Abstract
Ammonia-oxidizing archaea (AOA) have more importance in ammonia oxidation than ammonia-oxidizing bacteria (AOB) in acidic red soils. The aim of this study was to investigate if the abundance and composition of AOA could be altered by long-term application of organic manure in an acidic red soil. The abundance and composition of AOA were evaluated by polymerase chain reaction (PCR) and denaturing gradient gel electrophoresis (DGGE) targeting archaeal amoA genes after long-term (24-year) application of mineral fertilizer and/or organic manure. The treatments were: non-fertilized control, mineral nitrogen (N) fertilizer only, mineral N, phosphorus (P) and potassium (K) fertilizer only, organic manure only, and organic manure plus mineral NPK fertilizer. The abundance of archaeal amoA genes was significantly increased after the long-term application of organic manures, either with or without mineral NPK fertilizer. So were the Shannon and Richness diversity indices of AOA deduced from the DGGE patterns. Phylogenetic analyses showed that most of the AOA sequences from various fertilization treatments were affiliated with group 1.1b thaumarchaea and only one with the group 1.1a-associated thaumarchaea. Nitrification potential was significantly increased after the long-term application of organic manures in comparison with the non-fertilized control. Our results strengthened the importance of organic manure in promoting the growth of AOA and thus nitrification potential in the acidic red soils.
INTRODUCTION
Red soils (Ultisols and Oxisols in US Soil Taxonomy) are widely distributed in South and Central America, and South and Southeast Asia, and cover a land area of 3 billion ha in the world. Red soils are typical subtropical soils in southeast of China, which cover about 1.13 million km2 or 11.8% of the country’s land surface, and support 22.5% of the nation’s population (Zhao Citation2002). Red soils are heavily weathering and leaching soils, and are characterized by low pH and deficiencies in major nutrients, in particular phosphorus (P; Zhong and Cai Citation2007; He et al. Citation2008; Zhong et al. Citation2010). The red soils are eroded due to deforestation and intensive land use, resulting in low organic matter and crop yields (Zhao Citation2002). Hence, great efforts have been made since the mid-1980s to restore the degraded red soils, such as the establishment of a long-term fertilizer experiment. Our previous study showed that the long-term (21-year) application of organic manures, either with or without mineral nitrogen, phosphorus and potassium (NPK) fertilizer, could improve soil microbial biomass and functional diversity, and thus enhance maize (Zea mays L.) yields in an acidic red soil (Zhong et al. Citation2010). Yet little is known about key microbial communities involved in the nitrogen (N) cycle after the long-term application of organic manure and/or mineral fertilizer.
Oxidation of ammonia (NH3) to nitrite (NO2–) by ammonia-oxidizing prokaryotes, as a key step in the global N cycle in terrestrial ecosystems, is the rate-limiting step in nitrification (Kowalchuk and Stephen Citation2001; He et al. Citation2012). NH3 is used as a substrate for NH3 monooxygenase (AMO) during NH3 oxidation (Kowalchuk and Stephen Citation2001; He et al. Citation2012). The decrease of pH could reduce the concentration of NH3 exponentially in soil; thus the NH3 availability would be under the substrate threshold of ammonia-oxidizing bacteria (AOB) rather than ammonia-oxidizing archaea (AOA; Stopnisek et al. Citation2010; He et al. Citation2012). Recent studies have shown that AOA play a more important role than AOB in NH3 oxidation in low-pH soils (Nicol et al. Citation2008; Yao et al. Citation2011; He et al. Citation2012; Huang et al. Citation2012; Zhang et al. Citation2012). For example, archaeal amoA gene abundance and transcriptional activity increased when soil pH decreased (0.5 interval from 4.5 to 7.5), whilst bacterial amoA gene abundance was generally lower and transcriptional activity decreased with decreasing pH after a 46-y soil pH manipulation with lime or aluminum sulfate (Nicol et al. Citation2008).
Long-term (16-year) application of mineral NPK fertilizer plus organic manure changed the abundance and composition of AOA and nitrification potential in an acidic red soil (He et al. Citation2007). The population size and nitrification potential were greatest after the application of mineral NPK fertilizer plus organic manure, and least after the application of mineral N fertilizer only. The abundance and composition of AOA were more sensitive than AOB to long-term (19-year) application of mineral NPK fertilizer plus crop residues in an acidic paddy soil (Chen et al. Citation2011). The population size was highest after the application of mineral NPK fertilizer plus crop residues whilst lowest under the non-fertilized control. Yet the differences in the community structure of AOA are not fully understood between fertilization treatments. Moreover, it remains to be investigated what influence the organic manure exerted on the abundance and composition of AOA, since the interaction effects between mineral NPK fertilizer and organic manure are not well separated.
We therefore conducted a 24-year long-term fertilizer experiment to study if the abundance and composition of AOA and nitrification potential could be altered by long-term application of organic manure in an acidic red soil. Our objectives were to investigate the long-term effects of application of mineral N fertilizer only, mineral NPK fertilizer only, organic manure only, and organic manure plus mineral NPK fertilizer on the abundance and composition of AOA and nitrification potential in the acidic red soil. We then related these effects to better fertilization regimes and soil fertility management for such eroded red soils.
MATERIALS AND METHODS
Field site
The field site is located in Jinxian County, Jiangxi Province, China (28°15′30″N, 116°20′24″E), where the climate is a subtropical monsoon with annual mean temperature and precipitation of 17.5°C and 1537 mm, respectively. The long-term field fertilizer experiment has been established in an uncultivated wasteland since 1986, where the soil was derived from quaternary red clay, and the surface soil was completely eroded with lower organic carbon (C) content. Soil pH, organic C and nutrient contents as well as field planting history were reported previously by Zhong et al. (Citation2010). The experiment was conducted in triplicate according to a completely randomized design, with 10 fertilizer treatments: (1) the control (CK, no fertilization); (2) mineral nitrogen fertilizer (N); (3) mineral phosphorus fertilizer (P); (4) mineral potassium fertilizer (K); (5) mineral nitrogen and phosphorus fertilizer (NP); (6) mineral nitrogen and potassium fertilizer (NK); (7) mineral nitrogen, phosphorus and potassium fertilizer (NPK); (8) double mineral NPK fertilizer (2NPK); (9) organic manure (OM); and (10) organic manure plus mineral NPK fertilizer (OM+NPK). The N, P and K fertilizers and organic manure were applied in the form of urea (120 kg N ha–1 per year), calcium superphosphate (60 kg P2O5 ha–1 per year), potassium chloride (KCl; 120 kg K2O ha–1 per year), and composted pig manure (30 t ha–1 per year), respectively. Mineral fertilizers and organic manure were applied as basal fertilization before planting maize (Zea mays L.).
Soil sampling and chemical analysis
Soil samples were collected from five selected treatments including CK, N, NPK, OM and OM+NPK since we have shown that organic manure and/or balanced fertilization with N, P and K significantly improved soil microbial community and functional diversity, and thus enhanced crop growth and yields (Zhong et al. Citation2010). The soil samples were gathered from the 0–20 cm layer at six randomly selected points in each plot in July 2010 and mixed to yield a composite sample. Each treatment had three composite samples (replicates). Fresh composite samples were then stored at 4°C for microbiological and chemical N analysis within 1 week, and at –80°C for DNA extraction, respectively. Air-dried, sieved (< 2 mm) soils were used for chemical analysis.
Soil pH was measured with a glass electrode (soil:water = 1:2.5). Soil organic C was determined by the dichromate oxidation. Soil mineral N was extracted with 2 M KCl (soil:KCl = 1:4) for 1 h, and ammonium N (NH4+-N) and nitrate N (NO3–-N) were then determined with a Skalar SANplus Segmented Flow Analyzer (Skalar Analytic B.V., De Breda, The Netherlands) according to the manual. Olsen-P and ammonium acetate-extractable K (NH4OAc-K) were extracted with sodium bicarbonate and ammonium acetate (NH4OAc), and then determined by the molybdenum-blue method and a flame photometry, respectively (Lu Citation2000).
Soil DNA extraction
Soil DNA was extracted with a FastDNA Spin Kit for Soil (MoBio, Inc., Irvine, CA, USA), according to the manual. Briefly, 0.5 g soil (fresh weight) was added to a lysing matrix tube containing a mixture of ceramic and silica particles. After the addition of 978 µL sodium phosphate and 122 µL microtubule buffer to the lysing matrix tubes, genomic DNA was extracted with a FastPrep FP (Bio 101 Inc., Carlsbad, CA, USA) for 40 s at speed 6.0. A Geneclean procedure was then applied to purify the genomic DNA with a silica matrix and elution reagent (MP Biomedicals, LLC, Solon, OH, USA). The purified DNA was stored at –20°C for further analysis.
Real-time quantitative PCR of archaeal amoA genes
The copy numbers of archaeal amoA genes were determined in triplicates using a CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA). The primers Arch-amoAF and Arch-amoAR were used for polymerase chain reaction (PCR) amplification targeting the archaeal amoA genes (Francis et al. Citation2005). Real-time quantitative PCR was performed using the SYBR® Premix Ex TaqTM (TaKaRa, Tokyo, Japan) according to the manual. Briefly, each reaction contained 12.5 μL SYBR Premix Ex TaqTM, 0.2 μM each primer and 1 μL 10 fold-diluted DNA template in a final volume of 25 μL. Two-step thermal profiles consisted of 95°C at 10 s, followed by 40 cycles of 5 s at 95°C and 30 s at 60°C, with plate reading at 83°C for data acquisition. Product specificities were confirmed by melting curve analyses as the temperature ranged from 55 to 95°C, and were visualized in 1.5% agarose gels, which showed specific product bands at the expected size of 635 bp. Data analyses were performed with CFX Manager Software Version 2.0 (Bio-Rad Laboratories, Hercules, CA, USA). The parameter Ct (threshold cycle) was determined as the cycle number at which a statistically significant increase in the reporter fluorescence was detected. Archaeal amoA amplicon was generated from total DNA extracted from soil samples tested in this study. The PCR products were purified and then cloned into the pEasy-T1 Simple Cloning Vector (TransGen Biotech, Beijing, China). The positive clones were picked and sequenced. One clone containing correct insert was selected to extract plasmid DNA with the MiniBEST Plasmid Purification Kit (TaKaRa, Tokyo, Japan). The plasmid DNA concentration was determined on a SmartSpec 3000 Spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA), and the copy numbers of archaeal amoA genes were calculated directly from the concentration of the extracted plasmid DNA. Ten-fold serial dilutions of a known copy number of the plasmid DNA were subjected to the real-time quantitative PCR assays to generate external standard curves. PCR amplification efficiency of 82.1% was obtained from the amoA genes with R2 values of 0.989.
PCR and DGGE assays of archaeal amoA genes
The primers Arch-amoAF-GC and Arch-amoAR were used for the archaeal amoA gene amplification (Francis et al. Citation2005; Chen et al. Citation2008). PCR amplification was performed using a S1000TM Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA) in 50 µL reaction volumes containing 0.4 µM of each primer, 2.5 mM magnesium chloride (MgCl2), 0.2 mM of each deoxynucleotide triphosphate (dNTP), 1.25 U Taq DNA polymerase (TaKaRa, Dalian, China), and 1 µL of DNA template. The thermal profiles consisted of 94°C for 5 min, followed by 30 cycles of 94°C for 45 s, 55°C for 45 s and 72°C for 60 s, and a final extension at 72°C for 10 min. The PCR products were electrophoresed on 1.0% agarose gel to ascertain their size and quality, which showed specific product bands at the expected size of 675 bp.
Denaturing gradient gel electrophoresis (DGGE) was performed at 60°C with the DCode Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, CA, USA) according to the manual. The PCR products (approximately 200 ng) were directly loaded on 6% (weight/volume) polyacrylamide gels (acrylamide:bisacrylamide = 37.5:1) with a gradient of 30–65%, where a 100% denaturing solution was defined as 7 M urea and 40% formamide. The gels were electrophoresed at 100 V for 16 h in 0.5 × Tris-acetate-EDTA (TAE) buffer, and stained with 1:10,000 SYBR Green І (Molecular Probes, Eugene, OR, USA) for 30 min. The stained gels were then immediately photographed on an ultraviolet (UV) transilluminator with a charge-coupled device (CCD) camera (Bio-Rad Laboratories, Hercules, CA, USA). Digital images of the gels were further analyzed with Quantity One® 1-D Analysis Software (Bio-Rad Laboratories, Hercules, CA, USA). Removing the background intensity from each lane, the software performs a density profile through lanes, detects individual bands and matches bands occupying the same position in different lanes (Xue et al. Citation2006). The genetic diversities were analyzed by Richness (S), Shannon diversity (H) and Evenness (EH) indices (Xue et al. Citation2006).
Cloning, sequencing and phylogenetic analyses
Only numbered bands in the DGGE gel were excised for clone and sequencing analysis. The dominant bands in the DGGE gel were excised and placed in a sterile microcentrifuge tube. The polyacrylamide slice was then finely ground using a sterilized pipette tip, and 20 μL distilled water (dH2O) was added. The sample was left for 12 h at 4°C, and 1 μL of the gel suspension was used for PCR amplification using the primers Arch-amoAF/Arch-amoAR without a guanosine-cytosine clamp. The PCR product was purified with MiniBEST DNA Fragment Purification Kit (TaKaRa, Tokyo, Japan), and then cloned into the pEasy-T1 Simple Cloning Vector (TransGen Biotech, Beijing, China). The positive clones containing the insert in the correct size and mobility were selected and sequenced with an ABI PRISM 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). The nucleotide sequences of archaeal amoA genes determined or retrieved from the GenBank were aligned, and the neighbor-joining trees were constructed using MEGA version 4.0 with 1000 replicates to produce bootstrap values (Tamura et al. Citation2007). We used the nomenclature for archaeal amoA clusters as defined by Nicol et al. (Citation2008) and Zhang et al. (Citation2012). The sequences generated in this study have been submitted to the GenBank databases (accession numbers KC469620 to KC469632).
Nitrification potential
Nitrification potential was determined under natural pH or neutral pH according to Taylor et al. (Citation2010), with slight modifications. Fresh 5 g soil samples were added to 50 mL distilled water or phosphate buffer (pH 7.0) in a 150-mL measuring flask with ammonium sulfate solution at a rate of 1 mM NH4+ to achieve natural pH or neutral pH, respectively. Soil slurries were shaken at 200 rpm and maintained at 30 or 40°C for 48 h in dark. Subsamples of soil slurry were collected every 24 h and centrifuged at 12,000 rpm for 10 min, and NO2– and nitrate (NO3–) concentrations in the supernatants were determined immediately. NO2– concentration was spectrophotometrically determined at 540 nm by the Griess reagent colorimetric method, and NO3– concentration was done at 570 nm with Szechrome NAS (Polysciences, Inc., Warrington, PA, USA) according to the manual. The nitrification potential (nmol NO2– + NO3– g–1 dry weight (DW) d–1) was the accumulations of NO2– and NO3– over 24 h.
Statistics
All data were subjected to one-way analysis of variance (ANOVA), and differences in means (amoA gene copy numbers were Log10 transformed) between treatments were analyzed by the least significant difference (LSD) multiple comparison at the 5% level with SPSS 16.0 for Windows (IBM Co., Armonk, NY, USA). The correlation of AOA community structure to environmental factors was assessed using redundancy analysis (RDA). RDA was performed by inputting the “species data” (individual DGGE bands) and “environmental data” (soil pH, organic C, NH4+-N, NO3–-N, Olsen-P and NH4OAc-K) into the computer program CANOCO 4.5 (Microcomputer Power, Ithaca, NY, USA). A Monte Carlo permutation test was carried out based on 499 random permutations.
RESULTS
Soil chemical properties
Soil pH was significantly decreased after application of mineral N fertilizer only, while it increased after application of organic manures, either with or without mineral NPK fertilizer, compared to the control or mineral NPK fertilizer only (). Soil organic C was significantly increased after the application of organic manures, either with or without mineral NPK fertilizer, in comparison with the control, and also significantly increased after the application of mineral NPK fertilizer, only compared to the control. Soil NH4+-N was significantly increased after the application of organic manure plus mineral NPK fertilizer in comparison with the control, or with mineral N fertilizer only. Soil NO3–-N was significantly increased after the application of organic manures, either with or without mineral NPK fertilizer, compared to the control. Olsen-P was significantly increased after the application of organic manures, either with or without mineral NPK fertilizer, in comparison with the control, mineral N only or mineral NPK fertilizer only. NH4OAc-K was significantly increased after the application of organic manures, either with or without mineral NPK fertilizer, compared to the control or mineral N fertilizer only, and also significantly increased after the application of mineral NPK fertilizer only, compared to the control or organic manure only.
Table 1 Soil pH, organic carbon (C), ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3–-N), Olsen phosphorus (Olsen-P) and ammonium acetate-extractable potassium (NH4OAc-K) after long-term (24-year) treatments with mineral fertilizer and/or organic manure in an acidic red soil
Abundance of archaeal amoA genes
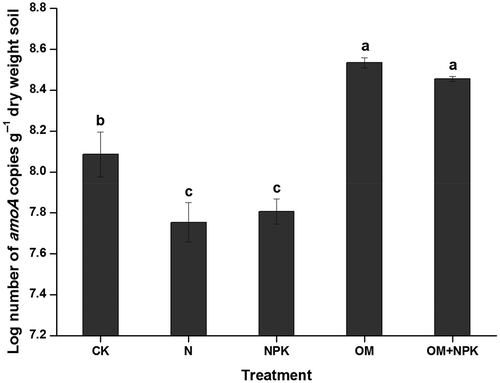
Archaeal amoA gene copy numbers ranged from 5.14 × 107 to 3.56 × 108 g–1 dry weight soil. Long-term (24-year) application of organic manures, either with or without mineral NPK fertilizer, changed the abundance of archaeal amoA genes (). The abundance of archaeal amoA genes was decreased after application of mineral N or NPK fertilizers, while it significantly increased after application of organic manures, either with or without mineral NPK fertilizer, compared to the control. Correlation analysis showed that the abundance of AOA (r = 0.888, n = 15, p < 0.001) was positively correlated with soil pH.
Figure 1 The abundance of archaeal amoA genes after long-term (24-year) treatments with mineral fertilizer and/or organic manure in an acidic red soil. Values (means ± standard error, SE, n = 3) followed by the same letter above the bars are not significantly different determined using least significant difference (LSD) test (p < 0.05). CK, N, NPK, OM and OM+NPK are the control; mineral nitrogen (N) fertilizer; mineral N, phosphorus (P) and potassium (K) fertilizer; organic manure; and organic manure plus mineral NPK fertilizer treatments, respectively.
Composition of archaeal amoA genes
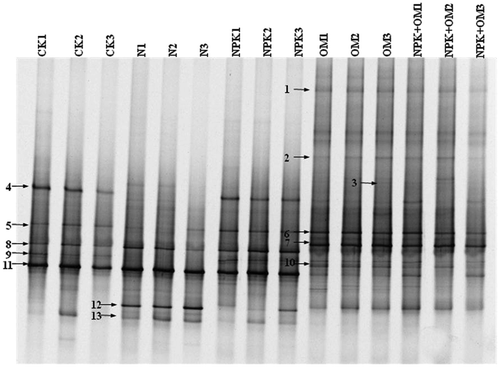
Long-term (24-year) application of organic manures, either with or without mineral NPK fertilizer, changed the composition of archaeal amoA genes (). The highest Shannon indices of archaeal amoA genes were found after application of organic manures, followed by application of mineral NPK fertilizer only, then application of mineral N fertilizer only, and were least in the control (). Richness indices differed in the following pattern: OM > OM+NPK > NPK > N > CK. Evenness indices were significantly increased after application of mineral N fertilizer only, compared to application of mineral NPK fertilizer only (p = 0.041), while neither was significantly different from the control.
Table 2 Diversity indices of ammonia-oxidizing archaea (AOA) after long-term (24-year) treatments with mineral fertilizer and/or organic manure in an acidic red soil
Figure 2 Denaturing gradient gel electrophoresis (DGGE) profiles of archaeal amoA genes after long-term (24-year) treatments with mineral fertilizer and/or organic manure in an acidic red soil. Bands with the same mobility in the DGGE gel were marked with the same number and excised for sequencing. CK, N, NPK, OM and OM+NPK are the control; mineral nitrogen (N) fertilizer; mineral N, phosphorus (P) and potassium (K) fertilizer; organic manure; and organic manure plus mineral NPK fertilizer treatments, respectively.
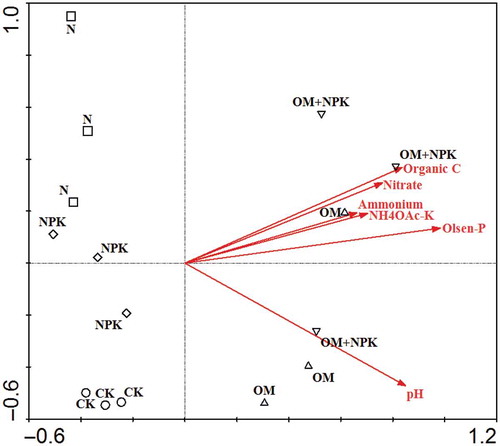
According to the main axis of the RDA ordination plot, the composition of archaeal amoA genes differed in organic manure treatments from mineral fertilizer only treatments and the control (). The percentage of the variance explained by the environmental variables was 70.4% along axis 1 and 13.0% along axis 2. The RDA graph illustrated that Olsen-P (p = 0.002) and pH (p = 0.022) were key environmental variables determining the community composition. Soil pH, organic C, NH4+-N, NO3–-N, Olsen-P and NH4OAc-K were positively correlated with the application of organic manures either with or without mineral NPK fertilizer, while they were negatively correlated with the control and application of mineral fertilizers only.
Figure 3 Redundancy analysis (RDA) of ammonia-oxidizing archaea after long-term (24-year) treatments with mineral fertilizer and/or organic manure in an acidic red soil. CK, N, NPK, OM and OM+NPK are the control; mineral nitrogen (N) fertilizer; mineral N, phosphorus (P) and potassium (K) fertilizer; organic manure; and organic manure plus mineral NPK fertilizer treatments, respectively. Abbreviations: organic C, organic carbon; NH4OAc-K, ammonium acetate-extractable potassium; Olsen-P, Olsen phosphorus.
Phylogenetic tree of archaeal amoA genes
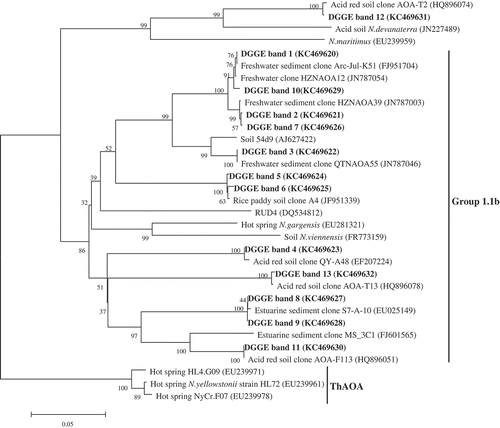
A neighbor-joining tree was constructed by depositing the nucleotide acid sequences of archaeal DGGE bands and their related sequences in GenBank ( and ). Most of the band sequences belonged to the soil and sediment lineage or the group 1.1b thaumarchaea, while only one belonged to the sediment and soil V cluster in marine lineage, or the group 1.1a-associated thaumarchaea. Bands 1, 2, 3, 7 and 10 were detected in organic manure treatments but not detected in any other treatment. Band 4 was not detected in the N treatment and showed less intensity in OM and OM+NPK treatments. Bands 8 and 13 were detected in the control, N and NPK treatments but not detected in organic manure treatments. Band 11 showed high intensity in the control, N and NPK treatments but less intensity in OM and OM+NPK treatments. Band 12 showed high intensity in N treatments but less intensity in NPK, OM and OM+NPK treatments, and was not detected in the control.
Figure 4 Phylogenetic relationships between partial archaeal amoA sequences retrieved from the denaturing gradient gel electrophoresis (DGGE) bands (in bold) in this study and the GenBank. The accession number for each sequence is shown in brackets. The scale bar indicates five changes per 100 nucleotide positions.
Nitrification potential
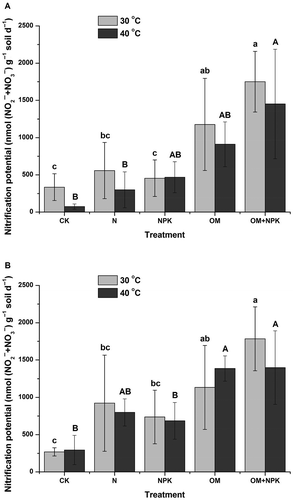
Under neutral pH, nitrification potential was significantly increased after application of organic manure plus mineral NPK fertilizer compared to the control or application of mineral N fertilizer only, at either 30°C or 40°C, and significantly increased after the application of organic manure compared to the control or application of mineral NPK fertilizer only at 30°C (). Under natural pH, the nitrification potential was significantly increased after the application of organic manures compared to the control, at either 30°C or 40°C, and significantly increased after the application of organic manure plus mineral NPK fertilizer compared to mineral fertilizers only at 30°C (). Moreover, the nitrification potential was significantly increased after the application of organic manures compared to the control or application of mineral NPK fertilizer only at 40°C.
Figure 5 Soil nitrification potential incubated under neutral pH (A) and natural pH (B) after long-term (24-year) treatments with mineral fertilizer and/or organic manure in an acidic red soil. Values (means ± standard error, SE, n = 3) followed by the same letter at 30°C (a, b, c) or 40°C (A, B, C) above the bars are not significantly different, determined using least significant difference (LSD) test (p < 0.05). CK, N, NPK, OM and OM+NPK are the control; mineral nitrogen (N) fertilizer; mineral N, phosphorus (P) and potassium (K) fertilizer; organic manure; and organic manure plus mineral NPK fertilizer treatments, respectively. Abbreviations: NO2–, nitrite; NO3–, nitrate.
DISCUSSION
The archaeal amoA gene copy numbers in our acidic red soil were comparable to those measured with real-time quantitative PCR in other agricultural soils (He et al. Citation2007; Chen et al. Citation2008; Shen et al. Citation2008). The AOA population sizes were observed to be highest in an acidic red soil (pH 3.7–6.0) receiving mineral NPK fertilizer plus organic manure while they were the lowest in the soil receiving mineral N fertilizer only (He et al. Citation2007). Moreover, the AOA population sizes were significantly increased after long-term application of mineral NPK fertilizer plus crop residues in comparison with the non-fertilized control in an acidic paddy soil (pH 5.1–5.4; Chen et al. Citation2011). Our results clarified that long-term application of organic manures, either with or without mineral NPK, could increase the AOA population size in our acidic red soil (pH 4.5–6.3) compared to mineral N fertilizer only or mineral NPK fertilizer only ().
The community structure of AOA was sensitive to different long-term fertilization treatments in the acidic red soils (He et al. Citation2007; Chen et al. Citation2011). Our results show that the application of organic manures, either with or without mineral NPK fertilizer, resulted in a notable shift in the community structure of AOA in comparison with mineral fertilizers only and the non-fertilized control ( and ). Moreover, the application of organic manures significantly increased the Shannon and Richness diversity indices compared to either mineral fertilizers only or the non-fertilized control. The growth of AOA was enhanced after the long-term application of organic manures, probably due to the availability of P and increased pH in soils receiving organic manures, which were key environmental factors governing the microbial community of AOA (; ). Moreover, the long-term application of organic manures improved soil structure and fertility, and enhanced water and nutrient supplying capacity, and thus increased plant growth and crop yields (Zhong et al. Citation2010). Hence, much more root exudates and residues were released into soils receiving organic manures, which might stimulate the growth of AOA as substrate, energy source or hormone (Arp et al. Citation2007; Hatzenpichler Citation2012; Stahl and De La Torre Citation2012).
DGGE bands of AOA were used for sequencing, and most of the sequences fell within the soil and sediment linage or the group 1.1b thaumarchaea (). The observation of bands 1, 2, 3, 7 and 10 in soils receiving organic manures indicated that these species might be sensitive to more acidic conditions in soils receiving mineral fertilizers only or the non-fertilized soils (). The dominant bands 6 and 7 in soils receiving organic manures suggested the preference of the two corresponding species in neutral pH conditions. In contrast, the unique presence of bands 8 and 13 in the non-fertilized soil and soils receiving mineral fertilizer only indicated their potential adaptation to low-pH soil and sensitivity to increased pH. Similarly, the dominant band 11 in these soils suggested the species preferably presented in low-pH soils. Only one sequence from band 12 fell within the sediment and soil V cluster in marine linage, or the group 1.1a-associated thaumarchaea, which was reported to be negatively correlated with soil pH. As expected, the highest intensity of band 12 was observed in the soil receiving mineral N fertilizer only, which caused the lowest soil pH (). The long-term application of organic manures could dramatically change the community structure as well as the population size of AOA, possibly because AOA was able to grow under autotrophic, mixotrophic and heterotrophic conditions (Hatzenpichler Citation2012; Stahl and De La Torre Citation2012).
Nitrification potential was suggested to be determined under conditions of non-limiting NH3 and oxygen (O2), and neutral pH (Taylor et al. Citation2010). However, soil pH was found to be significantly positive correlated with the abundance of archaeal amoA genes in this study. Hence, the nitrification potential was more reliably determined at natural pH that was almost the same as in situ soil pH. Recent studies also revealed that AOA had a more important role than AOB in NH3 oxidation of acidic soils tested through soil microcosm with a 13C-labeled C source (Zhang et al. Citation2012), or a 15N-labled N source (Huang et al. Citation2012), as did in situ field studies (Yao et al. Citation2011). Our results illustrated that the long-term application of organic manures, either with or without mineral NPK fertilizer, enhanced the nitrification potential in the acidic red soil, irrespective of culture pH and temperature. These results might suggest that the activity of AOA was significantly increased after the long-term application of organic manures in the acidic red soil. Moreover, the AOB population size may be increased, since soil pH and NH3 content were significantly increased after the long-term application of organic manures (), which were key ecological factors determining the AOB population size and activity (Kowalchuk and Stephen Citation2001; He et al. Citation2007; Shen et al. Citation2008). Hence, both AOA and AOB may contribute to the increased nitrification potential after the long-term application of organic manures.
CONCLUSION
Long-term (24-year) application of organic manures, either with or without mineral NPK fertilizer, could change the abundance and composition of AOA and nitrification potential in the acidic red soil. The abundance of archaeal amoA genes was significantly increased after the long-term application of organic manures, either with or without mineral NPK fertilizer. Most AOA sequences from various fertilization treatments were affiliated with group 1.1b thaumarchaea, and only one with the group 1.1a-associated thaumarchaea. The nitrification potential was increased after the long-term application of organic manures.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (41271255, 41001146) and the Major Program of Natural Science Research (12KJA170001), and the Academic Priority Development Program of Jiangsu Higher Education Institution of China.
REFERENCES
- Arp DJ, Chain PSG, Klotz MG 2007: The impact of genome analyses on our understanding of ammonia-oxidizing bacteria. Annu. Rev. Microbiol., 61, 503–528. doi:10.1146/annurev.micro.61.080706.093449
- Chen X, Zhang LM, Shen JP, Wei WX, He JZ 2011: Abundance and community structure of ammonia-oxidizing archaea and bacteria in an acid paddy soil. Biol. Fertil. Soils, 47, 323–331. doi:10.1007/s00374-011-0542-8
- Chen XP, Zhu YG, Xia Y, Shen JP, Jz H 2008: Ammonia-oxidizing archaea: important players in paddy rhizosphere soil? Environ. Microbiol., 10, 1978–1987.
- Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB 2005: Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA, 102, 14683–14688. doi:10.1073/pnas.0506625102
- Hatzenpichler R 2012: Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl. Environ. Microbiol., 78, 7501–7510. doi:10.1128/AEM.01960-12
- He JZ, Hu HW, Zhang LM 2012: Current insights into the autotrophic thaumarchaeal ammonia oxidation in acidic soils. Soil Biol. Biochem., 55, 146–154. doi:10.1016/j.soilbio.2012.06.006
- He JZ, Shen JP, Zhang LM, Zhu YG, Zheng YM, Xu MG, Di HJ 2007: Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol., 9, 2364–2374. doi:10.1111/j.1462-2920.2007.01358.x
- He JZ, Zheng Y, Chen CR, He YQ, Zhang LM 2008: Microbial composition and diversity of an upland red soil under long-term fertilization treatments as revealed by culture-dependent and culture-independent approaches. J. Soils Sediments, 8, 349–358. doi:10.1007/s11368-008-0025-1
- Huang R, Wu YC, Zhang JB, Zhong WH, Jia ZJ, Cai ZC 2012: Nitrification activity and putative ammonia-oxidizing archaea in acidic red soils. J. Soils Sediments, 12, 420–428. doi:10.1007/s11368-011-0450-4
- Kowalchuk GA, Stephen JR 2001: Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu. Rev. Microbial., 55, 485–529. doi:10.1146/annurev.micro.55.1.485
- Lu RK 2000: Methods of Soil Agricultural Chemical Analysis. China Agriculture Science & Technology Press, Beijing.
- Nicol GW, Leininger S, Schleper C, Prosser JI 2008: The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol., 10, 2966–2978. doi:10.1111/j.1462-2920.2008.01701.x
- Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ 2008: Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environ. Microbiol., 10, 1601–1611. doi:10.1111/j.1462-2920.2008.01578.x
- Stahl DA, De La Torre J 2012: Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol., 66, 83–101. doi:10.1146/annurev-micro-092611-150128
- Stopnisek N, Gubry-Rangin C, Höfferle S, Nicol GW, Mandic-Mulec I, Prosser JI 2010: Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl. Environ. Microbiol., 76, 7626–7634. doi:10.1128/AEM.00595-10
- Tamura K, Dudley J, Nei M, Kumar S 2007: MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol., 24, 1596–1599. doi:10.1093/molbev/msm092
- Taylor AE, Zeglin LH, Dooley S, Myrold DD, Bottomley PJ 2010: Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils. Appl. Environ. Microbiol, 76, 7691–7698. doi:10.1128/AEM.01324-10
- Xue D, Yao HY, Huang CY 2006: Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils. Plant Soil, 288, 319–331. doi:10.1007/s11104-006-9123-2
- Yao HY, Gao YM, Nicol GW, Campbell CD, Prosser JI, Zhang LM, Han WY, Singh BK 2011: Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Appl. Environ. Microbiol., 77, 4618–4625. doi:10.1128/AEM.00136-11
- Zhang LM, Hu HW, Shen JP, He JZ 2012: Ammnoia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J., 6, 1032–1045. doi:10.1038/ismej.2011.168
- Zhao QG 2002: Mechanism, temporal-spatial changes and controlling countermeasures of soil degradation in hilly red soil region of Southeastern China, 290–332. Science Press, Beijing.
- Zhong WH, Cai ZC 2007: Long-term effects of inorganic fertilizers on microbial biomass and community functional diversity in a paddy soil derived from quaternary red clay. Appl. Soil Ecol., 36, 84–91. doi:10.1016/j.apsoil.2006.12.001
- Zhong WH, Gu T, Wang W, Zhang B, Lin XG, Huang QR, Shen WS 2010: The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil, 326, 511–522. doi:10.1007/s11104-009-9988-y
