Abstract
Application of organic nitrogen (N) has been proposed as a method to reduce potential environmental pollution due to N loss without sacrificing grain yield. The mechanism responsible for organic N regulation of maize (Zea mays L.) N metabolism is nonetheless largely unknown. In this study, we compared the expression of genes related to N assimilation and remobilization during the grain filling stage in maize plants grown under field conditions. We applied five different N treatments, which consisted of N supplied in organic and/or inorganic forms at the following rates: 0/45, 0/120, 0/240, 120/120, and 240/0 kg ha−1 organic/inorganic N. Yield was found to increase with increasing N input, but no significant difference was found in grain yield among 0/240, 240/0 and 120/120 treatments. Organic N application tended to decrease N accumulation and to increase N utilization efficiency. Genes related to N assimilation activity in leaves, such as ZmNR1 and ZmFd-GOGAT1, were unaffected by different N forms. In contrast, genes related to N remobilization activity in leaves, such as ZmGS1.1 and ZmGDH1, were up-regulated, especially in old leaves, by the pure organic N supply treatment (240/0). These data suggest that application of pure organic N likely induces a N-deficiency response in maize plants, with a consequent increase in physiological N utilization efficiency due to up-regulation of key genes involved in N remobilization processes.
INTRODUCTION
Since the 1950s, synthetic nitrogen (N) fertilizers have been extensively used to increase maize (Zea mays L.) yield (Hirel et al. Citation2011). Also starting in the 1950s, agriculture in China has undergone a gradual transformation from traditional organic farming to modern chemical fertilizer-based operations (Ellis et al. Citation2000). The proportion of organic N fertilizers used on arable lands has declined from approximately 99.6% in 1949 to around 20.9% in 2000 (Zhu and Chen Citation2002). Many factors affect the amount of N input in fields, such as crop species, soil N content, and the timing and method of N application. Compared with synthetic N fertilizers alone, combined application of organic and synthetic N fertilizers has been found to improve crop yield and N uptake efficiency (Azeez et al. Citation2010; Zhao et al. Citation2011).
Because of the slow-release characteristics of organic N in soils, N uptake and utilization by plants under organic N supplementation may differ from those of plants treated with inorganic N. Expression levels of marker genes can be used as tools to evaluate plant N status and to subsequently determine optimal levels of N fertilizer application (Yang et al. Citation2011). For optimization of organic N application rates, however, the use of such markers is hampered by our limited understanding of the molecular response of N-related genes to organic N supply. Nevertheless, the roles of many enzymes involved in N assimilation have currently been elucidated. In leaves, the enzyme nitrate reductase (NR; EC [Enzyme Commission] 1.6.6.1) catalyzes the reduction of nitrate to nitrite—the first limiting step in the primary N assimilation process (Campbell Citation1999). NR transcript levels are likely correlated with N reduction ability in maize leaves (Martin et al. Citation2005). The enzyme glutamate synthase (GOGAT; EC 1.4.7.1), another key enzyme involved in the primary N assimilation process, assimilates ammonium into amino acids. Chloroplast-localized glutamine synthetas 2 (GS2) in combination with a ferredoxin-dependent isoenzyme (Fd-GOGAT) functions to assimilate ammonium (NH4+) restored in leaves (Masclaux-Daubresse et al. Citation2010; Hirel et al. Citation2011). Aspartate aminotransferase (AspAT; EC 2.6.1.1) is localized mainly in green tissues and plays a role in providing aspartate (Asp) for Asp-derived amino acid biosynthesis (de la Torre et al. Citation2006). Three genes encoding ZmAspAT1 and two genes encoding ZmAspAT2 have been identified in maize (Cañas et al. Citation2009). The enzyme Nicotinamide Adenine Dinucleotide -dependent (NAD-dependent) glutamate dehydrogenase (GDH; EC 1.4.1.2) is the key enzyme involved in the formation of ammonia (NH3) and 2-oxoglutarate through amino acid metabolism (Andrews et al. Citation2013). Two GDH genes in maize, ZmGDH1 and ZmGDH2, have been identified and found to be constitutively expressed throughout the whole plant (Cañas et al. Citation2009). The enzyme cytosolic glutamine synthetase (GS; EC 6.3.1.2) is involved in the N remobilization process and contributes significantly to N use efficiency in maize (Masclaux-Daubresse et al. Citation2010). Glutamine synthetase 1 (GS1) isoforms are newly expressed to synthesize glutamine in senescing leaves (Lothier et al. Citation2011). Five genes encoding GS1 have been identified in maize (Masclaux-Daubresse et al. Citation2010). Asparagine synthetase (AS; EC 6.3.5.4) can catalyze NH4+ assimilation in plants, and its expression is induced during leaf senescence. Four AS genes have been discovered in maize; the most important of these, ZmAS1, is expressed in leaves (Cañas et al. Citation2009).
Quantitative real-time polymerase chain reaction (qRT-PCR) is the simplest and most effective tool for gene expression analysis (Cañas et al. Citation2009). In the present study, we used qRT-PCR to test the expression of N-related genes in response to supplied organic N. We aimed to shed light on the molecular regulatory actions of applied organic N on maize N metabolism.
MATERIALS AND METHODS
Plant materials and experimental design
The field experiment was carried out in Dezhou, China (37.35° N, 116.57° E; altitude 21.36 m above sea level) in 2010 and 2011. The experimental design was a split-block experiment with three replications. The plots were 6 m long × 2.75 m wide. Five N treatments were applied, consisting of 0/45, 0/120, 0/240, 120/120, or 240/0 kg ha−1 organic/inorganic N. Fresh cow manure, used as organic N fertilizer, and urea (46% N), used as inorganic N fertilizer, were applied before sowing and subsequently plowed into the topsoil. Phosphorus (as triple superphosphate) and potassium (as potassium sulfate) were applied to all plots to achieve rates of 150 kg ha−1. However, if the 240 kg ha–1 manure plot had levels of P2O5 (Phosphorus pentoxide) and K2O (Potassium oxide) greater than 150 kg ha–1, then phosphorus and potassium were applied to all plots to achieve the same levels as in the fresh cow manure of 240 kg N ha–1. Maize hybrid cultivar NE9 was planted on June 23 in 2010 and June 20 in 2011, and harvested on October 6 in both years. The planting density was 60,000 plants ha−1. Soil physical and chemical characteristics at the onset of the experiment are listed in Table S1. Soil N contents at silking and maturity are given in Table S2.
Sampling and measurement
At maturity, two rows of plants were harvested for yield measurements. To determine grain moisture, grain was oven-dried and standardized to 14% moisture. Three plants from each plot were harvested at the soil surface, oven-dried at 70°C and weighed. N concentration was measured by the Kjeldahl method. Physiological N utilization efficiency (NUtE) was calculated as grain yield/N accumulation at maturity. Nitrogen harvest index (NHI) was calculated as grain N content/N accumulation at maturity × 100%.
For gene expression analysis, the ear leaf and the fourth leaf below the ear leaf, respectively, were harvested at silking (R1) and 28 days after silking (R4) in 2010. All leaf samples were harvested between 9 a.m. and 12 p.m. After removal of the main leaf midrib, leaves were frozen in liquid nitrogen (N2), ground to a homogenous powder, and stored at −80°C for subsequent RNA analyses. Total RNA samples were extracted using Trizol reagent and treated with an RNase-free DNase kit (Invitrogen) to remove DNA contamination, which was verified by the failure of PCR amplification using ZmACT1 intron primers. Following reverse transcription, qRT-PCR amplifications were performed using SYBR Green dye on a 7500 Real-Time PCR system (Applied Biosystems). The maize ZmTUB gene was used as an internal control (Gu et al. Citation2013). The primers used in the qRT-PCR analysis are listed in Table S3. The qRT-PCR products were sequenced and found to be homologous to their corresponding genes in GenBank.
Statistical analysis
The experimental data were analyzed by analysis of variance using the SAS statistical package (SAS Institute, Cary, NC, USA). Differences were compared by the least significant difference (LSD) test at the P ≤ 0.05 probability level.
RESULTS
Grain yield, N accumulation, NUtE and NHI
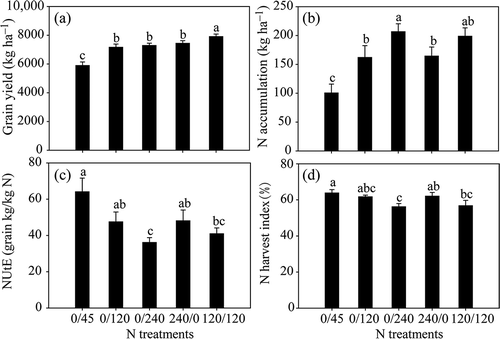
As shown in , grain yield increased with increasing levels of supplied N. When maize was grown at levels of 240 kg ha−1 N supply, no significant difference was observed among the 0/240, 240/0 and 120/120 treatments. This result indicates that the form of N—inorganic vs. organic—had no significant effect on maize grain yield. By contrast, plant N accumulation increased with increasing N supply (), while NUtE decreased (). Under sufficient N levels (240 kg ha−1), application of organic N decreased plant N accumulation but increased NUtE and NHI (). These results suggest that the slow N release that takes place under pure organic N treatment conditions may limit plant N uptake; the remobilization process in these plants may therefore be compensatory stimulation to enhance NUtE.
Figure 1 Grain yield, (a) nitrogen (N) accumulation, (b) N utilization efficiency (NUtE), (c) and N harvest index (d) of maize plants (Zea mays L.) grown under different N treatments. Bars indicate means ± standard error (SE) (n = 6). Significant differences at P < 0.05 are indicated by different letters.
Expression of N-related genes
Under the various N treatments, expression levels of ZmNR1, ZmFd-GOGAT1, ZmAspAT1.3, ZmGDH1, ZmGS1.1 and ZmAS1 varied significantly among leaf types (ear leaves and lower leaves [fourth leaves below ear leaves]) and developmental stages at harvest time (silking stage R1 and grain-filling stage R4).
Genes involved in N assimilation
In both ear leaves and lower leaves, ZmNR1 expression was higher at R4 than at R1 (, ). Among N treatments, high N supply caused a reduction in ZmNR1 expression, except for induction in lower leaves at R4 (). No significant difference was observed in ZmNR1 expression among high-N treatments (i.e., 240 kg ha−1).
Figure 2 Expression of genes ZmNR1 (a, b), ZmFd-GOGAT1 (c, d) and ZmAspAT1.3 (e, f) in ear leaves (a, c, e) and lower leaves (b, d, f) of maize plants (Zea mays L.) grown under different nitrogen (N) treatments. Transcript levels of ZmNR1, ZmFd-GOGAT1 and ZmAspAT1.3 were normalized to those of the control gene Alpha tubulin4, which were quantified by quantitative real-time polymerase chain reaction (PCR) using gene-specific primers. Bars indicate means ± standard deviation (SD) (n = 3). Significant differences within the same growth stage (silking or 28 days post-silking, DAS) at P < 0.05 are indicated by different letters.
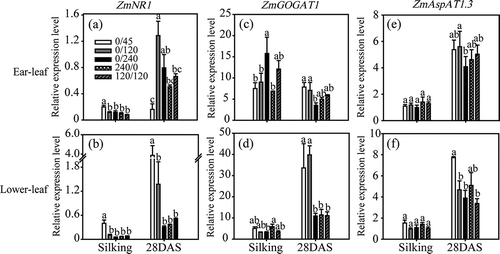
Expression of ZmFd-GOGAT1 in ear leaves was higher at R1 compared with R4 (). With increasing N supply, ZmFd-GOGAT1 was up-regulated at R1 and down-regulated at R4. At R1, ZmFd-GOGAT1 expression in ear leaves was higher under pure organic N supply than under pure inorganic supply, while the opposite situation was observed in lower leaves. At R4, application of organic N instead of inorganic N had no effect on ZmFd-GOGAT1 expression (, ).
ZmAspAT1.3 expression was higher at R4 than at R1. In both types of leaves at either developmental stage, no significant variation was observed in ZmAspAT1.3 expression among the various N treatments (, ).
Genes involved in N remobilization
ZmGDH1 expression was unaffected by developmental stage across all N treatments (, ). High N supply reduced ZmGDH1 expression in both ear leaves and lower leaves. At both R1 and R4 stages, no change in ZmGDH1 gene expression was detected among the 0/240,120/120 and 240/0 treatments, except that the corresponding expression at R4 in ear leaves supplied with pure organic N was higher than under inorganic N ().
Figure 3 Expression of genes ZmGDH1 (a, b), ZmGS1.1 (c, d) and ZmAS1 (e, f) in ear leaves (a, c, e) and lower leaves (b, d, f) of maize plants (Zea mays L.) grown under different nitrogen (N) treatments. Transcript levels of ZmGDH1, ZmGS1.1 and ZmAS1 were normalized to those of the control gene Alpha tubulin4, which were quantified by quantitative real-time polymerase chain reaction (PCR) using gene-specific primers. Bars indicate means ± standard deviation (SD) (n = 3). Significant differences within the same growth stage (silking or 28 days post-silking, DAS) at P < 0.05 are indicated by different letters.
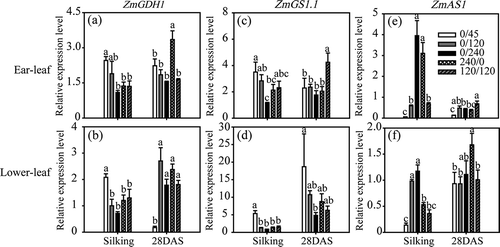
In lower leaves but not in ear leaves, ZmGS1.1 expression was higher at R4 than at R1 (, ). As N levels were increased, ZmGS1.1 expression was down-regulated in both ear leaves and lower leaves at R1, except that higher expression was observed at R4 in ear leaves supplied with a mixture of organic and inorganic N (120/120).
In ear leaves but not in lower leaves, ZmAS1 expression was higher at R1 than at R4 (, ). In ear leaves, the expression of ZmAS1 at R1 was higher under the 0/240 and 240/0 treatments than under the 120/120 treatment; at R1, expression was higher under the 120/120 treatment than under the 0/240 and 240/0 treatments (). In lower leaves, ZmAS1 was down-regulated under the 0/240 treatment compared with 120/120 at R1, while ZmAS1 expression at R4 was higher under the 240/0 treatment than under the 120/120 treatment (). Importantly, ZmAS1 expression was highly induced in lower leaves at R4 when pure organic N was supplied.
DISCUSSION
In our study, we found that insufficient N supply reduced grain yield and N accumulation while increasing NUtE and NHI (). Plants supplied with pure organic N (0/240) exhibited low N accumulation and high NUtE, similar to plants grown under insufficient N conditions. Thus, pure organic N supply, though containing the same amounts of total N as from inorganic N (240/0), seems unlikely to provide sufficient N to plants. Although soil total N content and concentration of ammonium-nitrogen (NH4+-N) did not differ among N treatments at any stage, the concentration of nitrate-nitrogen (NO3—N) was lower under the 240/0 treatment than under the 0/240 and 120/120 treatments at silking and maturity (Table S2). Previous studies have shown that maize and wheat (Triticum aestivum L.) plants have different physiological responses to slow-release N fertilizers and organic fertilizers (Salazar et al. Citation2005; Azadi et al. Citation2011; Hammad et al. Citation2011; Petersen and Sommer Citation2011). With respect to the distribution of N in plants, the greater NUtE observed under organic N treatment may be due to greater N remobilization from vegetative organs, especially from old leaves.
In senescing leaves, amino acids produced by protein degradation are translocated into the phloem in the form of glutamate or glutamine. GS1 and GDH genes are induced during leaf senescence in many plant species, suggesting that both genes are involved in N remobilization processes (Masclaux-Daubresse et al. Citation2008). In the present study, ZmGDH1 and ZmGS1.1 were down-regulated by increasing N levels, suggesting that N supply might delay N remobilization by reducing GDH and GS1 gene expression and their corresponding enzyme activities. Under pure organic N supply (240/0 treatment), ZmGS1.1 and ZmGDH1 expressions were higher than under the inorganic N (0/240) treatment, especially in old leaves at the grain-filling stage (–). This result is consistent with the hypothesis that N remobilization is higher in maize plants growing under pure organic N fertilization.
During the post-silking stage, most nitrates taken up by roots are reduced in the leaves before translocation into the grains (Dechorgnat et al. Citation2011). In our study, the expression of genes related to primary N assimilation (ZmNR1 and ZmGOGAT1) generally decreased with increasing N supply, suggesting the existence of a negative feedback effect of N status on the expression of these genes. On the other hand, N assimilation activity in leaves was unaffected by the two N forms. The expression of ZmAS1 was stimulated by increasing N supply, suggesting its function in asparagine N storage, but its expression differed from that observed in response to organic N supply (, ). This difference may be explained by the fact that Asn seemed to be a complementary storage amino acid easily altered by the balance between N assimilation and release in leaves. By contrast, ZmAspAT1.3 was not very responsive to N supply or different N forms, suggesting the lesser importance of ZmAspAT1.3 in the adaptation to varying N status levels.
In conclusion, application of organic N to maize plants tended to decrease N accumulation while increasing N utilization efficiency. The expression of genes involved in N assimilation in leaves, such as ZmNR1 and ZmFd-GOGAT1, was not affected regardless of whether organic or inorganic forms of N were applied. In contrast, genes related to N remobilization activity, such as ZmGS1.1 and ZmGDH1, were significantly induced, especially in old leaves, by pure organic N supply. These data suggest that maize plants likely respond to pure organic N supply in the same way as to insufficient N conditions. As a result, NUtE capacity is further enhanced by the up-regulation of genes involved in N remobilization processes.
SUPPLEMENTARY MATERIAL
The supplementary material for this article is available online from: http://dx.doi.org/10.1080/00380768.2014.970117
Supplemental Materials
Download MS Word (56.5 KB)ACKNOWLEDGMENTS
The authors gratefully acknowledge funding support from the European Seventh Framework Programme of the European Community for Research, Technological Development and Demonstration Activities for the Integrated Project NUE-CROPS FP7-CP-IP 222645. The views expressed in this publication are the sole responsibility of the authors, and do not necessarily reflect the views of the European Commission. Neither the European Commission nor any person acting on behalf of the Commission is responsible for any use that might be made of the information contained herein. This work was also supported by the National Basic Research Program (973 Program) of China (No. 2011CB100305) and the National Science Foundation of China (No. 31272233).
REFERENCES
- Andrews M, Raven JA, Lea PJ 2013: Do plants need nitrate? The mechanisms by which nitrogen form affects plants. Ann. Appl. Biol., 163, 174–199. doi:10.1111/aab.12045
- Azadi H, Schoonbeek S, Mahmoudi H, Derudder B, De Maeyer P, Witlox F 2011: Organic agriculture and sustainable food production system: main potentials. Agric. Ecosyst. Environ., 144, 92–94. doi:10.1016/j.agee.2011.08.001
- Azeez JO, Van Averbeke W, Okorogbona AOM 2010: Differential responses in yield of pumpkin (Cucurbita maxima L.) and nightshade (Solanum retroflexum Dun.) to the application of three animal manures. Bioresour. Technol., 101, 2499–2505. doi:10.1016/j.biortech.2009.10.095
- Campbell WH 1999: Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol., 50, 277–303. doi:10.1146/annurev.arplant.50.1.277
- Cañas RA, Quilleré I, Christ A, Hirel B 2009: Nitrogen metabolism in the developing ear of maize (Zea mays): analysis of two lines contrasting in their mode of nitrogen management. New Phytol., 184, 340–352. doi:10.1111/j.1469-8137.2009.02966.x
- de la Torre F, De Santis L, Suárez MF, Crespillo R, Cánovas FM 2006: Identification and functional analysis of a prokaryotic-type aspartate aminotransferase: implications for plant amino acid metabolism. Plant J., 46, 414–425. doi:10.1111/j.1365-313X.2006.02713.x
- Dechorgnat J, Nguyen CT, Armengaud P, Jossier M, Diatloff E, Filleur S, Daniel-Vedele F 2011: From the soil to the seeds: the long journey of nitrate in plants. J. Exp. Bot., 62, 1349–1359. doi:10.1093/jxb/erq409
- Ellis EC, Li RG, Yang LZ, Cheng X 2000: Changes in village-scale nitrogen storage in China’s Tai Lake Region. Ecol. Appl., 10, 1074–1089. doi:10.1890/1051-0761(2000)010[1074:CIVSNS]2.0.CO;2
- Gu RL, Duan FY, An X, Zhang FS, von Wiren N, Yuan LX 2013: Characterization of AMT-Mediated High-Affinity Ammonium Uptake in Roots of Maize (Zea mays L.). Plant Cell Physiol., 54, 1515–1524. doi:10.1093/pcp/pct099
- Hammad HM, Khaliq A, Ahmad A, Aslam M, Malik AH, Farhad W, Laghari KQ 2011: Influence of different organic manures on wheat productivity. Int. J. Agric. Biol., 13, 137–140.
- Hirel B, Tétu T, Lea PJ, Dubois F 2011: Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability, 3, 1452–1485. doi:10.3390/su3091452
- Lothier J, Gaufichon L, Sormani R, Lemaitre T, Azzopardi M, Morin H, Chardon F, Reisdorf-Cren M, Avice J.-C., Masclaux-Daubresse C 2011: The cytosolic glutamine synthetase GLN1;2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. J. Exp. Bot., 62, 1375–1390. doi:10.1093/jxb/erq299
- Martin A, Belastegui-Macadam X, Quilleré I, Floriot M, Valadier M-H., Pommel B, Andrieu B, Donnison I, Hirel B 2005: Nitrogen management and senescence in two maize hybrids differing in the persistence of leaf greenness: agronomic, physiological and molecular aspects. New Phytol., 167, 483–492. doi:10.1111/j.1469-8137.2005.01430.x
- Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A 2010: Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann. Bot., 105, 1141–1157. doi:10.1093/aob/mcq028
- Masclaux-Daubresse C, Reisdorf-Cren M, Orsel M 2008: Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol., 10(Suppl 1), 23–36. doi:10.1111/j.1438-8677.2008.00097.x
- Petersen SO, Sommer SG 2011: Ammonia and nitrous oxide interactions: roles of manure organic matter management. Anim. Feed Sci. Technol., 166–167, 503–513. doi:10.1016/j.anifeedsci.2011.04.077
- Salazar FJ, Chadwick D, Pain BF, Hatch D, Owen E 2005: Nitrogen budgets for three cropping systems fertilised with cattle manure. Bioresour Technol., 96, 235–245. doi:10.1016/j.biortech.2004.05.013
- Yang XS., Wu JR, Ziegler TE et al 2011: Gene expression biomarkers provide sensitive indicators of in planta nitrogen status in maize. Plant Physiol., 157, 1841–1852. doi:10.1104/pp.111.187898
- Zhao BQ, Li XY, Liu H, Wang BR, Zhu P, Huang SM, Bao DJ, Li YT, So HB 2011: Results from long-term fertilizer experiments in China: the risk of groundwater pollution by nitrate. NJAS-Wagen. J. Life Sci., 58, 177–183. doi:10.1016/j.njas.2011.09.004
- Zhu ZL, Chen DL 2002: Nitrogen fertilizer use in China - Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosys., 63, 117–127. doi:10.1023/A:1021107026067
