 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The wet–dry cycles of soil primarily drive carbon (C) dynamics in dry croplands that mainly experience sporadic rainfall events. We evaluated the in situ short-term (hourly) dynamics of soil carbon dioxide (CO2) efflux and microbial biomass, to compare the significance of a single rainfall event with/without C substrate to reveal the effects of a single rainfall on the soil C dynamics in clayey dry croplands in four different climates and ecosystems. The experiments were conducted on four clayey dry croplands as follows: Thailand (TH) and Tanzania (TZ) in tropical climates, and Kazakhstan (KZ) and Hungary (HG) in continental climates. Hourly measurements of soil CO2 efflux, in situ microbial biomass (MB) and in situ microbial activity (qCO2) were conducted after the application of simulated rainfall (W plots) and rainfall/glucose (WG plots) treatments. We also evaluated the easily mineralizable carbon (EMC) by incubation. The rainfall treatment caused an increase in the qCO2 but not in MB, causing a clear but short C flush in all W plots (10–37 h), while the WG treatment caused an increase both of qCO2 and MB, resulting in substantially longer and larger C flush in the WG plots (ca. 100 h). The ratio of the cumulative soil CO2 flux caused by rainfall treatment to EMC was larger in TH-W and TZ-W plots (8.2 and 4.9%, respectively) than in the KZ-W and HG-W plots (2.9 and 1.1%, respectively). In addition, applied glucose was more heavily mineralized in the TH-WG and TZ-WG plots (15.0 and 9.7%, respectively) than in the KZ-WG and HG-WG plots (6.4 and 3.4%, respectively), because of the different MB increment patterns for the first 24 h, i.e., immediate and large MB increments in TH and TZ, but not in KZ and HG. These results reveal a possible mechanism that causes the rapid decomposition of soil organic carbon and applied organic matter in the dry tropical cropland.
1 INTRODUCTION
Severe depletion of soil organic carbon (C) in the upper soil layers has resulted in land degradation and climate change worldwide (Post and Kwon Citation2000; Lal Citation2004), increasing the importance of efforts to increase soil C, such as soil C sequestration. Accurate prediction of soil C dynamics requires an understanding of soil carbon dioxide (CO2) dynamics. In regions with dry climates, the wet–dry cycles of soil primarily drive soil CO2 dynamics, through sporadic or periodic rainfall events (Austin et al. Citation2004; Schwinnin et al. Citation2004). Furthermore, climate change will probably result in a decrease in soil moisture in most regions of the Earth during this century (Meehl et al. Citation2007; Borken and Matzner Citation2009), causing many soils to be subjected to more frequent wet–dry cycles. As a result, evaluating soil CO2 dynamics during wet–dry cycles has become more important worldwide (Austin et al. Citation2004; Borken and Matzner Citation2009).
Many studies have addressed the mechanisms of short-term C dynamics after the rewetting of dry soil in relation to microbial dynamics. Based on laboratory experiments, rewetting of a dry soil stimulated microbial activity for 2–5 d (Mikha et al. Citation2005; Sponseller Citation2007), while similar rewetting of dry soil based on the field experiments stimulated microbial activity for only 6–24 h (Murphy et al. Citation1998; Sugihara et al. Citation2010b). Generally, increased microbial activity (qCO2) stimulated by rewetting of dry soil is considered to be the main reason for the soil C flush during a wet–dry cycle (Fierer and Schimel Citation2003; Wu and Brookes Citation2005), while other mechanisms, for example the contribution of increased microbial biomass (MB), have also been reported (Sugihara et al. Citation2010b). Sugihara et al. (Citation2010b) observed that a rewetting treatment of dry soil in Thailand in clayey and sandy cropland increased qCO2 but not MB in the former, and increased both qCO2 and MB, and resulted in larger C flush, in the latter. They found that the amount of mineralizable C per unit of either soil microbes or total C was much higher in sandy soil. Because rewetting with a greater C substrate treatment increased both MB and qCO2 in the clayey and sandy croplands, the larger mineralizable C ratio may cause the MB increment only in sandy cropland. These finding indicate that the amount of C substrate limited the MB increment during the wet–dry cycles, and the C flush would become substantially larger when MB increased during a wet–dry cycle. Therefore, the amount of mineralizable C would be important to predict soil CO2 dynamics during wet–dry cycles, in terms of controlling factors related to MB dynamics. However, Sugihara et al. (Citation2014) found little increase of MB and small C flush after the rewetting of dry soil with C substrate application in tropical sandy soil of Niger, where mineralizable C was quite scarce. They suggested that the unique soil microbial community present in Niger, which is probably adapted to C limitation, is not able to respond immediately to C application. This may be the cause of the lack of a rapid and robust MB reaction for applied C substrate, resulting in a small C flush in Niger. Based on these results and reports, we hypothesized that the C flush after the rewetting of dry soil with/without C substrate would differ in various regions with different amounts of mineralizable organic matter (OM), causing MB dynamics to vary during the wet–dry cycle from one region to another. Generally, levels of mineralizable OM were closely related with total C, and total C is, in turn, closely related to climatic patterns—i.e., temperature and rainfall distribution. That is, soils in a region with a cool climate should have a large amount of soil organic matter (SOM) and, hence, much more mineralizable OM than soils in a hot climate (Franzluebbers et al. Citation2000, Citation2001; Hevia et al. Citation2003). Therefore, the C flush occurring during a wet–dry cycle would be larger in the region with a cool climate when compared with that of a region with a hot climate.
Our objective was to evaluate and compare the relationship between in situ short-term C dynamics and microbial dynamics during a wet–dry cycle in different climate conditions, especially the related fluctuation in MB, after the rewetting of dry soil with or without C substrate application, between regions with cool and hot climates.
2 MATERIALS AND METHODS
2.1 Description of the study sites
The experiments were conducted on four croplands in regions with different climates. These include tropical climates, Thailand (TH) and Tanzania (TZ), and continental climates, Kazakhstan (KZ) and Hungary (HG), all having distinct dry and rainy seasons. TH and TZ were tropical climate cropland sites, the former located in Ban Rakpaendin, Chiang Rai, northern Thailand (19°49′N, 100°21′E; Fujii et al. Citation2009; Sugihara et al. Citation2010b), and the latter located at Sokoine University of Agriculture Morogoro in central Tanzania (6°50′S, 37°39′E; Sugihara et al. Citation2012). Both sites are classified as Am according to Köppen–Geiger climate classification (Peel et al. Citation2007). KZ and HG were continental climate cropland sites, the former located at the Barayev Kazakh Research and Production Center of Grain Farming, Shortandy, northern Kazakhstan (51°35′N, 71°03′E; Takata et al. Citation2008), and the latter located at the Research Institute of Karcag, Hungarian Academy of Science, Karcag, Eastern Hungary (47°17′N, 20°53′E). KZ and HG sites are clarified as Dwc and Dfb, respectively, according to Köppen–Geiger climate classification (Peel et al. Citation2007). All experimental sites were established in continuously cropped fields, except for the KZ site, which had lain fallow for 10 years. provides the annual precipitation and mean air temperature at all sites. The soils at the TH, TZ, KZ and HG sites are classified into Typic Haplustults, Kanhaplic Haplustluts, Typic Haplustoll and Typic Haplustoll, respectively (Soil Survey Staff Citation2006). also shows the chemical and physical properties of the surface soil (0–5 cm) from all sites. Soil texture of all sites were similar and clayey, i.e., Light clay (LiC). Although the silt contents in the KZ and HG are relatively higher than those in TH and TZ, the pattern of pF-soil moisture curve was mostly similar, especially at pF 1.0–3.0. Easily mineralizable carbon (EMC) was measured for each soil, and corrected before the field experiment, with reference to Kadono et al. (Citation2008). Samples in the amount of 20 g fresh soil, adjusted to 60% of water holding capacity, were incubated at 25°C in sealed plastic bottles with 1 M sodium hydroxide (NaOH) in duplicate. The amount of CO2 trapped in the alkali solution was measured by titration after 1, 3, 7, 14 and 28 d.
Table 1 Description of the climate and surface soil properties (0–5 cm) at each experimental site.
2.2 Field experimental design
The experiments were conducted at the end of the dry season, at the end of March 2004 in TH and mid October 2004 in TZ, and in the middle of the summer season, at the end of July 2004 in KZ and the middle of July 2003 in HG. We installed three experimental plots at each experimental site as follows (Sugihara et al. Citation2010b provides details of the experimental picture):
-
Control or C plots (TH-C, TZ-C, KZ-C and HG-C) without simulated rainfall.
-
Water treatment or W plots (TH-W, TZ-W, KZ-W and HG-W), with the addition of 10 mm of simulated rainfall at the start of each experiment.
-
Water and glucose treatment or WG plots (TH-WG, TZ-WG, KZ-WG and HG-WG), with the addition of 75 g C m−2 glucose solution as a C substrate mixed with the 10 mm of rainfall treatment. The WG plots were designed to allow the evaluation of the potential activities of soil microbes when compared with the W plot—that is, when the C substrate does not limit the microbial dynamics. On the basis of past similar studies, glucose is considered to be the representative C substrate, due to its easily mineralized and utilized characteristics for soil microbes; thus, we used glucose as a C substrate (Saggar et al. Citation1999; Wu and Brookes Citation2005).
Each plot was divided into two subplots to measure soil CO2 efflux and microbial biomass (MB), separately, as described below. The end of the dry season in TH and TZ, and the period after the harvest in HG, resulted in bare cropland fields at these sites for the experiments. For KZ, a fallow site, a cover of fallow grass was removed from the site 2 weeks before the experiments, and the field was allowed to stabilize, to reduce the effect of disturbance on the soil CO2 efflux. In all cases, all visible plant residues (some were found in all plots) were carefully removed from the soil surface prior to the experiments and before initiating the artificial rainfall treatment. The experiments began at 07:00 to allow the observation of the effects of the large evaporation rates in the initial stage of the experiment. The experiment began at 16:00 in HG because setting up and preparing for the experiment involved some difficulties. Following the irrigation, the W and WG plots were left to dry.
2.3 Environmental factors
The air and soil temperature at a depth of 5 cm and the volumetric soil moisture content (VMC) in the surface soils (0–15 cm) were continuously monitored at the C and W plots. This was done using a 107 thermistor (Campbell Scientific, Inc., Logan, UT, USA) for air and soil temperature measurements, and a CS616 probe (Campbell Scientific) for VMC measurements, connected to a CR10X data logger (Campbell Scientific). At only the HG site, VMC was measured at 5 cm using an AquaFlex probe connected to a CR10X data logger.
2.4 Soil CO2 efflux measurements
Soil CO2 efflux was measured using a closed-chamber system. For each experimental plot, three polyvinyl chloride (PVC) cylinders (13 cm in diameter × 15 cm in height) were inserted into the soil to a depth of 5 cm immediately after the rainfall treatment, for three sets of measurements. For each measurement, after the top of the cylinder was covered tightly with a plastic sheet and left for 30–40 min, a 50-mL gas sample was collected using a syringe, and then held in a previously evacuated 30-mL glass vial. At the same time, an air sample was collected to determine a baseline atmospheric CO2 concentration. The concentration of CO2 was measured using an infrared CO2 controller (ZFP9AA11; Fuji Electric, Tokyo, Japan) and the increase in CO2 concentration during the 30–40 min relative to the control sample was assigned as the soil CO2 efflux from the soil surface.
Temperature is widely recognized as one of the factors that controls soil CO2 efflux. Because our objective was to evaluate and compare the time course of soil CO2 efflux in different climates, we corrected the soil CO2 efflux data using a Q10 relationship (Fang and Moncrieff Citation2001) and converted the measured soil CO2 efflux values to that expected at 25°C (see Sugihara et al. Citation2010b for more information).
Sample collection was carried out after the simulated rainfall treatment at 3.5, 6, 9.5, 13.5, 25.5, 31, 37.5, 49.5, 55, 80.5, 102.5, 121.5, 129.5, 274 and 292 h at the TH site; at 0, 3, 5.5, 9, 12, 15, 26, 31, 35, 49, 58, and 98 h at the TZ site; at 0, 3, 6.5, 9.5, 12.5, 19.5, 25.5, 46.5, 54, 118.5, 125.5, 142.5 and 149.5 h at the KZ site; and at 0, 3, 5, 9, 15, 18, 24, 29.5, 42, 48.5 and 71 h at the HG site.
2.5 In situ microbial biomass and its activity
We measured MB in the field using the adapted substrate-induced respiration (SIR) method (Sugihara et al. Citation2010b). Our past study (Sugihara et al. Citation2010b) provides details related to the procedures. Simplified procedures were as follows: (1) the water content of the topsoil (0–5 cm) was adjusted to bring it to above 50% of its water holding capacity. After the soil was mixed well, the calculated amount of water was sprayed on; then, the soil was mixed again. (2) The glucose solution was applied using a syringe at a rate of 60 g C m−2. The topsoil was mixed uniformly to make sure that the glucose concentration was uniform in the soil. After the glucose application, we immediately inserted three PVC cylinders (the same ones as for the soil CO2 efflux measurements described above) into the soil to a depth of 5 cm. (3) Gas sampling and analysis were then performed, as described earlier.
Sample collection was carried out after the simulated rainfall at 5, 10, 14, 27, 39, 50, 55, 81, 123, 274 and 292 h at the TH site; at 4, 9.5, 12.5, 26, 35, 50, 58, and 98 h at the TZ site; at 4, 11, 15.5, 22.5, 47.5, 54.5, 119 and 151 h at the KZ site; and at 5, 9, 15.5, 19, 25, 31, 43.5, 49.5 and 71 h at the HG site.
To calculate the MB size based on our adapted SIR method, we used the following equation (Anderson and Domsch Citation1978):
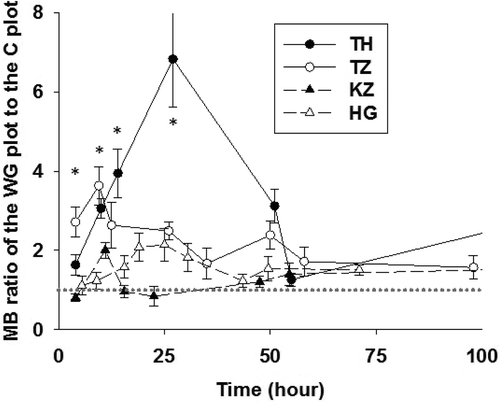
As our former study indicated, the adapted in situ SIR method used in the present study could be useful for comparing the fluctuation in i n situ-MB between the treatments, although the method could be improved to measure MB sizes more accurately (Sugihara et al. Citation2010b). Therefore, to discuss and compare the effect of the treatment on the behavior of i n situ-MB among the all sites, we calculated the ratio of i n situ-MB at the WG plots to the corresponding C plot (only for the HG site, W plot) at each site and sampling time ().
We also calculated qCO2 to determine the contribution of MB and microbial activity (qCO2) to the SOM dynamics separately, using the following equation (Anderson and Domsch Citation1993; Anderson and Joergensen Citation1997):
2.6 Statistical analyses
All statistical analysis were carried out in SYSTAT 13.0 (SYSTAT Software, Point Richmond, CA, USA). All data were expressed on a dry-weight basis. To assess the MB and qCO2 dynamics between the W and C plots of each site, we conducted a repeated-measures analysis of variance (ANOVA) for each site during the first 50 h. In addition, to comparing the MB increment size in the WG plot with the ratio of WG to C plots for the first 50 h, ANOVA was used to detect significant differences at each similar sampling time among the TH, TZ, KZ and HG sites. When ANOVA indicated a significant difference, mean comparisons were carried out using post hoc Tukey multiple-comparison tests. In all cases, P < 0.05 was considered significant.
3 RESULTS
3.1 Trends in air and soil temperature and soil moisture
The daily air temperature fluctuated at TH (16.1–36.5°C; mean 25.7°C), TZ (18.5–33.6°C; 26.0°C), KZ (2.1–23.1°C; 12.1°C) and HG (15.8–33.8°C; 24.3°C) (). Cloudy weather during the experimental period at the KZ site caused the average air temperature to be substantially lower than the general average air temperature of July (ca. 18°C) at the KZ site (Takata et al. Citation2007). The soil temperature substantially fluctuated, and soil temperature in the W plot was 2–5°C lower than that in the C plot, especially in the daytime during the first 3 d, owing to the heat of vaporization. The averaged soil temperatures for the first 3 d, at TH, TZ, KZ and HG, were, in the W plot 25.1, 30.0, 21.5 and 27.1°C, respectively, and in the C plot, 26.7, 31.9, 23.4 and 29.2°C, respectively.
Figure 1 Trends in air and soil temperature (5 cm depth; a, b, e, f) and volumetric moisture content of soil (0–15 cm depth; c, d, g, h) at the Thailand, Tanzania, Kazakhstan and Hungary sites. At the Hungary site, volumetric moisture content was measured at a depth of 5 cm in W plot, because of the limited species found with the logger sensor. Also, a small amount of rainfall (2.0 mm) was observed during the experiments (after 11 h). The data for Thailand (TH) were revised from Sugihara et al. (Citation2010b). C plot: Control plot; W plot: Water treatment plot.
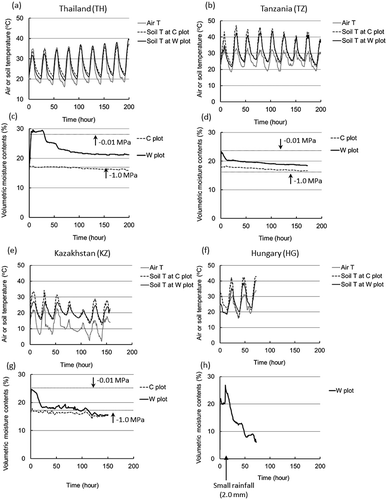
The soil was very dry before the experiments, lower than −1.0 MPa in most plots. The simulated rainfall treatment clearly increased the VMCs in the W plot of all sites, reaching nearly −0.01 MPa (). The VMCs in the TH-W and KZ-W plots remained high during the first 12–24 h and then decreased. The VMCs in the TZ-W plot decreased immediately after the treatment. In the HG site, a small amount of rainfall (2.0 mm) occurred at 11 h, resulting in increased VMC, although the VMC decreased continuously after the rainfall. The VMCs in the TH-W, TZ-W, KZ-W and HG-W plots were 29.3, 20.3, 18.3 and 16.1% after 24 h, respectively, and 22.8, 19.6, 18.1 and 6.1% after 72 h, respectively.
3.2 Fluctuation in soil CO2 efflux during a wet–dry cycle
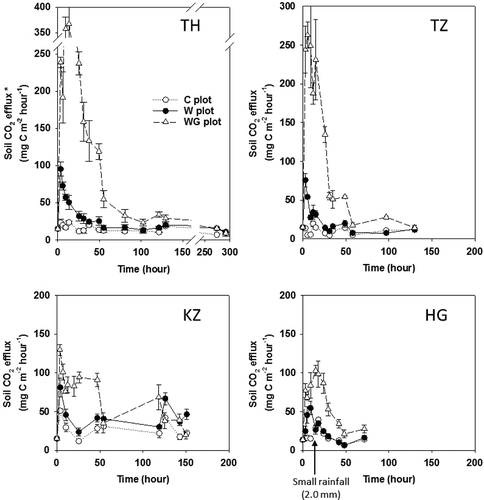
After the initial treatments, soil CO2 efflux in the W and WG plots at all sites immediately increased within a few hours, while soil CO2 efflux in the C plot remained stable (). Averaged soil CO2 effluxes in the TH-C, TZ-C, KZ-C and HG-C plots were 14.8, 10.7, 22.8 and 18.0 mg C m−2 h−1, respectively. The soil CO2 efflux rates in the TH-W, TZ-W, KZ-W and HG-W plots increased up to 95.2 (at 3.5 h), 75.8 (at 3 h), 81.1 (at 3 h) and 54.6 (at 9 h) mg C m−2 h−1, respectively, and in the WG treatment they increased up to 367.0 (at 13.5 h), 263.0 (at 5.5 h), 129.8 (at 3 h) and 102.9 (at 15 h) mg C m−2 h−1, respectively. After the clear peak of C flush in the W and WG plots of all sites was observed, the soil CO2 efflux rate clearly decreased to a similar value to that of the nearly C plot within 50–100 h at most sites. Only at the HG sites, soil CO2 efflux increased slightly in the HG-C and HG-W plots from 15 h to 24 h, because a small amount of natural rain fell (2.0 mm) at 11 h after the treatment began. The peak of soil CO2 efflux in the HG-W plot occurred at 9 h after the treatment began, and the effect of the small amount of natural rainfall started to decrease immediately. Subsequently, the soil CO2 efflux in the HG-C and HG-W plot fluctuated similarly from 15 to 24 h, so the effect of small natural rainfall was considered to be negligible, allowing a comparison of the effect of a single rainfall event on the C flush between the HG site and other sites.
Figure 2 Fluctuations in soil carbon dioxide (CO2) efflux* at each experimental site; Thailand (TH), Tanzania (TZ), Kazakhstan (KZ) and Hungary (HG). Bars indicate the standard error. Sugihara et al. (Citation2010b) provided the figures for the TH site. *The soil CO2 efflux rate was converted to that expected at 25°C based on the Q10 relationship (Fang and Moncrieff Citation2001). C plot: Control plot; W plot: Water treatment plot; WG plot: water and glucose treatment plot.
3.3 Fluctuation in microbial biomass and its activity during a wet–dry cycle
Average (mean ± standard deviation) in situ-MB values for the TH-C, TZ-C, KZ-C and HG-W plots were 4530 ± 690, 5150 ± 360, 5270 ± 310 and 5110 ± 250 mg C m−2 (0–5 cm), respectively (). In situ-MB in the C plot of each site certainly remained stable, and the coefficient of variance was 17.0, 20.7, 16.5 and 14.9%, respectively. Averaged in situ-qCO2 values for the TH-C, TZ-C and KZ-C plots were 0.0031 ± 0.00078, 0.0023 ± 0.00032 and 0.0047 ± 0.00070 g CO2-C g−1 MB-C h−1, respectively (). The estimated in situ-qCO2 in the HG-C plot was 0.0034 ± 0.00057 g CO2-C g−1 MB-C h−1, calculated by dividing averaged soil CO2 flux in the HG-C plot by averaged in situ-MB in the HG-W plot.
Figure 3 Fluctuations in in situ microbial biomass at each experimental site: Thailand (TH), Tanzania (TZ), Kazakhstan (KZ) and Hungary (HG). Bars indicate the standard error. Sugihara et al. (Citation2010b) provided the figure for the TH site. The values for in situ microbial biomass were measured and calculated according to the adapted SIR. C plot: Control plot; W plot: Water treatment plot; WG plot: water and glucose treatment plot.
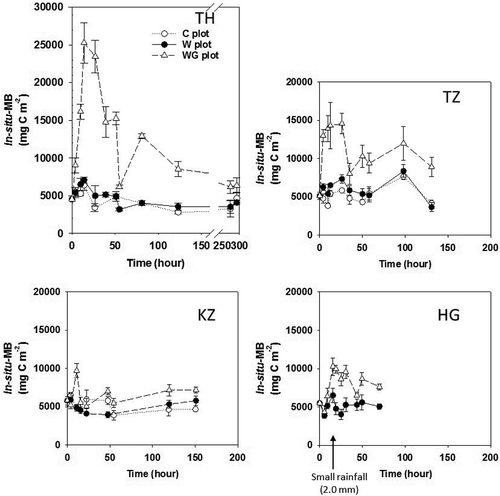
Figure 4 Fluctuations in in situ microbial activity (qCO2) at each experimental site: Thailand (TH), Tanzania (TZ), Kazakhstan (KZ) and Hungary (HG). Bars indicate the standard error. Sugihara et al. (Citation2010b) provided the figure for the TH site. Values for in situ microbial activity were measured and calculated according to the adapted SIR. C plot: Control plot; W plot: Water treatment plot; WG plot: water and glucose treatment plot.
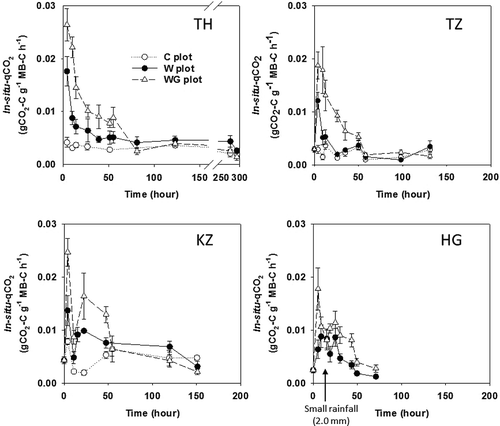
After the rainfall treatment, the in situ-MB in the W plot of all sites did not fluctuate significantly. However, after the WG treatment, the in situ-MB in the TH-WG and TZ-WG plots substantially increased, especially for the first 24 h (> 2.5 times that of each C plot), although the in situ-MB in the KZ-WG and HG-WG plots increased a little for first 24 h (ca. 1 ~ 2 times of each C plot; ). The increased in situ-MB in all of the WG plots decreased gradually with soil drying. Based on the ANOVA and post hoc Tukey multiple analysis for each sampling time, the MB increment ratios in TH-WG and TZ-WG were clearly larger than those in KZ-WG and HG-WG, throughout the first 24 h (P < 0.05). The in situ-qCO2 in the W and WG plots significantly increased after the treatments (P < 0.05), and rapidly decreased with soil drying. The in situ-qCO2 in the W and WG plots became similar to that of each C plot within 20–50 h ().
Figure 5 Fluctuations in the ratio of the WG plot to the C plot for in situ microbial biomass. The horizontal gray dotted line indicates a value of 1. Bars indicate the standard error. W plot data was used as an alternative for C plot only for the Hungary site. Values for in situ microbial biomass were measured and calculated according to the adapted SIR. * indicates the significant difference between the sites at each similar sampling time, based on ANOVA and post hoc Tukey test analysis (P < 0.05). TH: Thailand; TZ: Tanzania; KZ: Kazakhstan; HG: Hungary; C plot: Control plot; W plot: Water treatment plot; WG plot: water and glucose treatment plot.
4 DISCUSSION
4.1 Effect of one rainfall event on the soil C dynamics under the different climates
The rewetting of a dry soil treatment clearly increased the soil CO2 efflux in all of the W plots, and the entire C flush was mostly finished within 10–37 h, because the soil dried immediately. The qCO2 in all of the W plots immediately increased and mainly contributed to the C flush, but MB did not fluctuate clearly in all of the W plots. These results are consistent with previous studies, involving the wet–dry cycle treatments of clayey soil in laboratory experiments (Mikha et al. Citation2005; Pulleman and Tietema, Citation1999; Sponseller Citation2007). Therefore, this indicates that different climatic conditions would not affect the pattern or mechanisms of in situ C flush and in situ soil microbial dynamics during a wet–dry cycle in clayey cropland soils. When one considers the substantial increase in MB in all of the WG plots, the amount of C substrate should be expected to limit the MB increment during the wet–dry cycle in all of the clayey croplands studied here. In sandy soil ecosystems, Sugihara et al. (Citation2010b) observed the in situ MB increment after rainfall treatment only in Thailand. They explained the possible reasons for the observed increment in two ways: (1) different MB turnover rate, i.e., more rapid in sandy soil than clayey soil (Van Veen et al. Citation1984; Sugihara et al. Citation2010a); and (2) different accessibility of OM for soil microbes, i.e., better accessibility of sandy soil than that of clayey soil, because of the little and weak aggregate structure in sandy soil (Hassink Citation1994; Fierer and Schimel Citation2003). Based on these results, soil texture would be one of the important factors that controls MB dynamics during a wet–dry cycle, which determined the pattern and size of the C flush caused by a wet–dry cycle (Sugihara et al. Citation2010b). However, further studies are needed to confidently discuss this possibility, such as similar field experiments in sandy soil of a cool (or continental) climate region.
Because the C flush in all of the W and WG plots was mostly complete within the first 100 h after treatment, we estimated the cumulative soil CO2 efflux during this 100 h in all of the C, W and WG plots (). The difference in cumulative CO2 efflux between the W and C plots, which can be taken as the amount of C mineralization caused by a 10-mm rainfall event, was 1.3, 0.9, 1.4 and 0.3 g C m−2 100 h−1 in TH, TZ, KZ and HG, respectively. To properly compare the effect of a single rainfall event on the soil C dynamics for the different sites analyzed here, we divided the amount of C mineralization caused by 10 mm of rainfall by EMC and total C (). As a result, the ratio of mineralized C to EMC was found to be substantially larger in TH and TZ (8.2 and 4.9%) than that in KZ and HG (2.9 and 1.1%), and the ratio to total C was relatively larger in TH and TZ (0.15 and 0.09%) than that in KZ and HG (0.08 and 0.02%). The values were relatively small compared with our previous result, conducted in tropical sandy soil in Thailand, i.e., the ratio to total C was 1.3% (Sugihara et al. Citation2010b). As a result, our hypothesis was rejected; that is, the effect of a single rainfall event on soil C dynamics is larger in a tropical climate, where EMC and SOM are much lower, than that in a continental climate. This may occur because the size of C flush was similar in all regions while the amount of EMC or total C was smaller in the tropics, resulting in larger ratio in the tropical climates than in the continental climates. Franzluebbers et al. (Citation2000) observed a clearly higher ratio of mineralized C to MB in a hot climate than in a cool climate in the USA, although their studies were based on laboratory incubation. This indicates that the different soil microbial community which has a higher qCO2 in tropics may contribute to a similar or larger C flush effect in the tropical climate than in the continental climate in this study, although the value of qCO2 in this study does not clearly support this idea. In addition, the effect of different temperature conditions on soil microbial activity between the experimental period and annual averaged temperature, especially in continental climates, should also be considered, though we converted the measured soil CO2 efflux to that expected at 25°C, and therefore we must remove the effect of temperature difference, theoretically. These results, such as a similar or relatively larger C flush in tropical climate compared to a continental climate, may suggest the mechanisms of rapid turnover rate of SOM in the dry tropics (Palm et al. Citation2001), although the results were based on just one field experiment in the dry season, and, therefore, the same experiment should be conducted in other seasons, such as the middle or the end of the rainy season, to further explore the effect of climate.
Table 2 Cumulative soil CO2 efflux in the initial 100 h, and its ratio to easily mineralizable carbon, total carbon and added glucose.
4.2 Effect of C substrate application on the C flush under the different climates
The WG treatment clearly resulted in increases of both MB and qCO2 for the first 50 h, and this resulted in a substantially larger C flush compared with each respective W plot (). Based on our calculations, a substantially larger portion of the added glucose was mineralized in the tropical climate, i.e., TH (15.0%) and TZ (9.7%), than that in the continental climate, i.e., KZ (6.4%) and HG (3.4%). Because the qCO2 dynamics in each WG plot fluctuated similarly at all four sites, the different size of C flush for the WG plots must have been caused by the different MB dynamics, especially for the first 50 h, i.e., a faster and larger MB increment was observed in the TH-WG and TZ-WG plots than that in the KZ-WG and HG-WG plots. Based on the ANOVA and post hoc Tukey multiple analyses, the MB increments in the TH-WG and TZ-WG plots were clearly larger than those in the KZ-WG and HG-WG for the first 24 h (P < 0.05). Because the qCO2 in all of the WG plots was clearly high, especially for first 24 h, the larger MB increment in the TH-WG and TZ-WG plots for the first 24 h must have caused a larger C flush in those two plots than that in the KZ-WG and HG-WG plots. It remains unclear why soil microbes in the tropical climate can immediately respond to C addition and increase their MB substantially (although this did not occur in the continental climate); some possible mechanisms are provided below: (1) the different clay mineralogy and silt contents between TH/TZ and KZ/HG possibly resulted in different turnover rates of soil microbes or SOM mineralization, and (2) the different climate conditions possibly resulted in different soil microbial communities. For the first reason, we observed different clay mineralogy between the TH/TZ (Kaolinite, 1:1 clay mineral) and KZ/HG (smectite and/or vermiculite, 2:1 clay minerals) sites (Takata et al. Citation2007; Fujii et al. Citation2009). Denef et al. (Citation2002) reported the effect of clay mineralogy on soil aggregate stability in the laboratory, and found that 2:1 clay minerals substantially contributed to a well-developed soil aggregation when compared with that of 1:1 clay minerals, with the result that SOM mineralization occurs more slowly in the soil containing 2:1 clay minerals. In addition, silt content was also relatively high in KZ and HG, compared with TH and TZ, and, therefore, it may affect the soil microbial turnover rate (Hassink Citation1994; Sakamoto and Hodono Citation2000), although the clay contents were mostly the same for all sites, and it is still unclear whether such different silt contents did affect the soil microbial turnover rate or not. For the second reason, the different soil microbial communities in different climates and regions should also be considered (Steinberg et al. Citation1999; Waldrop and Firestone Citation2006). Further study is necessary to evaluate and clarify the above effects on MB dynamics after the rewetting of dry soil with a C substrate, and to evaluate the variations in the soil microbial community for both climatic regions analyzed here and its dynamics during the wet–dry cycle, if the above possibilities are to be discussed further (Denef et al. Citation2001; Six et al. Citation2002; Ford et al. Citation2007; Gordon et al. Citation2008).
5 CONCLUSION
We found that at the TH, TZ, KZ and HG sites, the ratio of the cumulative soil CO2 flux caused by a 10-mm rainfall to EMC was 8.2, 4.9, 2.9 and 1.1%, respectively, and the ratio to total C was 0.15, 0.09, 0.08 and 0.02%, respectively. This indicates that one rainfall event should be more critical and important for soil C dynamics in the tropics than that in a continental climate, possibly because of the smaller EMC and total C in soils of the tropical climate. In addition, applied glucose was also more substantially mineralized in the TH-WG (15.0%) and TZ-WG plots (9.7%) when compared with that of the KZ-WG (6.4%) and HG-WG plots (3.4%), because of the different MB increment pattern for the first 24 h after simulated rainfall, i.e., an immediate and large MB increment in TH and TZ, but not in KZ and HG. These results reveal a possible mechanism for the rapid decomposition of SOM or applied OM in the dry tropics in soils of clayey croplands.
ACKNOWLEDGMENTS
We wish to thank Prof. Roengsak Katawatin of Khon Kaen University, Prof. Kilasara Method and Prof. Msaky JJT of the Sokoine University of Agriculture, Dr. Kanat Akshalov of the Barayev Kazakh Research and Production Center of Grain Farming, and Prof. Tibor Toth of the Research Institute for Soil Science and Agricultural Chemistry of the Hungarian Academy of Sciences for their invaluable support to our field experiments. Our work was financially supported by the Japanese Society for the Promotion of Science’s KAKENHI Grant, Nos. #24255019 and #24228007.
REFERENCES
- Anderson JPE , Domsch KH 1978: A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. , 10, 215–221. doi:10.1016/0038-0717(78)90099-8
- Anderson TH , Domsch KH 1993: The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem., 25, 393–395. doi:10.1016/0038-0717(93)90140-7
- Anderson T-H , Joergensen RG 1997: Relationship between SIR and FE estimates of microbial biomass C in deciduous forest soils at different pH. Soil Biol. Biochem , 29, 1033–1042. doi:10.1016/S0038-0717(97)00011-4
- Austin AT , Yahdjian L , Stark JM , Belnap J , Porporato A , Norton U , Ravetta DA , Schaeffer SM 2004: Water pulses and biogeochemical cycles in arid and semiarid ecosystems. Oecologia , 141, 221–235. doi:10.1007/s00442-004-1519-1
- Borken W , Matzner E 2009: Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Global Change Biol. , 15, 808–824. doi:10.1111/j.1365-2486.2008.01681.x
- Denef K , Six J , Bossuyt H , Frey SD , Elliott ET , Merckx R , Paustian K 2001: Influence of dry-wet cycles on the interrelationship between aggregate, particulate organic matter, and microbial community dynamics. Soil Biol. Biochem., 33, 1599–1611. doi:10.1016/S0038-0717(01)00076-1
- Denef K , Six J , Merckx R , Paustian K 2002: Short-term effect of biological and physical forces on aggregate formation in soils with different clay mineralogy. Plant Soil , 246, 185–200. doi:10.1023/A:1020668013524
- Fang C , Moncrieff JB 2001: The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem., 33, 155–165. doi:10.1016/S0038-0717(00)00125-5
- Fierer N , Schimel JP 2003: A proposed mechanism for the pulse in Carbon Dioxide production commonly observed following the rapid rewetting of a dry soil. Soil Sci. Soc. Am. J., 67, 798–805. doi:10.2136/sssaj2003.0798
- Ford DJ , Cookson WR , Adams MA , Grierson PF 2007: Role of soil drying in nitrogen mineralization and microbial community function in semi-arid grasslands of north-west Australia. Soil Biol. Biochem. , 39, 1557–1569. doi:10.1016/j.soilbio.2007.01.014
- Franzluebbers AJ , Haney RL , Honeycutt CW , Arshad MA , Schomberg HH , Hons FM 2001: Climatic influences on active fractions of soil organic matter. Soil Biol. Biochem., 33, 1103–1111. doi:10.1016/S0038-0717(01)00016-5
- Franzluebbers AJ , Haney RL , Honeycutt CW , Schomberg HH , Hons FM 2000: Flush of carbon dioxide following rewetting of dried soil relates to active organic pools. Soil Sci. Soc. Am. J., 64, 613–623. doi:10.2136/sssaj2000.642613x
- Fujii K , Funakawa S , Hayakawa C , Sukartiningsih KT 2009: Quantification of proton budgets in soils of cropland and adjacent forest in Thailand and Indonesia. Plant Soil , 316, 241–255. doi:10.1007/s11104-008-9776-0
- Gordon H , Haygarth PM , Bardgett RD 2008: Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol. Biochem., 40, 302–311. doi:10.1016/j.soilbio.2007.08.008
- Hassink J 1994: Effect of soil texture on the size of the microbial biomass and on the amount of C and N mineralized per unit of microbial biomass in Dutch grassland soils. Soil Biol. Biochem., 26, 1573–1581. doi:10.1016/0038-0717(94)90100-7
- Hevia GG , Buschiazzo DE , Hepper EN , Urioste AM , Antón EL 2003: Organic matter in size fractions of soils of the semiarid Argentina. Effects of climate, soil texture and management. Geoderma , 116, 265–277. doi:10.1016/S0016-7061(03)00104-6
- Kadono A , Funakawa , Kosaki T 2008: Factors controlling mineralization of soil organic matter in the Eurasian steppe. Soil Biol. Biochem. , 40, 947–955. doi:10.1016/j.soilbio.2007.11.015
- Lal R 2004: Soil carbon sequestration impacts on global climate change and food security. Science , 304, 1623–1627. doi:10.1126/science.1097396
- Meehl MM , Stocker TF , Collins WD et al. 2007: Global climate projections. Climate change 2007: the physical science basis. In Contribution of Working Group 1 to the Fourth Assessment Report of the Intergoverment Panel of Climate Change, Eds. Solomon S , Qin D , Manning M et al., pp. 747–845. Cambridge University Press, Cambridge UK.
- Mikha MM , Rice CW , Milliken GA 2005: Carbon and Nitrogen mineralization as affected by drying and wetting cycles. Soil Biol. Biochem. , 37, 339–347. doi:10.1016/j.soilbio.2004.08.003
- Murphy DV , Sparling GP , Fillery IRP , McNeil AM , Braunberger P 1998: Mineralisation of soil organic nitrogen and microbial respiration after simulated summer rainfall events in an agricultural soil. Aust. J. Soil Res., 36, 231–246. doi:10.1071/S97043
- Palm CA , Giller KE , Mafongoya PL , Swift MJ 2001: Management of organic matter in the tropics: translating theory into practice. Nutr. Cycl. Agroecosys., 61, 63–75. doi:10.1023/A:1013318210809
- Peel M , Finlayson BL , McMahon TA 2007: Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci., 11, 1633–1644. doi:10.5194/hess-11-1633-2007
- Post WM , Kwon KC 2000: Soil carbon sequestration and land-use change: processes and potential. Global Change Biol., 6, 317–327. doi:10.1046/j.1365-2486.2000.00308.x
- Pulleman M , Tietema A 1999: Microbial C and N transformations during drying and rewetting of coniferous forest floor materials. Soil Biol Biochem., 31, 275–285. doi:10.1016/S0038-0717(98)00116-3
- Saggar S , Parshotam A , Hedley C , Salt G 1999: 14C-labelled glucose turnover in New Zealand soils. Soil Biol Biochem. , 31, 2025–2037. doi:10.1016/S0038-0717(99)00126-1
- Sakamoto K , Hodono N 2000: Turnover rate of microbial biomass carbon in Japanese upland soils with different textures. Soil Sci Plant Nutr., 46(2), 483–490.
- Schwinnin S , Sala OE , Loik ME , Ehleringer JR 2004: Thresholds, memory, and seasonality: understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia , 141, 191–193. doi:10.1007/s00442-004-1683-3
- Six J , Feller C , Denef K , Ogle SM , deMoraesa JC , Albrecht A 2002: Soil organic matter, biota and aggregation in temperate and tropical soils-Effects of no-tillage. Agronomie , 22, 755–775. doi:10.1051/agro:2002043
- Soil Survey Staff 2006: Keys to Soil Taxonomy, 10th ed. United States Department of Agriculture Natural Resources Conservation Service, Washington, DC.
- Sponseller RA 2007: Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Change Biol., 13, 426–436. doi:10.1111/j.1365-2486.2006.01307.x
- Steinberg Y , Zelles L , Bai QY , von Lützow M , Munch JC 1999: Phospholipid fatty acid profiles as indicators for the microbial community structure in soils along a climatic transect in the Judean Desert. Biol. Fetil. Soils , 28, 292–300. doi:10.1007/s003740050496
- Sugihara S , Funakawa S , Ikazaki K , Shinjo H , Kosaki T 2014: Rewetting of dry soil did not stimulate the carbon and nitrogen mineralization in croplands with plant residue removed in the Sahel, West Africa. Trop. Agr. Deveolp., 58(1), 8–17.
- Sugihara S , Funakawa S , Kilasara M , Kosaki T 2010a: Effect of land management and soil texture on seasonal variations in soil microbial biomass in dry tropical agroecosystems in Tanzania. Appl. Soil Ecol., 44, 80–88. doi:10.1016/j.apsoil.2009.10.003
- Sugihara S , Funakawa S , Kilasara M , Kosaki T 2012: Effects of land management on CO2 flux and soil C stock in two Tanzanian croplands with contrasting soil texture. Soil Biol. Biochem., 46, 1–9. doi:10.1016/j.soilbio.2011.10.013
- Sugihara S , Funakawa S , Kosaki T 2010b: In situ short-term carbon and nitrogen dynamics in relation to microbial dynamics after a simulated rainfall in croplands of different soil texture in Thailand. Soil Sci. Plant Nutr., 56, 813–823. doi:10.1111/j.1747-0765.2010.00516.x
- Takata Y , Funakawa S , Akshalov K , Ishida N , Kosaki T 2007: Influence of land use on the dynamics of soil organic carbon in northern Kazakhstan. Soil Sci. Plant Nutr., 53, 162–172. doi:10.1111/j.1747-0765.2007.00127.x
- Takata Y , Funakawa S , Yanai J , Mishima A , Akshalov K , Ishida N , Kosaki T 2008: Influence of crop rotation system on the spatial and temporal variation of the soil organic carbon budget in northern Kazakhstan. Soil Sci. Plant Nutr., 54, 159–171. doi:10.1111/j.1747-0765.2007.00217.x
- Van Veen JA , Ladd JN , Frissel MJ 1984: Modelling C and N turnover through the microbial biomass in soil. Plant Soil , 76, 257–274. doi:10.1007/BF02205585
- Waldrop MP , Firestone MK 2006: Response of microbial community composition and function to soil climate change. Microbial Ecol., 52, 716–724. doi:10.1007/s00248-006-9103-3
- Wu J , Brookes PC 2005: The proportional mineralization of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol. Biochem., 37, 507–515. doi:10.1016/j.soilbio.2004.07.043
