Abstract
Forest fires can change the greenhouse gase (GHG) flux of borea forest soils. We measured carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) fluxes with different burn histories in black spruce (Picea mariana) stands in interior Alaska. The control forest (CF) burned in 1920; partially burned (PB) in 1999; and severely burned (SB1 and SB2) in 2004. The thickness of the organic layer was 22 ± 6 cm at CF, 28 ± 10 cm at PB, 12 ± 6 cm at SB1 and 4 ± 2 cm at SB2. The mean soil temperature during CO2 flux measurement was 8.9 ± 3.1, 6.4 ± 2.1, 5.9 ± 3.4 and 5.0 ± 2.4°C at SB2, SB1, PB and CF, respectively, and differed significantly among the sites (P < 0.01). The mean CO2 flux was highest at PB (128 ± 85 mg CO2-C m−2 h−1) and lowest at SB1 (47 ± 19 mg CO2-C m−2 h−1) (P < 0.01), and within each site it was positively correlated with soil temperature (P < 0.01). The CO2 flux at SB2 was lower than that at CF when the soil temperature was high. We attributed the low CO2 flux at SB1 and SB2 to low root respiration and organic matter decomposition rates due to the 2004 fire. The CH4 uptake rate was highest at SB1 [–91 ± 21 μg CH4-C m−2 h−1] (P < 0.01) and positively correlated with soil temperature (P < 0.01) but not soil moisture. The CH4 uptake rate increased with increasing soil temperature because methanotroph activity increased. The N2O flux was highest [3.6 ± 4.7 μg N2O-N m−2 h−1] at PB (P < 0.01). Our findings suggest that the soil temperature and moisture are important factors of GHG dynamics in forest soils with different fire history.
Key words:
1. INTRODUCTION
Together with water vapor, carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) are major greenhouse gases (GHGs) (IPCC Citation2007). In general, aerated forest soils act as sources of CO2 and N2O, and as sinks of CH4 (e.g., IPCC Citation2007). Production and consumption of these GHGs are governed by biological processes (Paul Citation2007), and soil temperature, moisture and substrates are very important factors that affect gas dynamics (Hashimoto et al. Citation2011a). Therefore, disturbances such as forest fires that change soil properties can affect GHG dynamics.
Forest fires are an important cause of disturbance in boreal forests (Ahlgren and Ahlgren Citation1960; Van Cleve et al. Citation1986), and they are becoming more frequent in Alaska; about 2500 fires occurred in the 1950s, whereas more than 6000 occurred in the 1990s (Todd and Jewkes Citation2006). In interior Alaska, black spruce (Picea mariana) is the dominant species on north-facing slopes and in bottomlands, which have permafrost soils (Viereck et al. Citation1983). Forest floors are covered by thick moss and lichens, which are burned by forest fires. The burned areas are revegetated after fire (Longton Citation1988), first by pioneer moss species (e.g., Polytrichum juniperinum and Ceratodon purpureus) and subsequently by cup lichens (Cladina spp. and Cladonia spp.), which gradually replace the pioneer moss species. Then, as the forest matures, feather mosses (Pleurozium schreberi, Hylocomium splendens and sometimes Sphagnum spp.) appear and become dominant. The intensity of the forest fire affects the depth to which the organic layers are burned and thus the composition of the moss and lichen community on the forest floor after the fire. Furthermore, forest fires often accelerate permafrost thawing, thus increasing the thickness of the soil’s active layer in the summer following the fire (UNEP Citation2008).
Comparisons of black spruce forests before and after burning (e.g., Kim and Tanaka Citation2003) and chronosequence studies (e.g., O’Neill et al. Citation2006) have shown that wild fires decrease CO2 flux from the soil. Sawamoto et al. (Citation2000) showed that, in the larch (Larix cajanderi) forests of eastern Siberia, CO2 flux was decreased more in a severely burned forest than in a mildly burned forest. CO2 flux decreases after fire are caused by decreased root and microbial activity (Sawamoto et al. Citation2000; Kim and Tanaka Citation2003; O’Neill et al. Citation2006). In contrast, few studies have examined CH4 and N2O fluxes in black spruce forests (Kim and Tanaka Citation2003; Matson et al. Citation2009), and the information available on CH4 and N2O fluxes in relation to fire disturbance remains limited. Kim and Tanaka (Citation2003) reported that CH4 flux increased and N2O flux decreased within 2 years after a forest fire in a black spruce forest in interior Alaska. They suggested that the increased CH4 emissions may have been caused by permafrost thawing. Permafrost sometimes has a high organic matter content (Tarnocai et al. Citation2009) and a high CH4 concentration (Brouchkov and Fukuda Citation2002); thus, permafrost thawing can increase organic matter decomposition (UNEP Citation2008) and CH4 emissions from the soil (Schaefer et al. Citation2011). The objective of this study was to clarify the effects of soil temperature and moisture on GHG dynamics in black spruce forest in areas of interior Alaska with different forest fire histories.
2. MATERIALS AND METHODS
2.1 Study sites
We established four sites on north-facing slopes in black spruce (Picea mariana) stands (), approximately 50 km north of Fairbanks in interior Alaska, where the annual mean temperature and precipitation over 30 recent years were –2.5°C and 275 mm, respectively (ACRC Citation2014). A control forest (CF) and a partially burned forest (PB) were located on different slopes in the Caribou-Poker Creek Research Watershed (CPCRW; 65°10′N, 147°30′W), and two severely burned forests (SB1 and SB2) were located on the same slope in the Poker Flat Research Range (PFRR; 65°07′N, 147°28′W).
Table 1 Year of fire disturbance, organic layer depth (mean ± SD, n = 4) and topographical conditions at the study sites
At CF, which last experienced fire in 1920, the stand density, the mean diameter at breast height and the mean height are 4700 trees ha−1, 4.4 cm and 4.1 m, respectively. The forest floor was covered with feather mosses (P. schreberi and H. splendens), Sphagnum mosses and lichens (Cladina rangiferina and C. stellaris) (). At PB, the P. mariana stands had been partially burned in 1999 as patches. Two thirds of the forest floor remained unburned, and the overall stand density was 2900 trees ha−1, and in the unburned patches feather mosses and Sphagnum species were dominant (). Kim and Tanaka (Citation2003) measured gas fluxes within 2 years after the forest fire on the same slope. A severe forest fire in 2004 had affected both SB1 and SB2 (), accounting for 96 and 100% of study plots, respectively. Saplings of deciduous species such as poplar (Populus tremuloides) and birches (Betula spp.) were dominant during the study period. Charred moss covered 71% of the surface at SB1 and 56% at SB2 (). Thus, fire had consumed a larger area of forest floor mosses and organic layers on the ground at SB2 than at SB1. Pioneer moss species (P. juniperinum and Ceratodon purpureus) colonized the bare ground where there were no charred mosses; these moss-covered areas accounted for 44% of the ground surface of SB2 and 25% at SB1 (). Because of differences in fire intensity among the sites, the thickness of the organic layer differed among sites (), from 22 ± 6 cm at CF and 28 ± 10 cm at PB to 12 ± 6 cm at SB1, and 4 ± 2 cm at SB2. The depth of the active layer in summer also differed among the sites (), from 35–55 cm at CF and PB to more than 160 cm at SB1 and SB2. All soils were classified as Cryosols (IUSS Citation2006). Noguchi et al. (Citation2012) similarly reported the presence of a shallow active layer of 33 cm in unburned forest in the PFRR. Therefore, the deep active layer at SB1 and SB2 was the result of the 2004 fire.
Table 2 Coverage ratio (%) of forest floor vegetation at the study sites. Feather moss, mostly Pleurozium schreberi and Hylocomium splendens; lichen, mainly Cladina rangiferina and C. stellaris
2.2 Measurement of GHG fluxes
We measured CO2 flux in August 2009, and in July and September 2010. We measured CH4 and N2O fluxes only in August 2009. For gas flux measurements, we selected six moss patches and six lichen patches at CF, six patches of charred moss at PB and SB1, and six patches of pioneer moss at SB2 (). We used the closed chamber method to measure gas flux (Sawamoto et al. Citation2000) at about 10:00, 14:00, and 16:00 on 2 or 3 days out of 10. At each study site, cylindrical stainless steel chambers (25 cm high, 20 cm in diameter) were inserted into the ground surface to a depth of 3–5 cm. Live mosses and lichens were removed to prevent them from affecting respiration measurement. To determine the CO2 flux, a 500-mL gas sample was collected from the chamber into a Tedlar® bag before the lid was closed; the same amount of gas was collected 6 min after the lid was closed. The lid was then opened for at least 15 min to prevent gas diffusion from having any effect on the next series of measurements. At 0, 20, 40 and 60 min after the lid was closed again, a 40-mL gas sample for CH4 and N2O measurement was collected into a 30-mL vacuum glass bottle sealed with a butyl rubber stopper and plastic cap. At the same time as the gas samples were collected, a digital thermometer was used to measure soil temperature at a depth of 5 cm below the surface, and a Time-domain Reflectometer (TDR) (Hydrosense™, Campbell Scientific Australia Pty. Ltd.) was used to measure soil moisture (volumetric water content) at a depth of 0–12 cm near each chamber. The CO2 concentration in each bag was analyzed with a portable infrared gas analyzer (Fuji Electronic ZEP-9, Japan) within a day of sampling. The CO2 flux was then calculated from a two-point regression of CO2 concentration in accordance with the method of Sawamoto et al. (Citation2000). CH4 and N2O concentrations were determined using a gas chromatograph with a flame ionization detector and an electron-capture detector (Shimadzu GC-14B, Kyoto, Japan), respectively. The CH4 and N2O fluxes were then calculated by linear regression of the four sample measurements in accordance with the method of Morishita et al. (Citation2014).
2.3 Statistical analysis
The means and standard deviations of soil temperature, soil moisture and gas fluxes were calculated for each site by combining all collected data. Then, two-way analysis of variance (site × measurement month) followed by Fisher’s least-significant-difference test was used to compare mean values among the sites. The relationship between gas flux and soil temperature or moisture was investigated using Peason’s correlation coefficient test. Regression analysis was done by simple regression of CO2 flux on soil temperature. Statistical analyses were performed with Microsoft Excel statistical software ver. 2008 (SSRI Co. Ltd., Tokyo, Japan). To examine the effect of soil moisture on CO2 flux without the effect of soil temperature, adjusted CO2 flux (mg C m−2 h−1) at 5°C was calculated using a modification of the methods of Lavigne et al. (Citation2004). In brief,
where a is a coefficient estimated by nonlinear regression of CO2 flux on soil temperature at each site. The values of a are shown in the caption to .
3. RESULTS AND DISCUSSION
3.1 Soil temperature and moisture
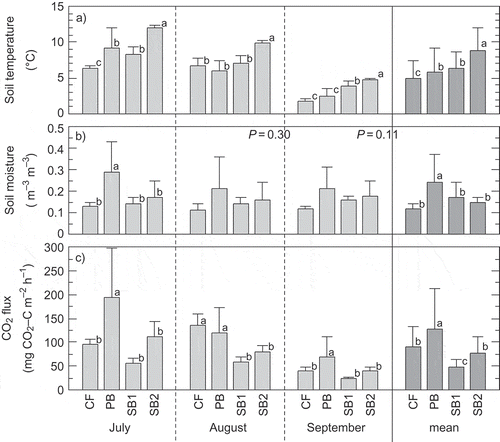
Soil temperature at SB2 was the highest among the sites in all months, whereas soil temperature at CF was generally lower than those at other sites (). Mean soil temperature was highest at SB2 (8.9 ± 3.1°C) and lowest at CF (5.0 ± 2.4 °C) (P < 0.01) (). Tsuyuzaki et al. (Citation2009) reported that surface albedo was lower at a burned site than at an unburned site in the PFRR, and that this difference in albedo affected soil temperature. We also considered that albedo was altered after forest fire, and this change was likely one of the factors explaining the soil temperature differences we observed between the severely burned forest (SB1 and SB2) and the control forest (CF). Iwahana et al. (Citation2005) found that a deep organic layer composed mainly of moss kept soil temperatures low in a larch forest in eastern Siberia. Thus, the thinner organic layers at SB1 and SB2 would have also caused the higher soil temperatures (). The mean soil moisture level was significantly higher at PB (0.24 ± 0.13 m3 m−3) than at the other sites () (P < 0.01). When the soil moisture data were analyzed for each sampling period, this significant difference was observed only for July. However, the trend in variation among the sites was similar through all sampling periods (). In addition, there were more live and dead patches of Sphagnum at PB than at the other sites (). This implies that PB was wetter than the other sites, because Sphagnum species prefer wet environments (Vitt et al. Citation1988).
Figure 1 (a) Mean soil temperature, (b) soil moisture and (c) carbon dioxide (CO2) flux from each site in each month and all data combined. Error bars are standard deviations (n = 18). Different letters within each row indicate significant differences (Fisher’s least-significance test, P < 0.05).CF = control forest, PB = partially burned, and SB = severely burned.
3.2 CO2 flux
The mean CO2 flux differed significantly among the sites (P < 0.01) () and decreased in the following order [mean ± SD mg carbon (C) m−2 h−1]: PB (128 ± 85) > CF (90 ± 42), SB2 (78 ± 47) > SB1 (47 ± 19). The mean CO2 flux at CF was similar to that at other unburned black spruce forest sites in interior Alaska (125–153 mg C m−2 h−1; reviewed by O’Neill et al. Citation2002). Within each site, CO2 flux was positively correlated with soil temperature (P < 0.01) (), and was lower at SB1 and SB2 than at CF and PB under high temperature conditions. In contrast, there was no relationship between CO2 flux and soil moisture at any of the sites (r2 = 0.01 to 0.21, P = 0.29 to 0.77). At SB1 and SB2, we attributed the low CO2 flux to decreased root respiration and organic matter decomposition. CO2 emissions from the soil are derived from root and microbial respiration (O’Neill et al. Citation2006). All trees at these sites, and the mosses and lichens on more than 95% of the area of these sites, had been killed (); as a result, soil respiration had decreased. O’Neill et al. (Citation2002) reported that root respiration in a mature black spruce stand contributed 75–78% of the total CO2 flux from the soil. However, CO2 flux did not differ significantly between CF and SB2; this was because decreased albedo (Chambers et al. Citation2005) and a thinner organic layer at SB2 (Iwahana et al. Citation2005) than at CF resulted in higher soil temperature at SB2 (). Higher soil temperature could also explain the higher CO2 flux at SB2 than at SB1 (). However, the adjusted CO2 flux at SB2 was lower than that at CF and did not differ from that at SB1 ().
Figure 2 Relationship between carbon dioxide (CO2) flux and soil temperature in each site. CF (control forest), y = 28.79e0.205x R2 = 0.75***; PB (partially burned), y = 50.47e0.124x R2 = 0.43**; SB1 (severely burned), y = 14.10e0.171x R2 = 0.52***; SB2 (severely burned), y = 21.36e0.134x R2 = 0.70***.
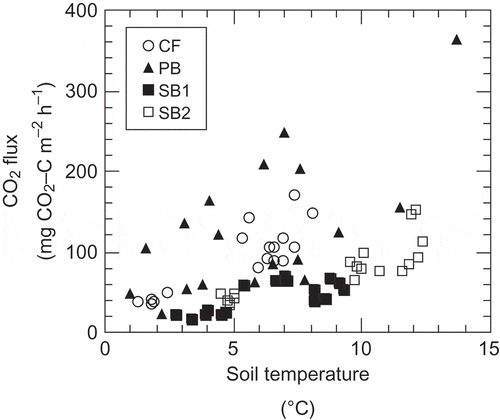
Figure 3 Relationship between adjusted carbon dioxide (CO2) flux and soil moisture. Regression line was drawn from only CF (control forest) and PB (partially burned) data, not including SB1 and SB2 (severely burned).
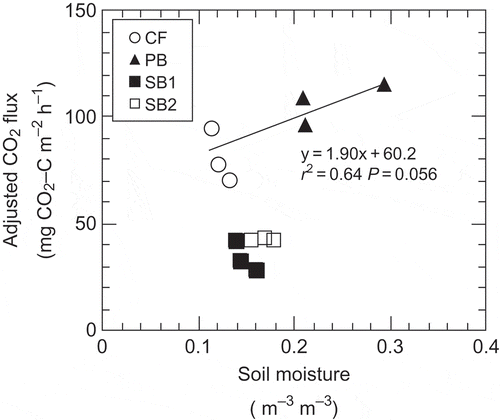
CO2 flux at PB was the highest among the sites (). Kim and Tanaka (Citation2003) estimated that CO2 flux decreased by 22 to 50% within 2 years after the forest fire at PB. The CO2 flux that we measured at PB was about 10 times that measured by Kim and Tanaka (Citation2003) immediately after the fire. O’Neill et al. (Citation2006) reported that the CO2 flux in a burned black spruce stand 10 years after a fire was half the flux in a mature stand. Thus, the CO2 flux not only recovered after the fire but also exceeded that before the fire. Further study is needed to clarify the mechanisms of this quick recovery, but there are some possible explanations. One is that soil moisture was also greater at PB than at other plots. Adjusted CO2 flux was positively correlated with soil moisture when we used the data from CF and PB (); this might partly explain the higher CO2 flux at PB than at CF. In addition, the higher soil temperature at PB than in the unburned CF plot would have resulted, in part, from a decrease in albedo through partial burning of the ground surface. O’ Neill et al. (Citation2006) suggested that increased soil moisture and soil temperature results in increased CO2 flux in burned black spruce stands, although their study sites experienced a higher intensity of forest fire than at PB. On the other hand, PB had a thicker organic layer than SB1 and SB2, probably because the fire intensity was much lower at PB than at SB1 and SB2 (). In addition, two thirds of the forest floor at PB remained unburned; these unburned areas could have been sources of the microbes involved in organic matter decomposition at the burned sites at PB. This implies that organic matter supply and microbial activity for decomposition were greater at PB than at SB1 and SB2. It has been suggested that the amount of easily decomposed organic material and the nutrient supply increase after fire (Vance Citation1996); the resultant increasing microbial activity levels can increase CO2 flux (Wüthrich et al. Citation2002). Thus, the soil moisture, temperature and nutrient supply levels at PB might have been favorable for the growth of roots and microorganisms that survived after the fire. This in turn might have given PB the greater CO2 flux than at our other study plots.
3.3 CH4 and N2O fluxes
At CF, the mean CH4 and N2O fluxes () were similar to those reported by Kim and Tanaka (Citation2003). However, at this site, the rate of CH4 uptake (i.e., the negative CH4 flux) was obviously greater, and the N2O emission rate lower, than those reported by Matson et al. (Citation2009). Differences in soil moisture conditions due to topographic differences may account for the CH4 and N2O flux differences. Black spruce stands occur on north-facing slopes and in bottomlands. The stands that we studied, and those studied by Kim and Tanaka (Citation2003), were on north-facing slopes, whereas Matson et al. (Citation2009) studied a bottomland forest in which Sphagnum spp. were dominant and soil moisture levels were higher (> 0.5 m3 m−3). Therefore, topographic differences must be considered in evaluating gas dynamics in black spruce forest.
Table 3 Methane (CH4) and nitrous oxide (N2O) fluxes at the study sites with soil temperature and moisture (mean ± SD, n = 6)
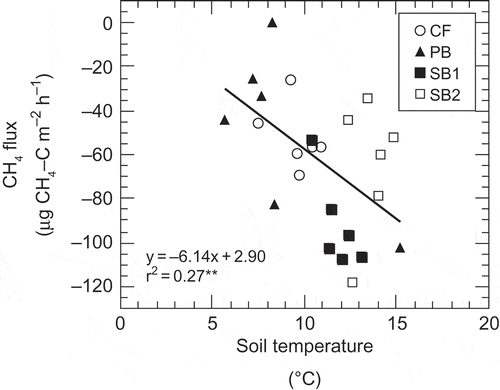
In boreal forests, CH4 uptake rates decrease after disturbances, such as fire (Kim and Tanaka Citation2003), clear-cutting (Morishita et al. Citation2005) or land-use change (Morishita et al. Citation2003). This is because such disturbances change the physicochemical properties of the soil, including the soil moisture conditions (Morishita et al. Citation2003, Citation2005). However, the CH4 uptake rate at SB1 (–91 ± 21 μg C m−2 h−1) was highest among the sites (P < 0.01) (). The variations that we observed in CH4 uptake rates could be explained by variations in soil temperature when we performed the analysis using data from all four sites combined. However, when we used the data from each site separately, the correlation was not significant (). The soil moisture conditions could also affect the CH4 uptake rate. A number of studies have reported that CH4 uptake rate decreases with increasing soil moisture content, owing to inhibition of CH4 diffusion from the atmosphere to the soil and the formation of anaerobic sites in the soil. In addition, Morishita et al. (Citation2014) found a positive correlation between CH4 flux and soil moisture in a larch forest in central Siberia; the soil moisture level at their site ranged from 0.15 to 0.70 m3 m−3. In our study, however, the range of soil moisture across all sites was narrow (0.10–0.25 m3 m−3), and the CH4 flux was not correlated with soil moisture (r = –0.07, P = 0.74). In fact, Morishita et al. (Citation2014) also found no clear relationship between CH4 flux and soil moisture when the soil moisture level was less than 0.4 m3 m−3. In addition, CH4 uptake generally occurs in the surface soils, rather than deeper soils, in boreal forests (e.g., Whalen and Reebrugh Citation1996, Saari et al. Citation1997); atmospheric CH4 concentration is a primary factor affecting the CH4 uptake rate (Hashimoto et al. Citation2011b), because atmospheric CH4 is an important substrate for methanotrophs in the surface soil. However, the mean atmospheric CH4 concentration at our four sites ranged from 1.93 to 1.97 ppmv and did not differ among sites. This suggests that the availability of atmospheric CH4 itself could not have accounted for the variation in CH4 uptake rate. Taken together, our findings suggest that soil temperature was a major parameter controlling CH4 uptake rate in black spruce stands with different fire histories.
Figure 4 Relationship between methane (CH4) flux and soil temperature.CF = control forest, PB = partially burned, and SB = severely burned.
N2O flux (3.6 ± 4.7 μg N m−2 h−1) was highest at PB among the sites (P < 0.01) (), and the standard deviation there was larger than at the other sites. Overall, N2O is produced by nitrification and denitrification processes, which are controlled by temperature and moisture conditions (Sahrawat and Keeny 1996). However, the N2O flux at our sites was not correlated with soil temperature (r = 0.17, P = 0.42) or soil moisture (r = –0.15 P = 0.48), within each site or across all four sites. After the forest fire at PB, N2O flux was reduced by 10 to 50% compared with before the fire (Kim and Tanaka Citation2003). Therefore, our data suggested that N2O flux had increased by the time of our study. Although we could not determine why the N2O flux was highest at PB, the effect of soil moisture should be considered. Although soil moisture levels during N2O flux measurement did not differ significantly among the sites (), PB was a wetter site than the others considering the data related to CO2 flux and our finding that the forest floor vegetation had a higher proportion of Sphagnum species ( and ). At PB, the variation in N2O flux was larger than that at other sites (). This suggests the existence of hot spots of N2O flux at PB from denitrification (Ambus and Zechmeister-Boltenstern Citation2007). However, our data were insufficient to reveal the mechanism behind the variation in N2O flux. In future studies, we will need to examine the spatial variation or seasonal change in N2O flux in relation to biotic and abiotic parameters so as to further elucidate the effect of fire intensity on N2O flux.
ACKNOWLEDGMENTS
We are grateful to Dr. Larry Hinzman and Dr. Masami Fukuda for their support of this study. We also thank Mr. Tadashi Sakata for assistance with the gas analyses. This study was partly supported by the Environment Research and Technology Development Fund (RF0902) of the Ministry of the Environment, Japan, and by a Grant-in-Aid for Scientific Research (B) (No. 22405027) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT).
REFERENCES
- ACRC (The Alaska Climate Research Center) 2014: Climate data in Fairbanks (Normal). http://climate.gi.alaska.edu/Climate/fairbanks (March, 2014)
- Ahlgren IF, Ahlgren CE 1960: Ecological effects of forest fires. Bot. Rev., 26, 483–533. doi:10.1007/BF02940573
- Ambus P, Zechmeister-Boltenetern S 2007: Denitrification and N-cycling in forest ecosystems. In Biology of the Nitrogen Cycle, Eds. Bothe H, Ferguson SJ, Newton WE, pp. 343–358. Elsevier, Amsterdam.
- Brouchkov A, Fukuda M 2002: Preliminary measurements on methane content in permafrost, central Yakutia, and some experimental data. Permafrost Periglacial Processes, 13, 187–197. doi:10.1002/ppp.422
- Chambers SD, Beringer J, Randerson JT, Chapin Iii FS 2005: Fire effects on net radiation and energy partitioning: contrasting responses of tundra and boreal forest ecosystems. J. Geophys.Res., 110, D09106.
- Hashimoto S, Morishita T, Sakata T, Ishizuka S, Kaneko S, Takahashi M 2011a: Simple models for soil CO2, CH4, and N2O fluxes calibrated using a Bayesian approach and multi-site data. Ecol. Modell., 222, 1283–1292. doi:10.1016/j.ecolmodel.2011.01.013
- Hashimoto S, Morishita T, Sakata T, Ishizuka S 2011b: Increasing trends of soil greenhouse gas fluxes in Japanese forests from 1980 to 2009. Sci. Rep., 1, 116. doi:10.1038/srep00116
- Ipcc 2007: Climate change 2007: The Scientific Basis: Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Houghton, J.T., Ding, Y., Griggs, D.J., et al. (Eds.), Cambridge University Press, Cambridge, 546 pp.
- IUSS (International Union of Soil Science) Working Group WRB 2006: World Reference Base for Soil Resources 2006. World Soil Resources Reports No. 103, Food and Agriculture Organization, Rome
- Iwahana G, Machimura T, Kobayashi Y, Fedorov AN, Konstantinov PY, Fukuda M 2005: Influence of forest clear-cutting on the thermal and hydrological regime of the active layer near Yakutsk, eastern Siberia. J. Geophys. Res., 110(G2), G02004.
- Kim Y, Tanaka N 2003: Effect of forest fire on the fluxes of CO2, CH4 and N2O in boreal forest soils, interior Alaska. J. Geophys. Res., 108, 8154. doi:10.1029/2001JD000663
- Lavigne MB, Foster RJ, Goodine G 2004: Seasonal and annual changes in soil respiration in relation to soil temperature, water potential and trenching. Tree Phys., 24, 415–424. doi:10.1093/treephys/24.4.415
- Longton RE 1988: Pattern, process and environment. In Biology of Polar Bryophytes and Lichens, Ed. Longton RE, pp. 66–105. Cambridge, NewYork.
- Matson A, Pennock D, Bedard-Haughn A 2009: Methane and nitrous oxide emissions from mature forest stands in the boreal forest, Saskatchewan, Canada. Forest Ecol. Manag., 258, 1073–1083. doi:10.1016/j.foreco.2009.05.034
- Morishita T, Hatano R, Desyatkin RV 2003: CH4 flux in an alas ecosystem formed by forest disturbance near Yakutsk, eastern Siberia, Russia. Soil Sci. Plant Nutr., 49, 369–377. doi:10.1080/00380768.2003.10410022
- Morishita T, Hatano R, Takahashi K, Kondrashov LG 2005: Effect of deforestation on CH4 uptake in Khabarovsk, far east Russia. Phyton, 45, 267–274.
- Morishita T, Matsuura Y, Kajimoto T, Osawa A, Zyryanova OA, Prokushkin AS 2014: CH4 and N2O dynamics of a Larix gmelinii forest in a continuous permafrost region of central Siberia during the growing season. Polar Sci., 8, 156–165. doi:10.1016/j.polar.2014.01.004
- Noguchi K, Dannoura M, Jomura M, Noguchi M, Matsuura Y 2012: High belowground biomass allocation in an upland black spruce (Picea mariana) stand in interior Alaska. Polar Sci., 6, 133–141. doi:10.1016/j.polar.2011.12.002
- O’Neill KP, Kasischke ES, Richter DD 2002: Environmental controls on soil CO2 flux following fire in black spruce, white spruce, and aspen stands of interior Alaska. Can. J. Forest Res., 32, 1525–1541. doi:10.1139/x02-077
- O’Neill KP, Richter DD, Kasischke ES 2006: Succession-driven changes in soil respiration following fire in black spruce stands of interior Alaska. Biogeochemistry, 80, 1–20. doi:10.1007/s10533-005-5964-7
- Paul EA 2007: Biochemistry and biogeochemistry. In Soil Microbiology, Ecology, and Biochemistry, Ed. Paul EA, pp. 303–467. Academic Press, Tokyo.
- Saari A, Martikainen PJ, Ferm A, Ruuskanen J, Boer de W, Troelstra SR, Laanbroek HJ 1997: Methane oxidation in soil profiles of Dutch and Finnish coniferous forests with different soil texture and atmospheric nitrogen deposition. Soil Biol. Biochem., 29, 1625–1632. doi:10.1016/S0038-0717(97)00085-0
- Sahrawat KL, Keeny DR 1986: Nitrous oxide emission from soils. In Advances in Soil Science, Ed. Stewart BA, volume 4, pp. 103–148. Springer, New York. doi:10.1007/978-1-4613-8612-4_2
- Sawamoto T, Hatano R, Yajima T, Takahashi K, Isaev AP 2000: Soil respiration in Siberian Taiga ecosystems with different histories of forest fire. Soil Sci. Plant Nutr., 46, 31–42. doi:10.1080/00380768.2000.10408759
- Schaefer K, Zhang T, Bruhwiler L, Barrett AP 2011: Amount and timing of permafrost carbon release in response to climate warming. Tellus B, doi:10.1111/j.1600-0889.2011.00527.x
- Tarnocai C, Canadell JG, Schuur EAG, Kuhry P, Mazhitova G, Zimov S 2009: Soil organic carbon pools in the northern circumpolar permafrost region. Global Biogeochem. Cycles, 23, GB2023. doi:10.1029/2008GB003327
- Todd SK, Jewkes HA 2006: Wildland fire in Alaska: A history of organized fire suppression and management in the last frontier, Agricultural and Forestry Experiment Station Bulletin No. 114, 64pp. University of Alaska Fairbanks
- Tsuyuzaki S, Kushida K, Kodama Y 2009: Recovery of surface albedo and plant cover after wildfire in a Picea mariana forest in interior Alaska. Climatic Change, 93, 517–525. doi:10.1007/s10584-008-9505-y
- UNEP 2008: The permafrost carbon feedback. In: Policy implications of warming permafrost, 18–20
- Van Cleve K, Chapin Iii FS, Flanagan PW, Vierick LA, Dyrness CT 1986: Forest ecosystems in the Alaskan taiga. Ecological Studies 57, 230pp. Springer-Verlag, New York.
- Vance ED 1996: Land application of wood-fired and combination boiler ashes: An overview. J. Environ. Qual., 25, 937–944. doi:10.2134/jeq1996.00472425002500050002x
- Viereck LA, Dyrness CT, Van Cleve K, Foote MJ 1983: Vegetation, soils, and forest productivity in selected forest types in interior Alaska. Can. J. Forest Res., 13, 703–720. doi:10.1139/x83-101
- Vitt DH, Marsh JE, Bovey RB 1988: Sphagnopsida (peat mosses). In A Photographic Field Guide to the Mosses, Lichens and Ferns of Northwest North America, Eds. Vitt DH, Marsh JE, Bovey RB, pp. 53–56. Lone Pine, Edmonton.
- Whalen SC, Reeburgh WS 1996: Moisture and temperature sensitivity of CH4 oxidation in boreal soils. Soil Biol. Biochem., 28, 1271–1281. doi:10.1016/S0038-0717(96)00139-3
- Wüthrich C, Schaub D, Weber M, Marxer P, Conedera M 2002: Soil respiration and soil microbial biomass after fire in a sweet chestnut forest in southern Switzerland. Catena, 48, 201–215. doi:10.1016/S0341-8162(01)00191-6
