Abstract
This study conducted flux measurements of carbon dioxide (CO2), nitrous oxide (N2O) and methane (CH4), estimated by 222Rn (Radon) and chamber methods in a cold-temperate northern Japanese grassland soil during summer and winter seasons. Our research aims to compare these fluxes of CO2, N2O and CH4 calculated by 222Rn and static chamber methods, and to understand the responses of fluxes by 222Rn and chamber methods to temperature. 222Rn fluxes ranged from 0.0046 to 0.0157 Bq m2 s−1, and the average was 0.0068 ± 0.0013 Bq m2 s−1 on sandy soil (> 50% sand). The average diffusion coefficients for CO2, N2O and CH4 calculated using the 222Rn method were 0.049 ± 0.008 cm2 s−1, 0.023 ± 0.005 cm2 s−1 and 0.054 ± 0.008 cm2 s−1, respectively, reflecting seasonality. CO2, N2O and CH4 flux measurements from the 222Rn method were in good agreement with those of the static chamber method, within the observed range of error, suggesting a high correlation coefficient of > 89% between the methods. Also, the temperatures of air and soil at 5-cm depth played a significant role in determining the fluxes of CO2 and CH4 measured by the 222Rn and chamber methods; meanwhile, the N2O flux displayed an inverse exponential relation to temperature. This suggests that N2O flux may be regulated by other factors such as soil water content and soil oxygen concentration. The contribution of winter fluxes of CO2, N2O and CH4 corresponded to 9, 51 and 3% of the annual fluxes of CO2, N2O and CH4, respectively, reflecting higher winter N2O production due to the constraint of oxygen in soil.
1. INTRODUCTION
Carbon dioxide (CO2), nitrous oxide (N2O) and methane (CH4) are important major trace gases as they absorb the Earth’s infrared radiation and contribute to global warming. CH4 and N2O have 23 and 296 times higher greenhouse warming potential than CO2 (Houghton et al. Citation2001), respectively. Further, CH4 is well known as a precursor of ozone destruction in the troposphere, and N2O is an agent in decreasing the stratospheric ozone level (Houghton et al. Citation2001).
In a natural environment, an aerated (i.e., unsaturated) soil plays a significant role in the emission of CO2 and N2O, and in the oxidation of atmospheric CH4. N2O production by aerated soils is 20% of the total global N2O source, and CH4 oxidation by soils contributes about 5% to the global CH4 sink (Houghton et al. Citation2001). CO2 is produced in soil by microbial decomposition of soil organic matter and by root respiration (Raich and Schlesinger Citation1992). These gases are principally transported to the soil air by molecular diffusion in aerated soil. The fluxes of CO2, N2O and CH4 at the soil surface and their concentrations in soil air depend on environmental factors such as temperature and soil moisture, as well as soil features including accumulated soil organic matter, soil microbial activity, soil type, soil pore space and so on (Dörr and Münnich Citation1987, Citation1990; Born et al. Citation1990; Nazaroff Citation1992; Dueñas et al. Citation1999; Kim and Tanaka Citation2002).
Gas transport in the soil was examined using the radioactive, inert, noble gas 222Rn (Radon, half-life: 3.82 d), which is produced by α-decay of 226Ra (Radium, half-life: 1602 y), and which has been used as a tracer of unsaturated soil in the method adopted by Born et al. (Citation1990), Dörr and Münnich (Citation1987, Citation1990), Trumbore et al. (Citation1990), Davidson and Trumbore (Citation1995), Dueñas et al. (Citation1999), and Kim and Tanaka (Citation2002). The 222Rn method requires significant additional labor compared to the static chamber method, because of the need to measure both depth profiles of 222Rn and other trace gases in the soil, and 222Rn flux at the soil surface. However, the 222Rn method does give significant information about trace gas diffusion in the soil throughout the year. Only a small fraction of 222Rn is produced by recoil from soil particles into the air, depending on the specific surface area of soil particles—and thus depending also on grain size distribution (Trumbore et al. Citation1990; Davidson and Trumbore Citation1995; Dueñas et al. Citation1999; Kim and Tanaka Citation2002). 222Rn flux at the soil surface is affected by 226Ra contents, as well as soil physical properties such as grain size distribution and resistance to molecular diffusion in the unsaturated soil zone. 222Rn flux at the soil surface and 222Rn concentration in soil air are also influenced by soil characteristics such as moisture content, tortuosity and porosity, which can be determined by measuring the 222Rn flux and concentration gradient at the same site. Further, the spatial variation of the 222Rn flux has been found to depend more on soil type than on the 226Ra activity of the soil material (Dörr and Münnich Citation1987, Citation1990; Dueñas et al. Citation1999). Further, the 222Rn method provides a proxy for temporal changes in diffusivity within the soil. This enables us to estimate rates of emission of CO2 and N2O, oxidation of CH4 through the soil to the atmosphere, production of CO2 and N2O within the soil using a one-dimensional vertical diffusion box model under a non-steady state condition, and mixing of 222Rn without any disturbance and contamination. Kim and Tanaka (Citation2002) previously reported the emission and production rates of N2O through snowpack to the atmosphere using this 222Rn method. To this point, previous reports have used the 222Rn method to estimate soil fluxes of CO2 and CH4. In response, we used 222Rn and static chamber methods to estimate fluxes of CO2, N2O and CH4 in a cold-temperate aerated grassland soil, located in northern Japan. The purposes of this study are (1) to compare the fluxes of CO2, N2O and CH4 calculated by 222Rn and static chamber methods, and (2) to understand 222Rn- and chamber-measured flux responses to temperature in our cold-temperate grassland soil of northern Japan, during summer and winter seasons.
2. THEORETICAL CONSIDERATIONS
The 222Rn method used to calculate fluxes of CO2, N2O and CH4 was based on a one-dimensional vertical model, as shown below. The diffusion transport of four gases (222Rn, CO2, CH4 and N2O) in the unsaturated soil zone are described by Fick’s first law:
Assuming horizontal homogeneity (i.e., advective transport = 0), our changes in soil air 222Rn concentrations with depth (z, taken as positive in the downward direction) and time (t) are described by the diffusion equation:
Assuming a steady-state condition, a vertically homogeneous QRn and a depth-independent diffusion coefficient (DRn), the 222Rn concentration profile is obtained by solving Eq. 3 with boundary condition CRn (z = 0) = 0, since the atmospheric 222Rn concentration is essentially equal to zero, and
:
The ratio of molecular diffusion coefficients is constant at a given temperature of 15°C (Dörr and Münnich Citation1987, Citation1990). The diffusion coefficient of CO2, N2O and CH4,
and
, can thus be calculated as
All equations are derived under the assumption that gas transport in the soil water phase can be neglected. This assumption is true for the most relevant gases (and especially for 222Rn, CO2, N2O and CH4), as the molecular diffusion coefficient of gases is 104 times smaller in water than in air, and solubility is on the order of 1 or even less (Nazaroff Citation1992), though we could not clearly measure soil moisture using this study.
3. MATERIALS AND METHODS
3.1 Research sites
The study site was located at the Institute of Low Temperature Science (H-1: 43°04’N, 141°20’E, 65 m above sea level) of the main campus of Hokkaido University, Sapporo, on the northern island of Japan. The site was typified by the brown forest and lowland soils of the characteristic Brown Lowland Soils (Fluvisol; Cultivated Soil Classification Committee Citation1995; IUSS Working Group WRB Citation2007), and by a cold-temperate climate. The average summer (July to August) and winter (January to February) temperatures in Sapporo for last 40 years are 21.2 ± 1.5°C and –3.8 ± 2.1°C, respectively. The average annual precipitation and the maximum snow depth for 40 years are 94 ± 30 cm and 98 ± 20 cm, respectively (Sapporo District Meteorological Observatory). The site H-1 is covered dominantly with orchard grass (Dactylis glomerata), and the soil shows an average bulk density of 0.69 ± 0.12 Mg m−3; 37% silt, 11% clay and 52% sand; and with 21.2 kg cm−2 and 2.05 kg cm−2 carbon and nitrogen organic matter by weight, respectively (Sapporo Regional Forest Office Citation1970). The study site had only been mowed—not fertilized, seeded or plowed—as part of site management. Grasslands porosity measurement varied from 0.152 to 0.274, according to the method of Osozawa and Kubota (Citation1987). A portable thermometer (Digital Thermometer, Iuchi Ltd., Japan) was used to measure temperatures in the air and the soil at 5 cm below the surface. The sampling interval was approximately 10 d, depending on the weather conditions, and was assumed to reach a steady-state condition for the 222Rn method. During the winter season of 1996–97, snow depths for Dec 24, 1996, and Jan 26, 1997, were 16 and 20 cm, respectively. Soil temperatures for these two observation days, during which the soil was still frozen, were –2.0 and –0.8°C, respectively.
3.2 Sampling by chamber and soil profile
222Rn fluxes from soil at site H-1 were measured simultaneously by monitoring of 222Rn in a static chamber placed over the soil. The flux chamber base was located within a few meters of in-soil probes, and had a stainless steel collar with a top diameter of 50 cm, a cross section area of 0.2 m2 and a height of 10 cm. This chamber was placed over the soil surface at the beginning of each measurement series, so as not to disrupt the soil surface. Air samples were drawn four to five times from the headspace in the chamber at 10–20 min intervals after deployment. The collected air sample was 10–20 mL, relatively small compared to the chamber volume.
Flux was calculated from the gradient of the relationship between concentration variations of 222Rn and the sampling period. The correlation coefficient (R2) was over 0.99, and the curve was linear (Livingston and Hutchinson Citation1995). These samples were introduced directly into the Lucas cell after radioactive equilibrium of 220Rn (Thoron: half-life 55 s) and the removal of water. Flux measurements and soil air sampling were carried out on clear afternoons.
222Rn flux from the soil surface can be calculated from the rate of concentration increase in the chamber:
Soil air samples for CO2, N2O, CH4 and 222Rn measurements were taken through a specially made concentric probe, preset at the selected depths a half month prior to the start of the series of air collections. The body of each probe consists of a modified stainless steel tube (0.2 cm inner diameter, 0.6 cm outer diameter, 100 cm length; GL Science, Inc., Japan). Probes were inserted vertically into the soil, and the horizontal distance between probes was approximately 10 cm. Sampling depths were at 5, 10, 20, 30, 40, 50, 70 and 100 cm during the summer season, and at 5, 10, 15, 20, 30 and 40 cm during winter—relatively shallower than summer, due to the frozen soil surface at site H-1. Probes were connected to polypropylene tubing, sealed with a rubber septum at the distal end. Ten milliliters of soil gas were collected with a plastic syringe from each probe and transferred to an aluminum foil bag (GL Science, Inc, Japan) for the analyses of CO2, N2O, CH4 and 222Rn.
3.3 Analyses of CO2, N2O, CH4 and 222Rn
These gas-filled bags were transported to the laboratory for analyses of CO2, N2O and CH4. CO2 concentrations were measured using a gas chromatograph equipped with a thermal conductivity detector (TCD-GC, GC-14B, Shimadzu Ltd., Japan), with a column packed with Porapack Q (80/100 mesh). Calibration was done with a series of standard gases containing 338 ± 7, 491 ± 10 and 5000 ± 100 ppmv CO2 in synthetic air (Nihon Sanso CO. Ltd., Japan). Calibration range precision was usually better than 1%. Soil air CO2 was analyzed within 10 h after sample collection, necessary due to the problem of sample preservation (data not shown), which was itself due to the higher CO2 transmissivity of the aluminum-made sampling bag. Concentrations of N2O were measured with a gas chromatograph equipped with an electron capture detector (ECD-GC, GC-14B, Shimadzu Ltd., Japan), with a column packed with Porasil Q (80/100 mesh). Calibration was conducted using a series of standard gases containing 302 ± 6, 784 ± 8 and 1308 ± 15 ppbv N2O in synthetic air (Nihon Sanso CO. Ltd., Japan). Calibration range precision was usually better than 2%. CH4 concentrations were measured with a gas chromatograph equipped with a flame ionization detector (FID-GC, GC-14B, Shimadzu Ltd., Japan), with a column packed with Molecular Sieve 13X (80/100 mesh). Calibration was conducted using a series of standard gases containing 0.79 ± 0.016, 1.61 ± 0.020 and 2.43 ± 0.048 ppmv CH4 in synthetic air (Nihon Sanso CO. Ltd., Japan). Calibration range precision was usually better than 3%.
Five milliliters of soil air for 222Rn measurements were collected from the soil probe in the same manner as for CO2, N2O and CH4 samples. This sampled air was immediately introduced into an evacuated phosphor-coated [ZnS(Ag)] Lucas cell (RDX-386, EDA Instruments Inc., Toronto, ON, Canada). Lucas cell air volume was 110 mL. 222Rn activity was measured using an alpha-ray scintillation counter (Model-2200 Scaler Ratemeter and Model-182 Radon Flask Counter, Ludlum Co., Ltd., Canada). 222Rn activities in the cell were counted after 3.5 h to attain radioactive equilibria with the short-lived daughter nuclides, including 218Po (Polonium, half-life: 3.05 m), 214Pb (Lead, half-life: 26.8 m), 214Bi (Bismuth, half-life: 19.7 m) 210Bi, and 214Po (half-life: 164 µs). The background value was counted for the evacuated cell after several repeated measurements of each gas sample. The background of each Lucas cell used ranged from 0.13 to 0.47 counts per minute (cpm). 222Rn activity was corrected for the background count of each cell and calibrated against a standard solution of 226Ra, of 12.9 Bq m−3. Uncertainty for the 222Rn measurements was about 7%, after we made 222Rn standard from a 226Ra standard solution in 20-L glass bottle and measured 222Rn activity a month later.
4. RESULTS
4.1 Concentration profiles of 222Rn, CO2, N2O and CH4
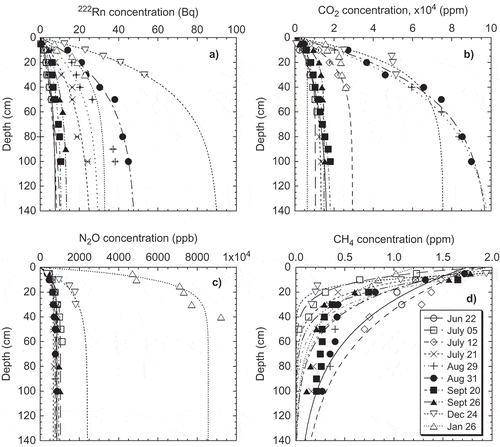
The concentration profiles of 222Rn, CO2 and N2O measured in aerated grassland soils increased exponentially with depth (–). Concentration gradients of 222Rn, CO2 and N2O in the aerated grassland soil were 10.8 ± 2.5 Bq m−3 cm−1, 520 ± 140 ppm cm−1 and 7.8 ± 1.7 ppb cm−1 through the observed soil column during the summer season, and 58 ± 25 Bq m−3 cm−1, 1160 ± 870 ppm cm−1 and 155 ± 26 ppb cm−1, respectively, during the winter. CH4 profile decreased with depth (), and the gradient was –0.021 ± 0.001 ppm cm−1 during the summer season and –0.040 ± 0.020 ppm cm−1 during the winter.
The profiles of these gases changed temporally throughout the year; production of 222Rn, CO2, and N2O, and CH4 oxidation, may be attributed to changes in effective pore space, depending on soil moisture, oxygen content and magnitude of soil microbial activity controlled by soil temperature. Generally, the profiles of 222Rn, CO2, N2O and CH4 during the winter season were much more different than during the summer.
4.2 Flux measurements by the chamber method
Fluxes of CO2, CH4 and N2O were also calculated using Eq. 16, and average CO2, CH4 and N2O fluxes were 38 ± 6.1 mg carbon (C) m2 h−1 (coefficient of variation, CV: 15%), 6.1 ± 0.8 µg nitrogen (N) m2 h−1 (12%) and –6.9 ± 1.1 µg C m2 h−1 (16%) during summer, and 4.4 ± 3.1 mg C m2 h−1 (72%), 8.5 ± 3.0 µg N m2 h−1 (35%) and –0.2 ± 0.2 µgC m2 h−1 (73%) respectively, during winter at site H-1. CO2 and CH4 fluxes during summer were 9- and 35-fold higher, respectively, than in winter. On the other hand, N2O flux during summer was slightly lower than during winter, which suggests that denitrification may have occurred due to constraint of soil oxygen by the frozen soil surface (Kim and Tanaka Citation2002), as shown in .
Figure 1 Concentration profiles of (a) 222Rn, (b) CO2, (c) N2O and (d) CH4, observed within northern Japanese grassland aerated soil during the summer and winter seasons. 222Rn, CO2, N2O, and CH4 profiles were fitted with exponential curves using the 222Rn method (R2 > 0.85). Each symbol denotes a sampling date of 1996/97.
4.3 222Rn flux from the soil surface
The 222Rn flux from the chamber method ranged from 0.0041 to 0.0157 Bq m2 s−1, with an average of 0.0068 ± 0.0013 Bq m2 s−1 on sandy soil (> 50% sand; CV 20%) over summer and winter seasons. According to Dörr and Münnich (Citation1990), in the temperate soils of western Germany, the wide variety of observed fluxes is caused by variations in 222Rn emanation rate, depending on the surface to volume ratio, as well as on the grain-size diameter of soil particles in different soil types, such as sand, loam, clay and silt. Thus, it is difficult to quantify the influence of soil moisture on tortuosity and emanation rate in this study.
The 222Rn fluxes measured in this study ranged between the lower 222Rn fluxes (0.0046–0.0093 Bq m2 s−1) of sandy soils (> 65% sand) and the higher 222Rn fluxes (0.0185–0.0278 Bq m2 s−1) of loamy and clay-like soils (> 35% clay) found in West Germany (Dörr and Münnich Citation1990). The value of the diffusion coefficient (DRn) ranged from 0.024 cm2 s−1 on September 26 to 0.093 cm2 s−1 on August 29, with an average of 0.050 ± 0.010 cm2 s−1 (CV: 21%). The average diffusion coefficient of 222Rn was similar to that estimated by Osozawa and Kubota (Citation1987) for the relative gas diffusion coefficient (0.048 ± 0.020 cm2 s−1), measured from soil horizons at the same site.
4.4 Fluxes of CO2, N2O and CH4 by the 222Rn method
Fluxes of CO2, N2O and CH4 were calculated using the 222Rn method and Eq. 14 and 15. Average diffusion coefficients of ,
and
were 0.049 ± 0.008 cm2 s−1 (CV 16%), 0.023 ± 0.005 cm2 s−1 (CV 20%) and 0.054 ± 0.008 cm2 s−1 (CV 15%) respectively, in aerated sandy soil, as shown in .
values ranged between 0.0013 cm2 s−1 in clay-like soil and 0.068 cm2 s−1 in sandy soil (> 68%), and
values varied from 0.0017 cm2 s−1 in clay-like soil and 0.069 cm2 s−1 in sandy soil (Dörr and Münnich Citation1990; Trumbore et al. Citation1990; Dueñas et al. Citation1999). However, there are few reports for the measurements of
from the 222Rn method, which yielded 0.002–0.046 cm2 s−1. The average relaxation depth of 222Rn was 152 ± 17 cm (CV: 11%) in sandy soil, which is similar to the value measured by Dorr and Munnich (Citation1990). The relaxation depths of CO2, N2O and CH4 are shown in , representing estimations of CO2, N2O and CH4 fluxes from the 222Rn method. The average relaxation depths of CO2, N2O and CH4 were 127 ± 14 cm (CV 9%), 211 ± 78 cm (CV 37%) and 152 ± 17 cm (CV 11%) respectively, at site H-1. CO2 and CH4 profiles showed similar relaxation depths, indicating similar depth distributions of the CO2 source and CH4 sink (Dörr and Münnich Citation1990; Dueñas et al. Citation1999). Contrary to the profiles of CO2 and CH4, the N2O profile is relatively shallower than the depths of CO2 and CH4, suggesting the depth of the N2O source may be shallower than that of CO2 in aerated sandy soil.
Table 1 Calculated diffusion coefficients, relaxation depths, and fluxes of CO2, N2O and CH4 by the 222Rn method in grassland aerated soil, northern Japan, during the summer and winter of 1996 and the winter of 1997
4.5 CO2, N2O and CH4 fluxes by the two methods
CO2, N2O and CH4 fluxes determined by the 222Rn method were compared with those estimated by the chamber method over grassland soils, and the ratios showing these comparisons are listed in . The ratios of CO2, N2O and CH4 fluxes during the summer season were somewhat higher than during the winter season. Ratios ranged from 0.84 to 1.64 for CO2, 0.70 to 1.20 for N2O and 0.57 to 1.44 for CH4 flux, respectively. CO2, N2O and CH4 fluxes calculated using both methods fluctuated temporally in the aerated grassland soil throughout the seasons. The magnitudes of temporal variation in CO2, N2O and CH4 fluxes were 20, 14 and 28% for the 222Rn method, and 21, 12 and 22% for the chamber method, respectively. The factor of temporal variation is used to quantify temporal variability in each flux throughout the year. Seasonal variations in CO2, N2O and CH4 from the two methods are related to fluctuations in environmental factors such as soil temperature, soil organic carbon, soil moisture, grain size and so on. Interestingly, there was no distinct difference in the ratio between summer and early winter, except for the data obtained for January 26, 1997. This may be due to the frozen ground during the winter season. Additional study will be required to estimate these fluxes, including for the winter season, for better understanding of these gases’ transport mechanism(s).
Table 2 The flux ratio of the 222Rn method to the chamber method in sandy soil, northern Japan
5. DISCUSSION
5.1 Comparison of CO2, N2O and CH4 fluxes by the two methods
CO2 flux estimated by the 222Rn method was slightly higher than that from the chamber method, suggesting that it may be due to some amount of soil moisture occurring during observation (Dörr and Münnich Citation1990; Dueñas et al. Citation1999). Unfortunately, the profile of soil moisture was not observed through this study, owing to the disturbance. Dorr and Munnich (Citation1990) and Dueñas et al. (Citation1999) also reported that CO2 and CH4 flux values measured by the 222Rn method tended to be higher than those measured by the chamber method, as observed in this study. On the other hand, N2O flux measured here by the chamber method is slightly higher than from the 222Rn method, which is due possibly to different soil types and environmental factors. A regression analysis in this study revealed that the differences between the two methods were not significant (p < 0.001). The validities of both flux measurement methods for CO2, N2O and CH4 were effectively confirmed. Specifically, CO2, N2O and CH4 fluxes estimated by the 222Rn method elucidated 89, 91 and 90% of the variability in CO2, N2O and CH4 flux measurements, respectively, from the chamber method within the aerated soil of site H-1. As a result, we found the 222Rn method analogous to the static chamber method for flux estimation of these greenhouse gases in this grassland of northern Japan.
Flux measurements of CO2, N2O and CH4, meanwhile, could be not represented due to the heterogeneity of the point-to-point site within grassland soils (Sommerfeld et al. Citation1996). To accurately estimate CO2, N2O and CH4 fluxes in heterogeneous systems, multiple CO2, N2O and CH4 flux measurements are needed to cover the target study site more widely. During winter, N2O fluxes estimated using both methods were much higher than those estimated during summer, suggesting that winter N2O flux may be attributed to the denitrification process from a deficiency of soil oxygen (Williams et al. Citation1992; Kim and Tanaka Citation2002), as well as an increase in soil moisture (Williams et al. Citation1992).
Concentrations of CO2, N2O and CH4 in the chamber varied linearly with time (R2 ≧ 0.99), suggesting that the results of these chamber measurements are indeed reliable (Livingston and Hutchinson Citation1995). Small concentration changes with a short sampling interval would not lead to large errors in flux determination. The static chamber method is well suited to easily estimate trace gas fluxes over a short period; however, this method can sufficiently explain neither the process of soil-originated CO2 and N2O production (Kim and Tanaka Citation2002), nor the depth-dependence distribution of the source-strength of CO2 and N2O within the soil.
5.2 Environmental factors regulating CO2, N2O and CH4 fluxes
Temperature and soil moisture are well-known environmental factors affecting CO2, N2O and CH4 fluxes in different soils. The relationships between temperature in air and soil at 5 cm depth and CO2, N2O and CH4 fluxes from the 222Rn and chamber methods are shown exponentially in . The correlation coefficients (R2) between air temperature and CO2 and CH4 fluxes by 222Rn and chamber methods were 0.73 and 0.77 for CO2 flux and 0.71 and 0.76 for CH4 flux, respectively, suggesting that air temperature accounts for > 70% of the variability in both CO2 and CH4 fluxes. Coefficients for soil temperature were 0.64 and 0.69 for CO2 flux, and 0.64 and 0.68 for CH4 flux, estimated by the 222Rn and chamber methods. On the other hand, N2O flux had an inversely exponential relationship to both air temperature (R2: 0.52 and 0.49) and soil temperature (R2: 0.54 and 0.50), as shown in and . This indicates that N2O flux magnitude is dependent on both soil moisture content (Williams et al. Citation1992) and soil oxygen concentration (Goreau et al. Citation1980; Williams et al. Citation1992; Kim and Tanaka Citation2002). As we measured neither soil moisture content nor oxygen concentration in the soil, it was difficult to figure the environmental factors affecting N2O flux in this study. However, Keeney et al. (Citation1979) and Zhou et al. (Citation2005) reported that N2O flux occurs in an inverse relationship with temperature, and Schimel et al. (1993) described temperature as a strong controller of microbially mediated production and consumption of trace gases. Other factors, such as substrate and moisture availability, could also often be limiting, to the extent that the temperature effect was not expressed. As a result, our findings suggest an inversely exponential relationship between N2O flux and temperature in this study.
Figure 2 Responses from fluxes measured by the 222Rn and chamber methods to air temperature (a, b, and c) and soil temperature at 5-cm depth (d, e and f) in aerated grassland soil. Negative CH4 denotes the oxidation of atmospheric CH4 to the soil. Solid and dotted lines denote exponential curves for 222Rn and chamber methods, respectively. Air and soil temperatures were significant, key factors in determining CO2 and CH4 fluxes, measured by 222Rn and the chamber method; however, N2O flux did not depend on temperature.
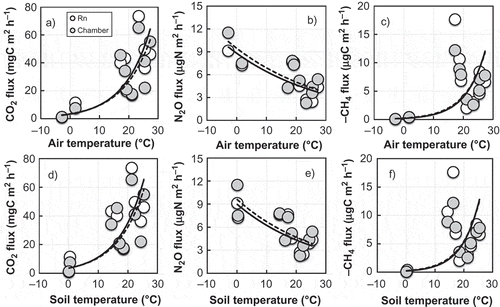
The Q10 value represents a temperature sensitivity of CO2, N2O and CH4 fluxes, and is defined as the ratio of reaction rate at an interval of 10°C, Q10 = exp (10 × β), where β is constant. The Q10 for air temperature was 2.98 and 2.79 for CO2, 0.73 and 0.74 for N2O and 4.58 and 4.21 for CH4, according to the 222Rn and chamber methods, respectively. Correspondingly, Q10 for soil temperature was 3.14 and 2.99 for CO2, 0.54 and 0.50 for N2O and 5.09 and 4.66 for CH4 for the 222Rn and chamber methods, respectively. Remarkably, Q10 for CH4 flux on soil temperature was much higher than for air temperature. Whalen and Reeburgh (Citation1990) suggested that CH4 oxidation rates can be measured by adding 14CH4 to a sample in a sealed incubation jar, supporting a decrease in 14CH4 and the increase in 14CO2. Our findings demonstrate that soil temperature has greater effect than air temperature in regulating the fluxes of CO2 and CH4, as well as also affecting the magnitude of soil microbial activity that controls the flux strengths of CO2 and CH4 in aerated grassland soils (Nazaroff Citation1992). Although the 222Rn and chamber methods were effective for the flux measuements of greenhouse gases, additional study is needed to better understand the production mechanism of CO2, N2O and CH4 oxidation in soil by using stable isotopes.
Seasonal fluxes of CO2, N2O and CH4 were 200 ± 22 and 19 ± 9.2 g C m2 season−1, 28 ± 2.8 and 30 ± 4.6 mg N m2 season−1 and –37 ± 5.2 and −0.8 ± 0.3 mg C m2 season−1, respectively, during the 215-d summer and 150-d winter in the aerated grassland soil of Northern Japan. The winter fluxes of CO2, N2O and CH4 represent 9, 51 and 3% of the annual fluxes, reflecting higher winter N2O production due to the constraint of soil oxygen (Kim and Tanaka Citation2002).
6. CONCLUSION AND FUTURE WORK
Flux measurements of CO2, N2O and CH4 estimated by the 222Rn and chamber methods were compared in cold-temperate grassland soil of northern Japan. From the results of the 222Rn method, average diffusion coefficients and relaxation depths of CO2 and CH4 were similar to previous values in aerated sandy soils (Dörr and Münnich Citation1990; Trumbore, Citation1990; Dueñas et al. Citation1999; Kim and Tanaka Citation2002). There are yet few reports for a diffusion coefficient and relaxation depth of N2O and, as a result, we first report here on these factors for our aerated grassland soil site. There is no significant difference between the CO2, N2O and CH4 fluxes measured by the 222Rn method and those by the chamber method, with a 95% confidence level. Soil temperature was a much more important factor than air temperature in determining fluxes of CO2 and CH4, and to a greater extent N2O flux, in aerated grassland soils.
The winter contributions from CO2, N2O and CH4 fluxes corresponds to 9%, 51 and 3% of total annual fluxes for CO2, N2O and CH4. Although there are few reports for winter N2O emission and observation frequency through the year of this study, emission should not be overlooked in estimations of regional nitrogen budget, due to higher winter N2O contribution to the atmosphere (Kim and Tanaka Citation2002).
ACKNOWLEDGMENTS
We appreciate our colleagues in the Laboratory of Marine and Atmospheric Geochemistry (MAG), Hokkaido University, for their cooperation throughout this study. We thank Dr. H. Narita at Tokai University for 222Rn analysis, and Nate Bauer (IARC), University of Alaska Fairbanks, for constructive editorial revisions of the manuscript.
REFERENCES
- Born M, Dorr H, Levin I 1990: Methane consumption in aerated soils of the temperate zone. Tellus, 42B, 2–8. doi:10.1034/j.1600-0889.1990.00002.x
- Cultivated Soil Classification Committee 1995: Classification of cultivated soils in Japan. Third approximation. Misc. Publ. Natl. Inst. Agro-Environ. Sci., 17, pp. 79.
- Davidson EA, Trumbore SE 1995: Gas diffusivity and production of CO2 in deep soils of the eastern Amazon. Tellus B, 47, 550–565. doi:10.1034/j.1600-0889.47.issue5.3.x
- Dörr H, Münnich KO 1987: Annual variation in soil respiration in selected areas of the temperate zone. Tellus, 39B, 114–121.
- Dörr H, Münnich KO 1990: 222Rn flux and soil air concentration profiles in West-Germany. Soil 222Rn as tracer for gas transport in the unsaturated soil zone. Tellus B, 42, 20–28. doi:10.1034/j.1600-0889.1990.t01-1-00003.x
- Dueñas C, Fernández MC, Carretero J, Liger E 1999: Methane and carbon dioxide fluxes in soils evaluated by 222Rn flux and soil air concentration profiles. Atmos. Environ., 33, 4495–4502. doi:10.1016/S1352-2310(99)00189-2
- Goreau TJ, Kaplan WA, Wofsy SC, McElroy MB, Valois FW, Watson SW 1980: Production of NO2- and N2O by nitrifying bacteria at reduced concentration of oxygen. Appl. Environ. Microbiol., 40, 526–532.
- Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA Ed. 2001: Climate Change 2001: The Scientific Basis. IPCC, pp. 239–253. Cambridge University Press, London, UK.
- IUSS Working Group WRB 2007: World Reference Base for Soil Resources 2006, first update 2007. World Soil Resources Reports No. 103. FAO, Rome, pp. 128.
- Keeney DR, Fillery IR, Marx GP 1979: Effect of temperature on the gaseous nitrogen products of denitrification in a silt loam soil. Soil Sci. Soc. Am. J., 43(6), 1124–1128. doi:10.2136/sssaj1979.03615995004300060012x
- Kim Y, Tanaka N 2002: Winter N2O emission rate and its production rate in soil underlying the snowpack in a sub-boreal region, Japan. J. Geophys. Res., 107(D19), 4406. doi:10.1029/2001JD000833
- Livingston GP, Hutchinson GL 1995: Enclosure-based measurement of trace gas exchange: applications and source error. In Biogenic Trace Gases: Measuring from Soil and Water, Ed. Matson PA, Harriss RC, pp. 14–51. Blackwell Scientific Publications, USA.
- Nazaroff WW 1992: Radon transport from soil to air. Rev. Geophys., 30(2), 137–160. doi:10.1029/92RG00055
- Osozawa S, Kubota T 1987: A simple method to determine gas diffusion coefficient in soil. Jpn. J. Soil Sci. Plant Nutr., 58, 528–535. (in Japanese)
- Raich JW, Schlesinger WH 1992: The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus B, 44, 81–99. doi:10.1034/j.1600-0889.1992.t01-1-00001.x
- Sapporo District Meteorological Observatory. http://www.jma-net.go.jp/sapporo
- Sapporo Regional Forest Office 1970: Regional forest soil survey report. 8, pp. 145. (in Japanese)
- Schimel JP, Holland EA, Valentine D 1993: Controls on methane flux from terrestrial ecosystems. In Agricultural Ecosystem Effects on Trace Gases and Global Climate Change, ASA Special Publication No. 55, Ed. Harper LA, Mosier AR, Duxbury JM, pp. 167–182. Agronomy Society of America, Madison, Wisconsin, USA.
- Sommerfeld RA, Massman WJ, Musselman RC, Mosier AR 1996: Diffusional flux of CO2 through snow: Spatial and temporal variability among alpine-sub-alpine sites. Glob. Biogeochem. Cycles, 10, 473–482. doi:10.1029/96GB01610
- Trumbore SE, Keller M, Wofsy SC, Costa JM 1990: Measurements of soil and canopy exchange rates in the Amazon rain Forest using 222Rn. J. Geophys. Res., 95, 16865–16873. doi:10.1029/JD095iD10p16865
- Whalen SC, Reeburgh WS 1990: Consumption of atmospheric methane by tundra soils. Nature, 346, 160–162. doi:10.1038/346160a0
- Williams EJ, Hutchinson GL, Fehsenfeld FC 1992: NOx and N2O emissions from soil. Glob. Biogeochem. Cycles, 6, 35–388. doi:10.1029/92GB02124
- Zhou C, Zhang D, Wang Y, Zhoe G, Liu S, Tang X 2005: Diurnal variations of greenhouse gas fluxes from mixed broad-leaved and coniferous forest soil in Dinghushan. Chin. For. Sci. Technol., 4(2), 1–7.
