Abstract
Soil fertility has been an important factor in sustainable plant production in native and agricultural fields in temperate climates such as that in Japan. Soil fertility is assessed based on the availability of nutrients, in particular inorganic nitrogen (N) and phosphorus (P), from soil-accumulated organic matter (SOM) via microbial immobilization and mineralization. However, the pool sizes of SOM in humid tropics such as those in Thailand are small and they are turned over rapidly; under such circumstances, the tropical soil fertility would soon be depleted. To meet the urgent requirement of plant nutrients for high plant productivity, we define a direct supply of plant nutrients (i.e., residue fertility) from raw plant and microbial residues. The residue fertility may be driven by the activities of soil fauna (e.g., earthworms, collembolans, termites) and micro-organisms (e.g., saprophytic fungi, protozoa, bacteria), and the released nutrients may be collected and absorbed directly by plant roots including root hairs, and via arbuscular mycorrhizal phyphae. Here, we propose the Ecosystem Fertility paradigm: the Ecosystem Fertility may consist of various ecological nutrient availabilities including both residue fertility and soil fertility. The structure and function of Ecosystem Fertility driven by the above-mentioned biodiversity in different ecosystems may supply not only inorganic N and P but also various forms of nutrients. However, the underlying mechanisms of the Ecosystem Fertility remain to be determined. For the quantification of the various activities and routes involved, the use of molecular and ecosystem approaches may be highly valuable.
SOIL FERTILITY: AN OLD PARADIGM FOR NUTRIENT AVAILABILITY
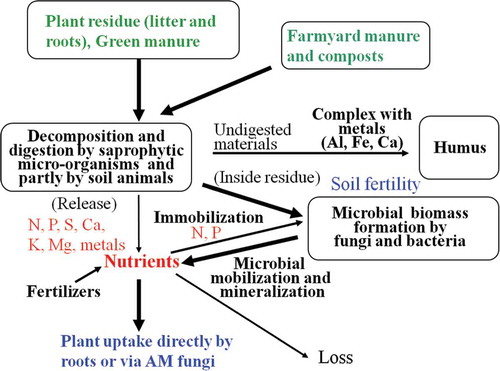
In temperate climates such as that in Japan, soil fertility has been an important factor in plant production in native and agricultural fields. As shown in , soil fertility is quantified based on the availability of nutrients – inorganic nitrogen (N) and phosphorus (P) in particular – which are produced via the immobilization–mineralization cycle by microbial activities from the soil organic matter (SOM) that has accumulated over long periods.
Although the mechanisms underlying the development of soil fertility are not yet completely understood, it is presumed that plant residues (litter and roots) and farmyard manure and composts in agricultural fields are digested to inorganic N and P by microbial enzymes, and the inorganic N and P thus produced, and those supplied as inorganic fertilizers, are partially transformed into microbial organic matter in the presence of carbon (C) resources (top and root residues and root exudates). The partially digested components (lignin, cellulose, polyphenols) are steadily changed to soil humus by polymerization and stabilization by forming complexes with metals [iron (Fe), aluminum (Al) and calcium (Ca)] under humid oxygen-deficient conditions (Hosono and Sase Citation1997). A major carbonaceous source of humus of Japan volcanic ash soils (Andosols) is derived from Japanese pampas grasses (Miscanthus spp.); they have been present for hundreds of thousands of years (Yamane Citation1973; Yoneyama et al. Citation2001; Hiradate et al. Citation2004).
Temperate soils accumulate plant nutrients (N, P) in small microbial-biomass pools (Smith and Paul Citation1990). With an adequate supply of water and optimal temperatures, microbial cells and cell walls (Marumoto et al. Citation1977), which are small fractions (a few percent) of the accumulated SOM, are seasonally mineralized to the soluble forms of N and P by microbial extracellular enzymes, proteases and deamidases/deaminases for organic N and phosphatases for organic P. The quantities of these nutrients comprise the soil fertility. Rewetting after drying at 30°C, which causes a type of cell rupture called the “flush effect,” greatly increased the quantities of water-soluble P from soil microbial biomasses in soils in England and Wales (Turner and Haygrath Citation2001). Potassium (K) and other nutrients in temperate zones are supplied from plant residues, animal wastes and soil minerals such as granitic parent material (Öborn et al. Citation2010).
ECOSYSTEM FERTILITY: A NEW PARADIGM FOR NUTRIENT AVAILABILITY
The pool sizes of SOM containing N and P in humid tropics such as those in Thailand and the Philippines are small – as little as one third to one tenth of those in Japan (Yoneyama et al. Citation2004, Citation2006) – and their turnover rates are fast (with half-times of 0.5–2 years). There are also small pools, which have extremely slow turnover rates (humus; Inoue Citation1986; Wu et al. Citation1998; Yoneyama et al. Citation2006). Under such circumstances, the nutrients (particularly N) contained in the soil microbial mass and clays would be soon depleted.
In the cultivated fields of the Northeast Thailand region, the amounts of above- and below-ground organic matter (i.e., plant residues) fluctuate widely over the seasons due to the large input but subsequent rapid decomposition of organic matter in tandem with the active carbon dioxide (CO2) loss by respiration (Funakawa et al. Citation2006; Matsumoto et al. Citation2008; Hidenori Wada, personal communication). Soil fauna such as ascaris, collembolans, termites and ants participated in the decomposition of the plant residues held in litter bags in Amazon rain forests (Cornu et al. Citation1997).
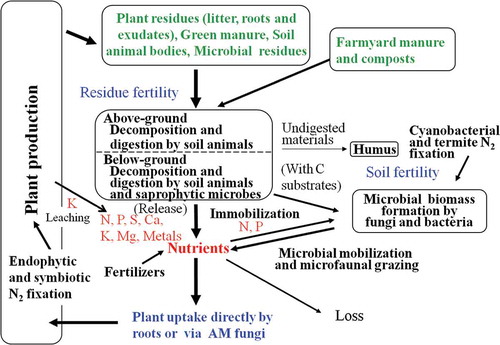
Nutrient resources other than SOM would be necessary to sustain the rapid and massive growth of plants in humid tropical fields. To meet the urgent requirement of nutrients by tropical plants, we propose a direct path (i.e., “residue fertility” system), not via the soil microbial mass, of the nutrients from raw root and microbial residues, which are the products of the previous cropping, in accord with the rapid turnover by the activities of soil fauna (e.g., earthworms, collembolans, mites, termites) and micro-organisms (e.g., saprophytic fungi, protozoa, bacteria) as important players. The processes of decomposition and digestion of residues by soil animals and saprophytic microbes may decompose and digest not only low-molecular compounds (sugars) but also high-molecular compounds such as cellulose and lignin to generate energy by respiration, and these processes may decrease C/N ratios; low C/N ratios of organic matter may suppress the microbial assimilation of nutrients (N and P). Excreta (casts) from detritivorous earthworms contain inorganic N (ammonium) derived from decomposed plant residue (Kawaguchi et al. Citation2011). N from the necromass of soil animals such as earthworms may be released to be taken up by crops (Whalen et al. Citation1999). In addition, the digestion (predation) of bacteria by protozoa (predators) may release nutrients (N), and mycorrhizal symbiosis increases the transfer of the mobilized N (amino acids and ammonium) and P (orthophosphate) to the host plant through the microbial loop (Koller et al. Citation2013). Mycorrhizal fungi may transfer nutrients; that is, N from dead nematodes (Perez-Moreno and Read Citation2001) and P from saprotrophic fungi (Lindahl et al. Citation1999). Thus, high species diversity may decrease the limitations of residue decomposition and increase the rates of nutrient release under both favorable and unfavorable conditions (Loeau et al. Citation2001).
Active faunal digestion of aboveground-localized residues before incorporation into the soil was suggested based on a simulation of organic matter dynamics in Thai and Nigerian soils (Tian et al. Citation1995; Shirato Citation2006). The quick decomposition of canavalia stems and leaves held in litter bags and the resulting N release from these residues caused the recovery (12%) of the plant residue N by maize (Zea mays L.) plants seeded under such residues, although the rest of the N was largely found in the SOM (Douxchamps et al. Citation2011). Rice plants grown in Philippine soils took up 25% of the 15N derived from the 15N-labelled rice (Oryza sativa L.) straws which had been incorporated in flooded soils (Yoneyama and Yoshida Citation1977). The N not absorbed by the crop is incorporated into the soil biomass or lost by leaching and denitrification. Thus, the above-ground plant residues are not good sources of plant nutrients, at least without incorporation into the soil.
Dourado-Neto et al. (Citation2010) demonstrated in 13 diverse tropical agroecosystems that the recoveries of plant residue 15N supplemented to subsequent upland crops were between < 1 and 31%, and that major crop N was derived from SOM. Important questions from their results are: what were the sources of such quickly plant-available SOM and what were the mechanisms or paths of the efficient availability? Based on our new paradigm of Ecosystem Fertility (), the sources may be fresh and raw below-ground materials (i.e., roots, their exudates, residues of fungi mycelium and faunal bodies), and they may be quickly decomposed by fauna and microbes and then release nutrients for current crops. The synchronization of the release of residue N and the demand for N by the crop is important for the efficient use of nutrients. The nutrients thus released may be largely collected and directly absorbed by plant roots and root hairs and via the arbuscular-mycorrhizal hyphae, prior to the immobilization–mineralization cycle involved in SOM turnover (). In the Ecosystem Fertility system in humid tropics (), the residue fertility and soil fertility systems in combination may be operative, and the contribution of the latter may be partial in nutrient availability.
The Ecosystem Fertility system may include other efficient nutrient captures. The leaching of nutrients from standing plants, in particular from senesced tissues, occurs for K and magnesium (Mg; Koelling and Kucera Citation1965), and this cycling may accelerate the turnover of the nutrients in plants. Nutrient cycling among neighboring plants via roots to roots is also an effective manner of quick nutrient capture by nutrient-deficient plants, such as N transfer from legumes to non-legumes (Chalk et al. Citation1996). N fixation by Symbionts and endophytes (Thaweenut et al. Citation2011) may contribute significantly as direct N sources of fixed N (amino acids) to plants.
Application of inorganic fertilizers and pesticides may reduce the soil biodiversity that underlies the efficiency of nutrient availability, as such fertilizer applications reduce the activity of microbial N fixation (Hartwig Citation1998). Recent high inputs of fertilizers and pesticides for higher cereal production may cause great changes to the Ecosystem Fertility system; high doses of fertilizers may affect the natural nutrient supply, and high pesticide exposures may kill insects and micro-organisms and reduce the efficiency of natural insect management (Tilman et al. Citation2002).
SLASH-AND-BURN CULTIVATION, A CASE OF ECOSYSTEM FERTILITY
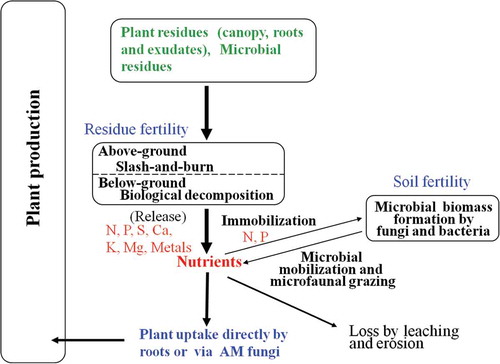
The Ecosystem Fertility scheme () can be applied to shifting cultivation (). In the Thai mountain areas, slash-and-burn sifting cultivation is practiced to enhance the accumulation of nutrients in the soils from the canopy and soil. The amounts of nutrients are high enough so that farmers can grow upland rice, cassava (Manihot esculenta L.) or maize for one or two seasons, but in order to recover the nutrients, fallow periods of around 10 years are required (Kyuma et al. Citation1985; Tulaphitak et al. Citation1985; Funakawa et al. Citation2006).
By burning the forest canopy and surface soil in the late dry season, soluble P, K and Ca obtained as ash, and mineral N (first as ammonium and subsequently as nitrate), are produced from the lyses of proteins and amides in the SOM and dead microbes (Tanaka et al. Citation2001). Such nutrients are further enriched in the wet season by decomposition of the raw below-ground residues like the remaining roots, with high faunal and microbial activities, but periods of frequent heavy rains are also critical for nutrient loss by leaching and soil erosion (Araki Citation2001). To maintain the nutrients in intensive cultivation systems, the return of organic matter to the soils, and the use of fertilizers on the crops have recently been practiced (Funakawa et al. Citation1997; Boonyanuphap et al. Citation2007).
IMPLICATIONS OF ECOSYSTEM FERTILITY IN ALL PLANT-PRODUCTION ECOSYSTEMS
The Ecosystem Fertility system includes both the residue fertility and soil fertility and other types of nutrient supplies. Various Ecosystem Fertility systems may be operated in dry tropics (Jones Citation1990) as well as in humid tropics, in temperate and boreal climates (Setälä et al. Citation1996; Heneghan et al. Citation1999) and in forest ecosystems (González and Seasted Citation2001), as well as in crop fields. Biodiversity for the digestion of plant and microbial residues and for the capture of nutrients may be composed of soil fauna, fungi and bacteria (Coleman Citation2001). The nutrients released from the residues include not only inorganic N and P, which are partially derived from soil fertility, but also various forms of nutrients including different N forms (amino acids, ammonium and nitrate), different P forms (orthophosphate, phospholipid and RNA, Noack et al. Citation2012), different sulfurs forms (S-amino acids and sulfate), various alkaline ions of K, Ca, Mg, Fe, zinc (Zn) and manganese (Mn), chelate complexes of metals [Fe and copper (Cu)], and borate and silicate. Boreal forest plants can take up amino acids directly (Näsholm et al. Citation1998) in contrast to the uptake of inorganic N by plants in the temperate and tropical climates.
FURTHER STUDIES NEEDED: NEXT STEPS
The components of the Ecosystem Fertility systems in various ecosystems have not yet been well examined. It is necessary to determine the underlying mechanisms and acting players (organisms and enzymes), and to quantify the intensities of each of the reaction paths. In such investigations, molecular and ecosystem approaches such as the transcriptome approach (Cardon and Gage Citation2006) and isotope tracing (Koller et al. Citation2013) may be very useful. Important research issues to be addressed are: (1) the elucidation of biodiversity involved in residue decomposition and nutrient release; (2) the clarification of the various processes of nutrient availability, including how nutrients are absorbed; (3) the identification of the diversity of Ecosystem Fertility in different ecosystems; and (4) for sustainability of plant productivity and biodiversity in the tropics, we need to determine what we can and should do to enhance natural processes with minimal anthropogenic consequences.
ACKNOWLEDGMENTS
TY thanks Dr. Hitoshi Shiga, who provided the initial idea regarding the soil fertility of tropical soils in 1974 when we were in the International Rice Research Institute, and Professor Kazutake Kyuma, who ensured the author’s continued interest in tropical soils.
REFERENCES
- Araki S 2001: Soil ecology under slash-and-burn shifting cultivation. In Tropical Soils, Ed. Kyuma K, pp. 300–346, Nagoya University Press, Nagoya. (in Japanese).
- Boonyanuphap J, Sakurai K, Tanaka S 2007: Soil nutrient status under upland farming practice in the Lower Northern Thailand. Tropics, 16, 215–231. doi:10.3759/tropics.16.215
- Cardon ZG, Gage DJ 2006: Resource exchange in the rhizosphere: molecular tools and the microbial perspective. Ann. Rev. Ecol. Evol. Syst., 37, 459–488. doi:10.1146/annurev.ecolsys.37.091305.110207
- Chalk PM 1996: Nitrogen transfer from legumes to cereals in intercropping. In Dynamics of Roots and Nitrogen in Cropping Systems of the Semi-Arid Tropics, Eds. Ito O, et al., pp. 351–374. JIRCAS, Japan.
- Coleman DC 2001: Soil biota, soil systems, and progresses. Encycl. Biodiv., 5, 305–314. doi:10.1006/rwbd.1999.0305
- Cornu S, Luizão F, Rouiller J, Lucas Y 1997: Comparative study of litter decomposition and mineral element release in two amazonian forest ecosystems: litter bag experiments. Pedobiologia, 41, 456–471.
- Dourado-Neto D, Powlson D, Abu Bakar R et al. 2010: Multiseason recoveries of organic and inorganic nitrogen-15 in tropical cropping systems. Soil Sci. Soc. Am. J., 74, 139–152. doi:10.2136/sssaj2009.0192
- Douxchamps S, Frossard E, Bernasconi SM, van der Hoek R, Schmidt A, Rao IM, Oberson A 2011: Nitrogen recoveries from organic amendments in crop and soil assessed by isotope techniques under tropical field conditions. Plant Soil, 341, 179–192. doi:10.1007/s11104-010-0633-6
- Funakawa S, Tanaka S, Shinjyo H, Kaewkhongkha T, Hattori T, Yonebayashi K 1997: Ecological study on the dynamics of soil organic matter and its related properties in shifting cultivation systems of Northern Thailand. Soil Sci. Plant Nutr., 43, 681–693. doi:10.1080/00380768.1997.10414793
- Funakawa S, Hayashi Y, Tazaki I, Sawada K, Kosaki T 2006: The main functions of the fallow phase in shifting cultivation by Karen people in Northern Thailand – a quantitative analysis of soil organic matter dynamics. Tropics, 15, 1–27. doi:10.3759/tropics.15.1
- González G, Seastedt TR 2001: Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology, 82, 955–964. doi:10.1890/0012-9658(2001)082[0955:SFAPLD]2.0.CO;2
- Hartwig UA 1998: The regulation of symbiotic N2 fixation: a conceptual model of N feedback from the ecosystem to the gene expression level. Persp. Plant Ecol. Evol. Syst., 1, 92–120. doi:10.1078/1433-8319-00054
- Heneghan L, Coleman DC, Zou X, Crossley Jr. DA, Haines BL 1999: Soil microarthropod contributions to decomposition dynamics: tropical-temperate comparisons of a single substrate. Ecology, 80, 1873–1882.
- Hiradate S, Nakadai T, Shindo H, Yoneyama T 2004: Carbon source of humic substances in some Japanese volcanic ash soils determined by carbon stable isotopic ratio, δ13C. Geoderma, 119, 133–141. doi:10.1016/S0016-7061(03)00257-X
- Hosono M, Sase T 1997: Preliminary discussion on the genesis of Kuroboku soils. Quaternary Res., 29, 1–9. (in Japanese).
- Inoue T 1986: Characteristics and productivity of red-yellow soils in Thailand. Pedol. Jpn., 30, 54–67. (in Japanese).
- Jones JA 1990: Termites, soil fertility and carbon cycling in dry tropical Africa: a hypothesis. J. Trop. Ecol., 6, 291–305. doi:10.1017/S0266467400004533
- Kawaguchi T, Kyoshima T, Kaneko N 2011: Mineral nitrogen dynamics in the casts of epigeic earthworms (Metaphire hilgendorfi: Megascolecidae). Soil Sci. Plant Nutr., 57, 387–395. doi:10.1080/00380768.2011.579879
- Koelling MR, Kucera CL 1965: Dry matter losses and mineral leaching in bluestem standing crop and litter. Ecology, 46, 529–532. doi:10.2307/1934887
- Koller R, Scheu S, Bonkowski M, Robin C 2013: Protozoa stimulate N uptake and growth of arbuscular mycorrhizal plant. Soil Biol. Biochem., 65, 204–210. doi:10.1016/j.soilbio.2013.05.020
- Kyuma K, Tulaphitak T, Pairintra C 1985: Changes in soil fertility and tilth under shifting cultivation. I. General description of soil and effect of burning on the soil characteristics. Soil Sci. Plant Nutr., 31, 227–238. doi:10.1080/00380768.1985.10557429
- Lindahl B, Stenlid J, Olsson S, Finlay R 1999: Translocation of 32P between interacting mycelia of a wood-decomposing fungus and ectomycorrhizal fungi in microcosm systems. New Phytol., 144, 183–193. doi:10.1046/j.1469-8137.1999.00502.x
- Loreau M, Naeem S, Inchausti P, Bengtsson J et al. 2001: Biodiversity and ecosystem functioning: current knowledge and future challenges. Science, 294, 804–808. doi:10.1126/science.1064088
- Marumoto T, Kai H, Yoshida T, Harada T 1977: Relationship between an accumulation of soil organic matter becoming decomposable due to drying of soil and microbial cells. Soil Sci. Plant Nutr., 23, 1–8. doi:10.1080/00380768.1977.10433016
- Matsumoto N, Paisancharoen K, Hakamata T 2008: Carbon balance in maize fields under cattle manure application and no-tillage cultivation in Northeast Thailand. Soil Sci. Plant Nutr., 54, 277–288. doi:10.1111/j.1747-0765.2007.00223.x
- Näsholm T, Ekblad A, Nordin A, Giesler R, Högberg M, Högberg P 1998: Boreal forest plants take up organic nitrogen. Nature, 392, 914–916. doi:10.1038/31921
- Noack SR, McLaughlin MJ, Smernik RJ, McBeath TM, Armstrong RD 2012: Crop residue phosphorus: speciation and potential bio-availability. Plant Soil, 359, 375–385. doi:10.1007/s11104-012-1216-5
- Öborn I, Edwards AC, Hillier S 2010: Quantifying uptake rate of potassium from soil in a long-term grass rotation experiment. Plant Soil, 335, 3–19. doi:10.1007/s11104-010-0429-8
- Perez-Moreno J, Read DJ 2001: Nutrient transfer from soil nematodes to plants: a direct pathway provided by the mycorrhizal mycelial network. Plant Cell Environ., 24, 1219–1226. doi:10.1046/j.1365-3040.2001.00769.x
- Setälä H, Marshall VG, Trofymow JA 1996: Influence of body size of soil fauna on litter decomposition and 15N uptake by poplar in a pot trial. Soil Biol. Biochem., 28, 1661–1675. doi:10.1016/S0038-0717(96)00252-0
- Shirato Y 2006: Validation and modification of soil organic matter models in arable soils in Japan and Thailand. Bull. Agro-Environ Sci., 24, 23–93. (in Japanese with English summary).
- Smith JL, Paul EA 1990: The significance of soil microbial biomass estimations. In Soil Biochemistry, Eds. Bollag J-M, Stotzky G, Vol. 6, pp. 357–396. Marcel Dekker, New York.
- Tanaka S, Ando T, Funakawa S, Sukhrun C, Kaewkhongkha T, Sakurai K 2001: Effect of burning on soil organic matter content and N mineralization under shifting cultivation system of Karen people in Northern Thailand. Soil Sci. Plant Nutr., 47, 547–558. doi:10.1080/00380768.2001.10408418
- Thaweenut N, Hachisuka Y, Ando S, Yanagisawa S, Yoneyama T 2011: Two seasons’ study on nifH gene expression and nitrogen fixation by diazotrophic endophytes in sugarcane (Saccharum spp. hybrids): expression of nifH genes similar to those of rhizobia. Plant Soil, 338, 435–449. doi:10.1007/s11104-010-0557-1
- Tian G, Brussaard L, Kang BT 1995: Breakdown of plant residues with contrasting chemical compositions under humid tropical conditions: effects of earthworms and millipedes. Soil Biol. Biochem., 27, 277–280. doi:10.1016/0038-0717(94)00182-Z
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S 2002: Agricultural sustainability and intensive production practices. Nature, 418, 671–677. doi:10.1038/nature01014
- Tulaphitak T, Pairintra C, Kyuma K 1985: Changes in soil fertility and tilth under shifting cultivation. II. Changes in soil nutrient status. Soil Sci. Plant Nutr., 31, 239–249. doi:10.1080/00380768.1985.10557430
- Turner BL, Haygarth PM 2001: Phosphorus solubilization in rewetted soils. Nature, 411, 258. doi:10.1038/35077146
- Whalen JK, Parmelee RW, McCartney DA, Vanarsdale JL 1999: Movement of N from decomposing earthworm tissue to soil, microbial and plant N pools. Soil Biol. Biochem., 31, 487–492. doi:10.1016/S0038-0717(97)00252-6
- Wu J, O’Donnell AG, Syers JK, Adey MA, Vityakon P 1998: Modelling soil organic matter changes in ley-arable rotations in sandy soils of Northeast Thailand. Eur. J. Soil Sci., 49, 463–470. doi:10.1046/j.1365-2389.1998.4930463.x
- Yamane I 1973: The meaning of Miscanthus sinensis in the formation of Kuroboku soil. Pedologist, 17, 84–94. (in Japanese with English summary).
- Yoneyama T, Dacanay EV, Castelo O, Kasajima I, Park YH 2004: Estimation of soil organic carbon turnover using natural 13C abundance in Asian tropics: A case study in the Philippines. Soil Sci. Plant Nutr., 50, 599–602. doi:10.1080/00380768.2004.10408517
- Yoneyama T, Nakanishi Y, Morita A, Liyanage BC 2001: δ13C values of organic carbon in cropland and forest soils in Japan. Soil Sci. Plant Nutr., 47, 17–26. doi:10.1080/00380768.2001.10408364
- Yoneyama T, Okada H, Chongpraditnum P, Ando S, Prasertsak P, Hirai K 2006: Effects of vegetation and cultivation on δ13C values of soil organic carbon and estimation of its turnover in Asian tropics: a case study in Thailand. Soil Sci. Plant Nutr., 52, 95–102. doi:10.1111/j.1747-0765.2006.00001.x
- Yoneyama T, Yoshida T 1977: Decomposition of rice residue in tropical soils I. Nitrogen uptake by rice plants from straw incorporated, fertilizer (ammonium sulfate) and soil. Soil Sci. Plant Nutr., 23, 33–40. doi:10.1080/00380768.1977.10433019