Fe was not measured by the present CE-MS analysis.
Fe-chelate complexes in xylem sap
In the non-graminaceous plants, the SE-HPLC analysis revealed two Fe peaks at the 23–24-min and 26–27-min fractions (), while in the graminaceous plants, three Fe peaks at 23–24 min, 26–27 min, and 30 min were found (, ). In reference to the retention times of complexes of Fe-citrate, Fe-NA and Fe-DMA, the 26–27-min Fe may correspond to Fe-citrate, and the 30–31-min Fe to Fe-DMA. NA may combine to Fe (von Wiren et al. Citation1999), but no peak for Fe-NA was found at the 34-min fraction—which was observed by the standard Fe-NA—in any of the xylem sap samples.
Regarding the 23–24-min Fe peak found in all xylem sap samples, high-molecular-weight Fe-containing proteins are the candidates. However, the xylem saps from rice () and castor bean (unpublished) treated with ProteinaseK did not cause any changes of Fe peaks at the 23–24-min fraction. The Fe peak at the 23–24-min fraction may be not ascribed to the Fe-containing proteins. Rellán-Álvarez et al. (Citation2010), using HPLC-ICP-MS (high pressure liquid-inductively coupled plasma-mass spectrometry) for Fe-citrate at pH 5.5, typical of xylem sap, reported that Fe-citrate complexes could assume two forms, favoring tri-Fe(III), tri-citrate complex (Fe3Cit3) at high Fe:cit ratios and favoring binuclear Fe(III)-cit species (Fe2Cit2) at lower Fe:cit ratios. They identified an Fe peak from Fe3Cit3 in the xylem sap of Fe-deficient/resupplied tomato plants (Solanum lycopersicum Mill.). In another examination (unpublished) of castor bean xylem sap from May- and October-grown plants, we found that the May plants had higher Fe concentrations in the xylem sap compared to the October plants, and the 23-min Fe peak in the May plants was higher than that in the October plants.
The citrate concentration of soybean xylem sap (2170 µM, ) in the present study was more than that of the standard solution (500 µM citrate plus 18 µM FeCl3) and both the standard solution and soybean xylem sap showed strong Fe peaks at the 27 min fraction (for soybean, e1, e2 and e3 in ). On the other hand, the free citrate concentration of castor bean xylem sap (3.9 µM) was low and a strong Fe peak was found at the 23-min fraction (f2 and f3 in ). The xylem saps from maize (305 µM citrate) and barley (247 µM) had medium levels of citrate concentrations and varied Fe peaks at 23–24 and 27 min (). Based on these findings, we suspect that the 23–24-min Fe peak corresponds to high-molecular-weight Fe3Cit3 and that the 26–27-min Fe peak corresponds to low-molecular-weight Fe2Cit2.
The 30-min Fe peak may correspond to the Fe-PSs, and graminaceous plants only had the 30-min Fe peak. The 30-min Fe peak in the xylem saps from rice and maize plants (, ) may be predominantly Fe-DMA, and those from barley plants may be Fe-MA since MA was found predominantly in barley xylem sap ().
In conclusion, both graminaceous and non-graminaceous plants contain large Fe peaks at 23–24 min corresponding to Fe3Cit3 and at 26–27 min corresponding to Fe2Cit2, and the ratios of those peaks may be regulated by citrate concentrations, favoring Fe3Cit3 at low citrate and Fe2Cit2 at high citrate. The Fe peak at the 30-min fraction corresponding to Fe-PSs was clearly found in graminaceous plants. The mean distribution of each Fe-chelate molecule was calculated from their Fe-peak heights and is shown in . At high Fe:cit ratios (rice, barley, tomato, castor bean) Fe3Cit3 was more abundant than Fe2Cit2 as predicted by Rellán-Álvarez et al. (Citation2010). The formation of Fe-PSs in the xylem saps of graminaceous plants may increase the proportion of Fe-chelate complexes and decrease the proportion of free Fe ions.
Uptake of Fe into the root cells and transport to the xylem sap
shows the schemes of Fe uptake into the root cells from the environment (rhizosphere soil), symplasmic short-distance transport through the root cells, loading into xylem vessels and the long-distance transport via the xylem for non-graminaceous (Strategy I) and graminaceous (Strategy II) plants.
Figure 4 Uptake, symplastic transport and xylem transport of iron (Fe) in non-graminaceous (Strategy-I) and graminaceous plants (Strategy-II).
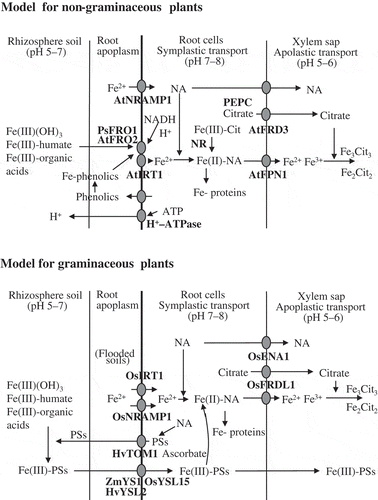
In soils, there are several iron forms (Fe-humate, Fe-organic acids, Fe-siderophores) which can be available for plants. Phenolics are secreted from Fe-deficient plants to mobilize apoplastic Fe (Jin et al. Citation2007). For acquisition of iron from Fe(III)-humate (Cesco et al. Citation2002) and Fe(III)-siderophores (Castignetti and Smarrelli Citation1986), and Fe(III)(OH)3 in Strategy-I plants, the Fe(III) must first be reduced to Fe2+ by protons in the root cell membranes by AtFRO2 (Robinson et al. Citation1999) and PsFRO1 (Waters et al. Citation2002) and transferred into the root cells by IRT1 (Eide et al. Citation1996).
In Strategy-II plants, the Fe(III)-chelate complexes in soils may be exchanged to form Fe-PSs; the PSs are excreted by the transporter of mugineic acid family phytosiderophores 1 (TOM1) from rice and HvTOM1 from barley (Nozoye et al. Citation2011). Although HvYSL2 (Araki et al. Citation2011) and ZmYS1 (Curie et al. Citation2001) localized at the root epidermis of barley and maize transport Fe(III)-PSs from the soils into the root cells, it is difficult to state whether the “standard reductase” is completely absent in graminaceous plants. Kannan (Citation1971) reported the presence of a carrier for Fe2+ uptake in rice plants; recently, OsNRAMP1 and AtNRAMP1 (Curie et al. Citation2000) were reported to be divalent metal transporters, localized at plasma membranes and function to take up Fe2+, and Ishimaru et al. (Citation2006) reported that rice plants take up both Fe2+ by OsIRT1 and Fe(III)-DMA by OsYSL15 in flooded soil conditions.
In root cells, iron may be symplasmically transported in the forms of Fe-NA, Fe-citrate and Fe-PSs to the xylem. To use iron for root metabolism, the ferric iron of Fe(III)-citrate, if present, can be reduced to ferrous by NADH-nitrate reductase (Redinbangh and Campbell Citation1983), and the ferric iron of Fe(III)-PSs can be reduced to ferrous form by ascorbate, forming Fe(II)-NA (Weber et al. Citation2008).
The citrate which is produced for instance by phosphoenolpyruvate carboxylase (López-Millán et al. Citation2009) and excreted by citrate transporters AtFRD3 (Durrett et al. Citation2007) and OsFRDL1 (Yokosho et al. Citation2009) into the xylem fluid may be combined with Fe(III) and form two forms of Fe-citrate, Fe3Cit3 and Fe2Cit2, in both non-graminaceous and graminaceous plants. The iron in the xylem may be effluxed from cells by AtFPN1 (Morrissey et al. Citation2009). NA in the root cells may be effluxed by OsENA1 to the xylem (Nozoye et al. Citation2011), although NA does not bind to iron in the xylem. In the graminaceous species, Fe-PSs may work for the efficient and stable transport of Fe.
ACKNOWLEDGMENTS
We thank Professor Naoko K. Nishizawa for her kind provision of nicotianamine and 2’-deoxymugineic acid.
References
- Alam S, Kamei S, Kawai S 2001: Metal micronutrients in xylem sap of iron-deficient barley as affected by plant-borne, microbial and synthetic metal chelators. Soil Sci. Plant Nutr., 47, 149–156. doi:10.1080/00380768.2001.10408377
- Ando Y, Nagata S, Yanagisawa S, Yoneyama T 2013: Copper in xylem and phloem saps from rice (Oryza sativa): the effect of moderate copper concentrations in the growth medium on the accumulation of five essential metals and a speciation analysis of copper-containing compounds. Func. Plant Biol., 40, 89–100. doi:10.1071/FP12158
- Araki R, Murata J, Murata Y 2011: A novel barley yellow stripe 1-like transporter (HvYSL2) localized to the root endodermis transports metal-phytosiderophore complexes. Plant Cell Physiol., 52, 1931–1940. doi:10.1093/pcp/pcr126
- Brown JC 1978: Mechanism of iron uptake by plants. Plant Cell Environ., 1, 249–257. doi:10.1111/j.1365-3040.1978.tb02037.x
- Brown JC, Tiffin LO 1965: Iron stress as related to the iron and citrate occurring in stem exudates. Plant Physiol., 40, 395–400. doi:10.1104/pp.40.2.395
- Castignetti D, Smarrelli J 1986: Siderophores, the iron nutrition of plants, and nitrate reductase. FEBS Lett., 209, 147–151. doi:10.1016/0014-5793(86)81100-0
- Cesco S, Nikolic M, Römheld V, Varanini Z, Pinton R 2002: Uptake of 59Fe from 59Fe-humate complexes by cucumber and barley plants. Plant Soil, 241, 121–128. doi:10.1023/A:1016061003397
- Chaney RL, Brown JC, Tiffin LO 1972: Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol., 50, 208–213. doi:10.1104/pp.50.2.208
- Curie C, Alonso JM, Le Jean ML, Ecker JR, Briat J-F 2000: Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem. J., 347, 749–755. doi:10.1042/0264-6021:3470749
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat J-F, Walker EL 2001: Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature, 409, 346–349. doi:10.1038/35053080
- Durrett TP, Gassmann W, Rogers EE 2007: The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol., 144, 197–205. doi:10.1104/pp.107.097162
- Eide D, Broderius M, Fett J, Guerinot ML 1996: A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc. Nat. Acad. Sci. USA, 93, 5624–5628. doi:10.1073/pnas.93.11.5624
- Ishimaru Y, Suzuki M, Tsukamoto T, et al. 2006: Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J., 45, 335–346. doi:10.1111/j.1365-313X.2005.02624.x
- Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ 2007: Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol., 144, 278–285. doi:10.1104/pp.107.095794
- Kannan S 1971: Kinetics of iron absorption by excised rice roots. Planta, 96, 262–270. doi:10.1007/BF00387445
- Kato M, Ishikawa S, Inagaki K, Chiba K, Hayashi H, Yanagisawa S, Yoneyama T 2010: Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.). Soil Sci. Plant Nutr., 56, 839–847. doi:10.1111/j.1747-0765.2010.00514.x
- Kruger C, Berkowitz O, Stephan UW, Hell R 2002: A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J. Biol. Chem., 277, 25062–25069. doi:10.1074/jbc.M201896200
- López-Millán AF, Morales F, Gogorcena Y, Abadía A, Abadía J 2009: Metabolic responses in iron deficient tomato plants. J. Plant Physiol., 166, 375–384. doi:10.1016/j.jplph.2008.06.011
- Morrissey J, Baxter IR, Lee J, Li L, Lahner B, Grotz N, Kaplan J, Salt DE, Guerinot ML 2009: The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell, 21, 3326–3338. doi:10.1105/tpc.109.069401
- Nikolic M, Römheld V 1999: Mechanism of Fe uptake by the leaf symplast: is Fe inactivation in leaf a cause of Fe deficiency chlorosis? Plant Soil, 215, 229–237. doi:10.1023/A:1004786211779
- Nishiyama R, Kato M, Nagata S, Yanagisawa S, Yoneyama T 2012: Identification of Zn-nicotianamine and Fe-2’-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol., 53, 381–390. doi:10.1093/pcp/pcr188
- Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK 2011: Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J. Biol. Chem., 286, 5446–5454. doi:10.1074/jbc.M110.180026
- Pich A, Scholz G, Stephan UW 1994: Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes. Nicotianamine as possible copper translocator. Plant Soil, 165, 189–196. doi:10.1007/BF00008061
- Redinbaugh MG, Campbell WH 1983: Reduction of ferric citrate catalyzed by NADH: nitrate reductase. Biochem. Biophys. Res. Commun., 114, 1182–1188. doi:10.1016/0006-291X(83)90687-3
- Rellán-Álvarez R, Giner-Martínez-Sierra J, Orduna J, Orera I, Rodriguez-Castrillön JÁ, García-Alonso JI, Abadía J, Álvarez-Fernandez A 2010: Identification of a tri-iron(III), tri-citrate complex in the xylem sap of iron-deficient tomato resupplied with iron: new insights into plant iron long-distance transport. Plant Cell Physiol., 51, 91–102. doi:10.1093/pcp/pcp170
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML 1999: A ferric-chelate reductase for iron uptake from soils. Nature, 397, 694–697. doi:10.1038/17800
- Romheld V, Marschner H 1986: Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol., 80, 175–180. doi:10.1104/pp.80.1.175
- Schmidke I, Kruger C, Frommichen R, Scholz G, Stephan UW 1999: Phloem loading and transport characteristics of iron in interaction with plant-endogenous ligands in castor bean seedlings. Physiol. Plant, 106, 82–89. doi:10.1034/j.1399-3054.1999.106112.x
- Takagi S 1976: Naturally occurring iron-chelating compounds in oat- and rice-root washing. I. Activity measurement and preliminary characterization. Soil Sci. Plant Nutr., 22, 423–433. doi:10.1080/00380768.1976.10433004
- Takagi S, Nomoto K, Takemoto T 1984: Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr., 7, 469–477. doi:10.1080/01904168409363213
- Tiffin LO 1966a: Iron translocation. I. Plant culture, exudate sampling, iron-citrate analysis. Plant Physiol., 41, 510–514. doi:10.1104/pp.41.3.510
- Tiffin LO 1966b: Iron translocation. II. Citrate/iron ratios in plant stem exudates. Plant Physiol., 41, 515–518. doi:10.1104/pp.41.3.515
- von Wiren N, Klair S, Bansal S et al. 1999: Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants. Plant Physiol., 119, 1107–1114. doi:10.1104/pp.119.3.1107
- Waters BM, Blevins DG, Eide DJ 2002: Characterization of FRO1, a pea ferric-chelate reductase involved in root iron acquisition. Plant Physiol., 129, 85–94. doi:10.1104/pp.010829
- Weber G, von Wirén N, Hayen H 2008: Investigation of ascorbate-mediated iron release from ferric phytosiderophores in the presence of nicotianamine. Biometals, 21, 503–513. doi:10.1007/s10534-008-9137-8
- Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF 2009: OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol., 149, 297–305. doi:10.1104/pp.108.128132
