Abstract
Foliar urea application provides an alternative strategy for minimizing the risk of nitrogen (N) over-fertilization in green tea (Camellia sinensis L.) production. Solution and pot experiments were conducted with the objective to evaluate the utilization efficiency of foliar applied urea and the impact of plant nitrogen (N), potassium (K), magnesium (Mg) and sulfur (S) nutritional status. In the solution experiment, the dynamic absorption and translocation of foliar N was followed by scheduled samplings at 2 and 6 h, 1, 2, 3, 7 and 13 d after application. In the pot experiment, foliar urea-N absorption was measured for tea plants supplied with adequate nutrients (Control, CK) or low levels of respective nutrients (–N, –K, –Mg, and –S) in soil. The results showed that foliar-15N uptake rate was the highest within the earliest 6 h and the 15N amount in treated leaves reached a maximum within 2 d after application in the solution experiment. The absorbed 15N was mainly transported to young shoots, and the export rate became significant after 1 d. The total 15N absorption in plants increased significantly within 2 d and increased slightly but constantly afterwards until 13 d. In the pot experiment, the low supply of nutrients except S significantly decreased their concentrations in mature leaves. The Ndff values in urea-treated leaves and young shoots were 8.1–16.4% and 7.1–19.2%, respectively, and were the highest in the –N treatment compared to the other treatments, suggesting the most remarkable contribution of foliar N application to the N status of –N plants. Low N (–N) supply reduced the total 15N absorption and its allocation to young shoots. Low K (–K) supply significantly diminished the 15N amount in young shoots without affecting the total absorption. Total 15N absorption in tea plants was decreased by low S (–S) but unaffected by low Mg (–Mg) status. The amount of 15N in the young shoots correlated closely (r = 0.94, p < 0.001) with its biomass and also with the biomass of whole above-ground plants, to a lesser extent (r = 0.51, p < 0.05). It was concluded that foliar urea-N was rapidly absorbed and exported mainly to the young shoots, representing the major sink. The utilization efficiency of foliar-N was reduced by depleted N, K and S nutritional status which weakened the N sink strength resulting from poor young shoot growth.
INTRODUCTION
Foliar application of nutrients is considered more efficient than soil fertilization and urea for its uncharged nature and ready passage through cuticle, and thus has been the most commonly and extensively used fertilizer method over fields and horticultural crops (Gooding and Davies Citation1992; Cheng et al. Citation2002). The efficiency of foliar urea application is affected by a number of factors, including the nutritional status of plants, the developmental stage and age of leaves, properties of leaf surface, weather conditions and application time (Fageria et al. Citation2009; Fernández and Eichert Citation2009). The effect of plant nitrogen (N) status on foliar N uptake has been investigated by a number of experiments but the results were inconclusive. For example, in Hydrangea and apple (Malus domestica Borkh.), absorption and translocation of foliar urea-N in fall were more efficient in plants with low N status than in those with higher N status, which was explained by high N feedback inhibition in the latter plants (Cheng et al. Citation2002; Bi and Scagel Citation2008). A similar effect of N status has been observed in citrus (Lea-Cox and Syvertsen Citation1995). By contrast, N-deficient citrus leaves demonstrated lower foliar N absorption than N-sufficient leaves due to increased epicuticular wax concentration in the former leaves (Bondada et al. Citation2001). Klein and Weinbaum (Citation1984) also showed that translocation of foliar urea-N from the treated leaves to new shoots and roots was reduced by N deficiency, and explained it as a result of decreasing sink demand. In some other works, N deficiency exhibited little effect on foliar urea absorption or the partitioning of absorbed N (Bowman and Paul Citation1989). On the other hand, there were fewer studies reporting the influence of other nutrients than N nutrition on the uptake and mobilization of foliar urea-N (e.g. Sekhon et al. Citation1990; Tea et al. Citation2007). Utilization of foliar-applied urea is also dependent upon the assimilation of ammonia (NH3) released from its hydrolyzation through the glutamine synthetase - glutamine oxoglutarate aminotransferase (GS-GOGAT) pathway via glutamine synthetase in the cytosols and efficient N export from the treated leaves to other organs via phloem passage (Dong et al. Citation2002; Witte et al. Citation2002; Xia and Cheng Citation2004). It was reported that loading and transport of amino acids in phloem is inhibited by potassium (K) and magnesium (Mg) deficiency (Cakmak and Kirkby Citation2008; Ruan et al. Citation2012). It is therefore speculated that K and Mg deficiency likely in turn interfere with the absorption and export of foliar N from the fertilization leaves. Due to a synergistic effect of N and sulfur (S) nutrition in plants, simultaneous foliar application of N and S fertilizers increased their recoveries and protein content in wheat (Triticum aestivum L.) (Tea et al. Citation2007). By contrast, the uptake of other nutrients was changed by foliar nutrition, requiring subsequent modification of the fertilization program (Scagel et al. Citation2008).
Tea (Camellia sinensis L.) is one of the most popular beverages in the world. Its quality is dependent upon the contents of free amino acids, polyphenols or catechins, and caffeine in the harvested young shoots. In China, a major portion of tea plantations are located on highly leached, strongly acidic red soils (Ferrosols, Luvisols and Ferralisols), and low inherent fertility is a widespread factor limiting tea productivity. N fertilizer is frequently applied in tea plantations to maintain sustainable yield production with desirable quality components (Tachibana et al. Citation1996; Venkatesan et al. Citation2004; Kamau et al. Citation2008; Ruan et al. Citation2010). Due to the important contribution of free amino acids to tea quality, a high amount of N fertilizer has been frequently applied in green tea production (Tachibana et al. Citation1996; Ruan et al. Citation2010). Over-fertilization of N exceeding plant requirements imposes low N use efficiency and severe environmental hazards including soil acidification, potential nitrate (NO3–) leaching and gaseous N emissions (Tachibana et al. Citation1996; Tokuda and Hayatsu Citation2004; Oh et al. Citation2006; Kamau et al. Citation2008). Some previous works revealed the importance of sufficient and balanced nutrition, with an emphasis on N and other nutrients including K, Mg and S (Ruan et al. Citation1998, Citation2013). Urea applied to mature leaves of tea plants is readily absorbed and assimilated into amino acids, caffeine and protein in the young shoots (Karasuyama et al. Citation1985). Therefore, foliar urea application provides an alternative fertilization strategy minimizing the potential risk of nutrient leaching loss compared with conventional soil fertilization (Gooding and Davies Citation1992; Dong et al. Citation2005). The objectives of this study were to evaluate the utilization efficiency of foliar-applied urea and the effect on N, K, Mg and S nutritional status of tea plants.
MATERIALS AND METHODS
Experiment 1: solution experiment investigating absorption of foliar urea-15N
Tea seedlings with 8–10 mature leaves (mean dry weight about 2.3 g per plant) raised from germinated seeds of clone Longjing 43 in nutrient solutions for 3 months were subjected to foliar urea application. Each of three plants was cultivated in a plastic pot filled with 4 L of continuously aerated nutrient solution. The compositions of nutrient solutions were (mmol L−1) 0.75 ammonium sulfate [(NH4)2SO4], 0.75 calcium nitrate [Ca(NO3)2], 0.1 potassium monobasic phosphate (KH2PO4), 0.45 potassium sulfate (K2SO4), 0.4 magnesium sulfate (MgSO4), 0.05 calcium chloride (CaCl2) and micronutrients (μmol L−1) ethylenediaminetetraacetic acid iron (EDTA-Fe) 6.3, 1.5 manganese sulfate (MnSO4), 1.0 zinc sulfate (ZnSO4), 0.2 copper sulfate (CuSO4), 10 boric acid (H3BO3), 0.07 ammonium molybdate [(NH4)6Mo7O24] and 25 aluminum sulfate [Al2(SO4)3] (Ruan et al. Citation2010). The pH of nutrition solutions were maintained at pH 5.0 ± 0.2 with a custom-built pH stat system with 0.1 mol L−1 sodium hydroxide (NaOH) or hydrochloric acid (HCl). Plants were placed in a naturally lit glasshouse (air temperature 22–34°C) provided with additional light (SON-T Agro, Philips) to ensure a minimum light intensity of 220 µmol m−2 s−1 at canopy level. The relative humidity was maintained at around 70% by a humidifier.
Volumes of 0.6 mL urea (15N enrichment 90%) solution at concentration of 1% [weight/volume (w/v), with 0.1% surfactant Triton X100], equivalent to 2.76 mg N pot−1, was applied carefully using a paintbrush to both surfaces of the four uppermost mature leaves of each plant (ages about 60 d) and, in total, 12 leaves of three plants per pot. Surfactant was added to facilitate uptake of foliar fertilization (Fernández and Eichert Citation2009). Each application was performed to a specified volume of urea solution per pot with a pre-equilibrated paintbrush. Freely absorbed solution was drained out of the brush by turning and pressing it manually against the wall of a glass beaker until there was no visible effluent, before and after every application, to make certain that the exact volume of urea solution was applied. A preliminary test showed that this volume of urea solution can be completely applied to 12 leaves without the formation of droplets. Each of four pots (as four replicates) was sampled at 2 and 6 h, 1, 2, 3, 7 and 13 d after urea application. Application of foliar urea was performed at the time that the terminal buds started opening and extending. The plants were immediately separated into roots, young shoots, urea-treated leaves, other leaves and stems. Averaged specific leaf weight (cm2 g−1) was obtained from the collective area of disks taken by a punch of known diameter and dried weight.
Experiment 2: pot experiment investigating the influence of nutritional status on foliar urea-15N absorption
Each of two rooted-cuttings (clone Longjing 43) was planted in a plastic pot containing 4 kg of a mixture of soil and perlite (2:1, by volume) in February, and received no extra fertilizers this year (Year 1). The soil contained 15.6 mg g−1 organic matter, 1.1 mg g−1 total N, 66 and 36 mg kg−1 exchangeable K and Mg (1 mol L−1 ammonium acetate (NH4OAC), pH = 7), 62 mg kg−1 S (0.5 mol L−1 calcium monobasic phosphate [Ca(H2PO4)2]) and soil pH was 4.8. The plants were exposed to different treatments of nutrients starting in June of the second year (Year 2). There were five treatments, i.e. control receiving full nutrients and treatments receiving a reduced supply of nutrients N (–N), K (–K), Mg (–Mg) and S (–S), to establish low nutritional status. Total amounts of nutrients N, P, K, Mg and S in the control treatment were 3.0, 0.66, 1.2, 0.45 and 1.08 g pot−1, respectively. The –N treatment received a total N of 0.6 g pot−1 and, in treatments of –K, –Mg and –S, the corresponding nutrients (K, Mg and S) were completely omitted, while other nutrients were applied at exactly the same doses as in the control treatment. The nutrients were supplied as urea, (NH4)2SO4, ammonium dibasic phosphate [(NH4)2HPO4], ammonium nitrate (NH4NO3), potassium nitrate (KNO3), K2SO4, MgSO4, magnesium nitrate [Mg(NO3)2], and KH2PO4 depending on the treatments. The nutrients were separated into six applications, two times in June and July of Year 2, and two times in March and two times in April of Year 3. For even distribution within the pots, nutrients were dissolved in 100 mL of deionized water and applied, followed by 300 mL deionized water applied to each pot. To facilitate the formation of frames and canopy, the plants were pruned to a height of about 20 cm above the soil, in late February of Year 3. Leaves and stems recovered about 1 month after pruning. All nutrient treatments consisted of four replications (pots), except for the –S treatment carrying three replications only. The plants were placed in a greenhouse with growth conditions similar to those of the solution experiment. Plants were watered when necessary with deionized H2O throughout the experiment.
In June of Year 3, 20 mature leaves per pot (leaf age about 50 d, 10 leaves per plant) were selected from the canopy surface and labeled for foliar urea application. A total amount of 34.5 mg N pot−1 in the form of urea (15N enrichment of 5.59%) was applied to leaves in three applications (first application on June 3 and 4) at weekly intervals. The foliar urea was applied at the time that the buds started breaking but were not yet extending. In each application, 2.5 mL of a 1% (w/v, with 0.1% surfactant Triton X100) urea solution was applied carefully using a paintbrush to both surfaces of leaves. Higher volumes of urea solution per leaf were applied here to match the larger size of plants than in the solution experiment. Repeated coating during each application, after the surface of leaves was dried, was needed in order to completely apply the specified amount of urea solution. The young shoots were plucked whenever reached the stage of a bud with two leaves, a common standard for green tea production. The first plucking took place 4 d after the last foliar urea application. A few young shoots (4–5 per pot) were preserved to the stage of three leaves. The third leaves from the bud were subjected to the measurement of activity of glutamine synthetase (GS, EC 6.3.1.2), while other parts were combined to the samples of young shoots. GS in leaves was extracted and measured by the transferase assay as previously described (Ruan et al. Citation2010). One unit enzyme activity corresponds to the formation of 1 μmol γ-glutamyl hydroxamate per gram fresh material per minute. The whole plants were sampled 33 d after the first foliar urea application, and separated into mature leaves receiving urea application, other mature leaves and stems.
Cleansing, preparation and analysis of samples
The urea-treated leaves were firstly washed in dilute detergent solution with a soft paintbrush to remove residual urea adhering to the leaf surfaces, and with deionized water 3 times afterwards. Other plant materials were washed 3 times in deionized water. The plant samples were subsequently blotted dry using tissue paper and dried in an air-forced oven at 70°C for 2 d. Plant samples were milled in a Retsch ball mill (Retsch GmbH, Haan, Germany) to fine powders. Plant samples were measured for K, Mg and S by inductively coupled plasma atomic emission spectrometry (IRIS-AP, Thermo Jarrel Ash Corp., USA) after digestion by mixed concentrated acids nitric acid-perchloric acid (HNO3–HClO4). Total N and 15N abundance were determined in an elemental analyzer (Carlo Erba, Milano, Italy) coupled to an isotope ratio mass spectrometer (Finigan Corp., Bremen, Germany). The Ndff (N derived from foliar fertilizer) and foliar fertilizer 15N amount in plant organs and recovery were calculated according to the following equations:
15N amount in plant organ = Ndff × total N concentration × organ biomass
Recovery rate (%) = 15N amount in plant/Foliar 15N applied × 100
In the solution experiment, the uptake rate of foliar applied N was calculated as the difference of total 15N amount in the plants between two consecutive samples divided by duration of hours per leaf area. The amount of exported 15N to other organs was the sum of 15N in organs other than in treated leaves, and the export rate was calculated as their amount difference between two consecutive sampling divided by area of treated leaves and duration in hours. The area of leaves was calculated from specific leaf area and dry matter weight.
Statistical analysis
Data were subjected to one-way analysis of variance (ANOVA) and least significant difference (LSD) by means of SigmaStat for Windows Version 3.5 (Systat Software Inc., San Jose, CA 95110, USA). A two-way ANOVA was performed on pooled data of the solution experiment, taking into account the urea treatment and sampling dates, to test the effect of foliar application on total N in treated leaves.
RESULTS
Urea-N absorption by leaves of tea plants in the solution experiment
Biomass and N content
The plant biomass production increased significantly (from 2.34 ± 0.29 g plant−1 at the first sampling to 3.63 ± 0.31 g plant−1 by day 13, the last sampling), with the increment mainly contributed from the growth of young shoots (0.95 ± 0.05 g plant−1) (). At the last sampling (by day 13), the young shoots had reached the developmental stage of 3–5 leaves. The biomass production of stems also increased significantly during this period, while those of leaves and roots (data not shown) changed insignificantly. Total N concentration of urea-treated leaves increased to the highest level by 2 d after urea application, and decreased thereafter to the beginning level until the last day considered (). The total N concentrations in the mature leaves were higher than values observed from field grown plants (e.g., 26.0–27.2 mg g−1 of plants fertilized with N at 300–450 kg ha−1 in Venkatesan et al. Citation2004), but were at levels comparable to those in our earlier experiment (41.0–44.4 mg g−1 in Ruan et al. Citation2007). This might be explained by sufficient N nutrition [and ammonium (NH4+) as well] in the solution experiments. Due to the limited N amount, application of urea to leaves slightly increased (p > 0.05) the concentration of N in the treated leaves compared to those without application on each sampling date. But a two-way ANOVA on pooled data taking into account of urea treatment and sampling dates showed a significant effect of foliar application (Fdate = 7.76, p < 0.001; Furea = 11.01, p = 0.002). To overcome these limitations, the 15N tracer technique was employed to investigate N uptake and mobilization from foliar nutrition. Total N concentrations in young shoots, roots and stem decreased from the beginning to the end of sampling (), which is in line with earlier findings that the total N content of young shoots decreased gradually with flush growth, and those in leaves, stem and roots tended to decrease when the tea plants became older (Feldheim et al. Citation1986). On the other hand, it was also likely that the decreasing N concentrations in stem and roots were caused by N remobilization from these organs to support the rapid growth of the young shoots. The earlier experiment demonstrated that about 70% of N in the young shoots was contributed from remobilization from vegetative organs, which was absorbed before sprouting, while the remainder was newly absorbed during the extension process (Okano et al. Citation1994).
Figure 1 (a) Dry matter production, and total nitrogen (N) concentration in (b) leaves and (c) other organs of tea (Camellia sinensis L.) plants receiving foliar urea application [means ± standard deviation (SD), solution experiment]. Bars of least significant difference (LSD) value and NS indicate significant and insignificant difference at p < 0.05, respectively.
![Figure 1 (a) Dry matter production, and total nitrogen (N) concentration in (b) leaves and (c) other organs of tea (Camellia sinensis L.) plants receiving foliar urea application [means ± standard deviation (SD), solution experiment]. Bars of least significant difference (LSD) value and NS indicate significant and insignificant difference at p < 0.05, respectively.](/cms/asset/df770df3-a7e6-407b-a81e-50ca7b1eec01/tssp_a_1027134_f0001_b.gif)
Ndff and 15N amounts in plants
Nitrogen derived from foliar fertilizer (Ndff) of treated leaves increased to the maximum within 3 days after application and then decreased constantly by the final sampling day (). The amount of 15N in the treated leaves increased considerably within 2 d after urea application and started to decrease, though slightly, thereafter until day 13, indicating stronger export than absorption (). Ndff of young shoots increased dramatically in the first 3 d and changed only a little thereafter (). The Ndff in stem, roots and other untreated mature leaves increased with much lower magnitudes (values below 0.3, 0.2 and 0.05%, respectively; , b). The 15N amounts in young shoots continued to increase throughout the sampling days, as did those in stems and roots (, ). Little 15N was found in other leaves not receiving urea application (). The total absorption of 15N in the plants increased significantly in the first 2 d and was then much slower afterwards (). By day 13, about 75% of applied urea-N was recovered in the plants, and the proportions of 15N amounts in treated leaves, young shoots, other mature leaves, stem and roots were 47.7, 43.7, 0.7, 2.7 and 5.1% of the total 15N uptake, respectively.
Uptake and export rate of foliar 15N
The uptake rate of foliar applied 15N, calculated on the basis of area of treated leaves and intervals between two consecutive samplings, was much higher for the first day than for other days, and decreased with the time after application (). The highest uptake rates were found in the earliest 2 or 6 h after application. The 15N export rates out of the treated leaves were quite low in the first few hours, increased considerably by day 1 and reached the highest levels by day 3 (). The amount of 15N export and its proportion in the total absorption increased with time after application. By day 13, about 53% of absorbed N was exported out of the treated leaves.
Figure 4 (a) Uptake and (b) export rate of foliar applied urea isotope nitrogen-15 (15N), by leaves of tea (Camellia sinensis L.) plants calculated at sampling intervals [means ± standard deviation (SD), solution experiment]. Bars of least significant difference (LSD) value indicate significant difference at p < 0.05.
![Figure 4 (a) Uptake and (b) export rate of foliar applied urea isotope nitrogen-15 (15N), by leaves of tea (Camellia sinensis L.) plants calculated at sampling intervals [means ± standard deviation (SD), solution experiment]. Bars of least significant difference (LSD) value indicate significant difference at p < 0.05.](/cms/asset/4b3e2a13-df1b-40c1-b005-a73c0aa8f48f/tssp_a_1027134_f0004_b.gif)
Influence of nutritional status on foliar urea absorption in the pot experiment
Nutrient concentrations and biomass production
Low supplies of N, K and Mg in soil significantly affected their nutritional status in tea plants as indicated by decreasing concentrations in mature leaves (). Low supply of one nutrient also caused changes in concentrations of other nutrients in the mature leaves. The K and S concentrations were significantly increased by low N supply. The Mg concentration was increased when K supply was diminished. However, the concentrations of S and other nutrients in the mature leaves were unaffected by status of S supply (). The growth of young shoots reached the developmental stage of one bud with two young leaves about 18 d after the first foliar urea application. The growth of young shoots was inhibited by low supply of N or K (). The whole aerial production was repressed significantly by low N but only slightly (p > 0.05) by low K. Effects of low Mg supply on biomass production were insignificant. Low S supply significantly reduced biomass production of whole aerial parts, and of young shoots to some extent (p > 0.05). The activity of GS was inhibited significantly by low N supply, while it was unaffected by other nutrient omission treatments ().
Table 1 Concentrations of nutrients in mature leaves, activity of glutamine synthetase (GS) and biomass of plants supplied with variable levels of nutrients [means ± standard deviation (SD), pot experiment]
Ndff and 15N amounts and recovery in plants
In the plants receiving low N, K or Mg nutrients, total N concentrations in urea-treated leaves were significantly higher than in untreated leaves, showing significant effects of foliar urea application (). Ndff in all organs was the largest in the –N treatment and was unaffected by other nutrient treatments (). The amounts of 15N in tea plants and their recovery rates are presented in . Plant roots were not recovered because the solution experiment showed that only a small amount of foliar applied N was transported to the roots. The 15N amounts in young shoots and total 15N absorption were generally the largest in the control treatment and significantly decreased in the treatment with low N supply. Low supply of K repressed 15N amount significantly in young shoots but only marginally (p > 0.05) the total 15N absorption. Low Mg application had no significant effect on 15N absorption in plants. Low S application diminished total 15N amount significantly, but only slightly (p > 0.05) in young shoots.
Table 2 Nitrogen derived from fertilizer (Ndff, %) in organs of plants supplied with variable levels of nutrients [means ± standard deviation (SD), pot experiment]
Table 3 Amounts of 15N absorption and mobilization of foliar applied urea N in tea (Camellia sinensis L.) plants supplied with variable levels of nutrients [means ± standard deviation (SD), pot experiment]
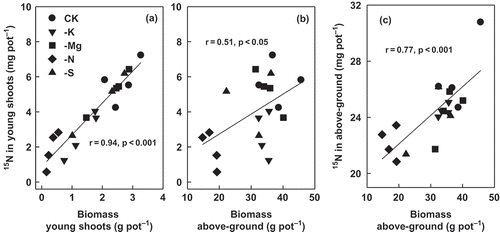
A proportion of 29–36% of total absorbed 15N was transported to other organs from the treated leaves, and the mobilization proportion was unaffected by any of the nutrient treatments (). The recovery rate of foliar-applied N was the largest in plants of the control (CK) and the smallest in plants supplied with low N and S nutrition. The amount of 15N in the young shoots correlated closely and significantly (r = 0.94, p < 0.001) with their biomass and also the biomass of whole above-ground parts to a lesser extent (r = 0.51, p < 0.05; ). Similarly, the absorption of foliar 15N in the whole aerial parts of plants correlated significantly (r = 0.77, p < 0.001) with the biomass production ().
DISCUSSION
Ready uptake of foliar urea-N and contribution to N nutrition in tea plants
The objective of the solution experiment was to investigate the dynamic uptake and partition of foliar N by tea plants. A low amount of highly enriched 15N-labeled urea was applied with the consideration not to significantly influence the N status of plants. In the pot experiment, however, a much larger amount of foliar urea-N was applied to evaluate its contribution to the N status of plants and the effects of nutrients supplied from the soil. The consequence of different strategies was well reflected by the values of Ndff in plants ( and ). In the former case, the Ndff value was less than 2% in the urea-treated leaves and was calculated to be 0.54–0.78% (0.66 ± 0.11% by day 13) in the whole plants, indicating a relatively small contribution of foliar 15N. The solution experiment showed that absorption of foliar-applied urea by tea plants took place immediately, because Ndff and the 15N amount in treated leaves increased significantly within 2 h after urea application ( and ). Meanwhile, the absorption rates were the highest in the earliest few hours (). Previous experiment also showed that leaves of young apple trees rapidly absorbed N from urea during the first 2 d after foliar application (Dong et al. Citation2002). The absorbed urea is hydrolyzed in the cytosols by urease, although urea persisted for over 36–72 h in plant leaves after foliar spray (Bowman and Paul Citation1990; Witte et al. Citation2002). In the pot experiment, the Ndff values in urea-treated leaves and young shoots were 8.1–16.4% and 7.1–19.2%, respectively, which were much larger than those in the solution experiment (). Meanwhile, the total N concentrations in treatments –K, –N and –Mg were higher in leaves receiving foliar urea than in those without (). These results demonstrated that foliar-applied N significantly improved the plant N nutrition in the pot experiment. The highest Ndff values of leaves and young shoots in the low N treatment compared to those in other treatments suggest that the foliar N made the most remarkable contribution in the N-deficient plants (). The N recovery reached about 60% within 2 d, about 75% by 13 d in the solution experiment, and was in the range of 64–78% in the pot experiment. This demonstrates high recovery efficiency of foliar feeding compared to soil fertilization (Gooding and Davies Citation1992; Tachibana et al. Citation1996; Kamau et al. Citation2008; Fageria et al. Citation2009). The plant 15N recovery rates in the solution and pot experiments remained at comparable levels despite different N concentrations of mature leaves ( and ).
Partitioning of foliar N in tea plants
The absorbed N from foliar urea application was readily transported to other organs, mainly in the form of amino acids (Dong et al. Citation2002; Xia and Cheng Citation2004). Export of foliar-applied N to other organs took place concomitantly within 2 h, and the export rate became significant 1 d after application in the present experiment (). Transition of export surpassing absorption in the treated leaves occurred 2 d after foliar application, as the N amount of foliar origin in the treated leaves turned to a gentle decrease (). About 53% or 29–36% of the total 15N was transported to other organs in the solution and pot experiments, respectively. Nevertheless, only a limited amount of urea-derived 15N was transported to roots in the nutrient solution experiment (), suggesting that root was a comparatively weak competitor for foliage-fed N (Morris and Weaver Citation1983; Karasuyama et al. Citation1985; Witte et al. Citation2002). Our finding is different from those of some other previous studies showing remarkable partitioning to the roots of, for example apple, peach and nectarine (Prunus persica L. Batsch var. nectarina) (Tagliavini et al. Citation1998; Cheng et al. Citation2002; Dong et al. Citation2002). The discrepancy in these findings might be explained by differences in application time and the growth stage of plants (Uscola et al. Citation2014). In the latter cases, foliar N was often applied in autumn before natural remobilization of N to roots and branches for storage (Tagliavini et al. Citation1998; Cheng et al. Citation2002; Dong et al. Citation2002). In our case, the foliar urea was applied just before bud breaking. The exported N was mainly found in the young shoots, representing the major sink for N redistribution.
Influence of plant N status on the efficiency of foliar N nutrition
Absorption and translocation of foliar applied urea-N are affected by a number of factors including the N status of plants, which has been focused on in many previous experiments (Klein and Weinbaum Citation1984; Bowman and Paul Citation1989; Lea-Cox and Syvertsen Citation1995; Bondada et al. Citation2001; Cheng et al. Citation2002; Bi and Scagel Citation2008). Foliar N uptake and mobilization were decreased by high plant N status due to a feedback regulation (Cheng et al. Citation2002). Thus, foliar application of urea may be more effective when N availability via soil had been sub-optimal for plant growth, and therefore is not recommended for crops of normal N status (Goodings and Davis Citation1992). However, in the present pot experiment, N deficiency significantly reduced the absorption and recovery of foliar urea-N (). Such decrease has been attributed to greater epicuticular wax concentration and smaller leaf areas intercepting foliar nutrients in N-deficient than in N-sufficient leaves, leading to lower foliar N absorption (Bondada et al. Citation2001; Fageria et al. Citation2009). The wax properties of leaves were not determined in the present study, whereas different uptake rates per unit leaf area were observed for –N (67.3 ± 11.3 μg cm–2) and CK (48.7 ± 8.1 μg cm–2) treatments. Consequently, smaller leaf area and absorption area (336.0 ± 41.6 cm2 per plant) in the –N treatment than those supplied with sufficient N (559.4 ± 59.9 cm2 per plant) was compensated to a certain extent by a higher uptake rate in the –N treatment (Bi and Scagel Citation2008). The present work shows that the difference in total 15N absorption was mainly contributed from the component in young shoots, whereas there were no significant differences among the 15N amounts in the mature leaves and stems (). The correlation analysis revealed a close and significant relationship between the biomass production and foliar 15N uptake (), likely suggesting that the reduced sink metabolic strength imposed by plant growth due to N deficiency decreased the absorption and translocation of foliar urea-N (Klein and Weinbaum Citation1984). The reduced GS activity might also be indicative of weaker N metabolism in the young shoots of N-deficient (–N) tea plants. This is in line with the observation that N uptake by tea plants under field conditions was regulated by dry mass production of young shoots (Kamau et al. Citation2008). The present finding provides an alternative explanation to the previously reported positive interactions of foliar urea application in combination with higher levels of soil-applied N fertilizer (Gooding and Davis Citation1992). Such a phenomenon has been observed for other nutrients such as K. In olive (Olea europaea L.) plants, the uptake rate of foliar applied K (Rb) was decreased by K deficiency, while the uptake increased with the K concentration in nutrient solution (Restrepo-Diaz et al. Citation2008).
Influence of plant K, Mg and S status on the efficiency of foliar N nutrition
Translocation of assimilated N from foliar urea-N to organs other than treated leaves is mainly dependent upon phloem transport. The present experiment therefore hypothesized that absorption of foliar N might be negatively affected by inhibited phloem loading and translocation induced by K or Mg deficiency (Cakmak and Kirkby Citation2008). In tea plants, Mg nutrition was an important factor regulating the long-distance mobility of amino acids and sugars in the phloem, especially when remobilization of storage N and carbon (C) reserves occurs to support the spring growth of young shoots (Ruan et al. Citation2012). The status of plant K and Mg was reduced by omission of nutrient supply, as indicated by nutrient concentrations in the plant leaves and plant growth in the case of K (). However, the total 15N absorption was only slightly (p > 0.05) decreased by low K and Mg supplies, suggesting that transport might not be a key factor. It was frequently reported that carbohydrates accumulated in leaves of K- or Mg-deficient plants (Cakmak and Kirkby Citation2008), possibly providing an unexpected advantage in assimilating foliar-applied N, which consumes non-structural carbohydrates (Xia and Cheng Citation2004). The 15N partition to the young shoots was significantly decreased by the low-K treatment, which is likely explained by reduced biomass accumulation and a consequently lower sink N strength, as discussed above for N. The absorption of urea-N was reduced in plants of the –S treatment, showing that adequate S nutrition is needed for better utilization of foliar-applied N. As S-containing amino acids and proteins synthesized therefrom represent the major metabolic sink for S, the N × S interaction is easily understood. In agreement, in wheat a synergistic effect was obtained by simultaneous foliar application of N and S fertilizers (Tea et al. Citation2007). Low S supply significantly reduced the biomass production of whole aerial parts, but caused no evident change of its concentration in the mature leaves, implying that the latter might not be a good indicator of plant S status.
In conclusion, the present experiments showed that urea was rapidly absorbed by tea leaves and exported mainly to the young shoots, representing the major sink. Insufficient supply of nutrients N, K and S negatively affected the absorption and utilization of foliar urea fertilization by weakening sink N strength imposed by plant growth due to nutrient deficiency. It is anticipated that improvement of amino acids in young shoots by foliar urea application (Karasuyama et al. Citation1985) became less efficient because of decreasing N absorption or partitioning to young shoots due to low plant N, K and S status.
ACKNOWLEDGEMENTS
The authors thank B. Biegler for analyzing 15N abundance, and Lifeng Ma for analysing nutrients in plant samples. This study was financially supported by the China Natural Science Foundation (30771251), the German Science Foundation (DFG Sa359/22), the Earmarked Fund for China Agriculture Research System (CARS 23) and the Innovation Project of Chinese Academy of Agricultural Sciences.
REFERENCES
- Bi G, Scagel CF 2008: Nitrogen uptake and mobilization by Hydrangea leaves from foliar-sprayed urea in fall depend on plant nitrogen status. HortScience, 43, 2151–2154.
- Bondada BR, Syvertsen JP, Albrigo LG 2001: Urea nitrogen uptake by citrus leaves. HortScience, 36, 1061–1065.
- Bowman DC, Paul JL 1989: The foliar absorption of urea-N by Kentucky bluegrass turf. J. Plant Nutr., 12, 659–673. doi:10.1080/01904168909363981
- Bowman DC, Paul JL 1990: The foliar absorption of urea-N by tall fescue and creeping bentgrass turf. J. Plant Nutr., 13, 1095–1113. doi:10.1080/01904169009364138
- Cakmak I, Kirkby EA 2008: Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant., 133, 692–704. doi:10.1111/j.1399-3054.2007.01042.x
- Cheng L, Dong S, Bates T 2002: Urea uptake and nitrogen mobilization by apple leaves in relation to tree nitrogen status in autumn. J. Hort. Sci. Biotech., 77, 13–18.
- Dong S, Cheng L, Scagel CF, Fuchigami LH 2002: Nitrogen absorption, translocation and distribution from urea applied in autumn to leaves of young potted apple (Malus domestica) trees. Tree Physiol., 22, 1305–1310. doi:10.1093/treephys/22.18.1305
- Dong S, Neilsen D, Neilsen GH, Fuchigami LH 2005: Foliar N application reduces soil NO3–N leaching loss in apple orchards. Plant Soil, 268, 357–366. doi:10.1007/s11104-004-0333-1
- Fageria NK, Filho MPB, Moreira A, Guimarães CM 2009: Foliar fertilization of crop plants. J. Plant Nutr., 32, 1044–1064. doi:10.1080/01904160902872826
- Feldheim W, Yongvanit P, Cummings PH 1986: Investigation of the presence and significance of theanine in the tea plant. J. Sci. Food Agric., 37, 527–534. doi:10.1002/jsfa.2740370604
- Fernández V, Eichert T 2009: Uptake of hydrophilic solutes through plant leaves: current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci., 28, 36–68. doi:10.1080/07352680902743069
- Gooding MJ, Davies WP 1992: Foliar urea fertilization of cereals: a review. Fertil. Res., 32, 209–222. doi:10.1007/BF01048783
- Kamau DM, Spiertz JHJ, Oenema O, Owuor PO 2008: Productivity and nitrogen use of tea plantations in relation to age and genotype. Field Crops Res., 108, 60–70. doi:10.1016/j.fcr.2008.03.003
- Karasuyama M, Yoneyama T, Kobayashi H 1985: 15N study on the fate of foliarly applied urea nitrogen in tea plant (Camellia sinensis L.). Soil Sci. Plant Nutr., 31, 123–131. doi:10.1080/17470765.1985.10555223
- Klein I, Weinbaum SA 1984: Foliar application of urea to olive, translocation of urea nitrogen as influenced by sink demand and nitrogen deficiency. J. Am. Soc. Hort. Sci., 109, 356–360.
- Lea-Cox JD, Syvertsen JP 1995: Nitrogen uptake by Citrus leaves. J. Am. Soc. Hort. Sci., 120, 505–509.
- Morris DR, Weaver RW 1983: Absorption and translocation of foliarly applied 15N by soybeans. Agron. J., 75, 572–574. doi:10.2134/agronj1983.00021962007500030036x
- Oh K, Kato T, Li ZP, Li FY 2006: Environmental problems from tea cultivation in Japan and a control measure using calcium cyanamide. Pedosphere, 16, 770–777. (2006). doi:10.1016/S1002-0160(06)60113-6
- Okano K, Komaki S, Matsuo K 1994: Remobilization of nitrogen from vegetative parts to sprouting shoots of young tea (Camellia sinensis L.) plants. Japan. J. Crop Sci., 63, 125–130. doi:10.1626/jcs.63.125
- Restrepo-Diaz H, Benlloch H, Fernandez-Escobar R 2008: Plant water stress and K+ starvation reduce absorption of foliar applied K+ by olive leaves. Sci. Hortic., 116, 409–413. doi:10.1016/j.scienta.2008.03.004
- Ruan JY, Gerendás J, Haerdter R, Sattelmacher B 2007: Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea plants (Camellia sinensis (L.) O. Kuntze). Ann. Bot., 99, 301–310. doi:10.1093/aob/mcl258
- Ruan J, Härdter R, Gerendás J 2010: Impact of nitrogen supply on carbon/nitrogen allocation: a case study on amino acids and catechins in green tea (Camellia sinensis (L.) O. Kuntze) plants. Plant Biol., 12, 724–734. doi:10.1111/j.1438-8677.2009.00288.x
- Ruan JY, Ma LF, Shi YZ 2013: Potassium management in tea plantations: its uptake by field plants, status in soils and efficacy on yields and quality of teas in China. J. Plant Nutr. Soil Sci., 176, 450–459. doi:10.1002/jpln.201200175
- Ruan JY, Ma LF, Yang YJ 2012: Impact of magnesium nutrition on accumulation and transport of amino acids in tea plants. J. Sci. Food Agric., 92, 1375–1383. doi:10.1002/jsfa.4709
- Ruan JY, Ye Y, Wu X, Härdter R 1998: Effect of potassium, magnesium and sulphur applied in different forms of fertilisers on free amino acid content in leaves of tea (Camellia sinensis L.). J. Sci. Food Agric., 76, 389–396. doi:10.1002/(SICI)1097-0010(199803)76:3<389::AID-JSFA963>3.0.CO;2-X
- Scagel CF, Bi G, Fuchigami LH, Regan RP 2008: Rate of nitrogen application during the growing season and spraying plants with urea in the autumn alters uptake of other nutrients by deciduous and evergreen container-grown Rhododendron cultivars. HortScience, 43, 1569–1579.
- Sekhon BS, Thapar S, Atwal A, Singh R 1990: Effect of foliar application of urea on the enzymes and metabolites of nitrogen metabolism in mycorrhizal moong plants under different phosphorus levels. Plant Physiol. Biochem., 28, 393–397.
- Tachibana N, Ikeda T, Ikeda K 1996: Changes in nitrogen uptake with aging and under heavy application of nitrogen in tea plants. Japan. J. Crop Sci., 65, 8–15. doi:10.1626/jcs.65.8
- Tagliavini M, Millard P, Quartieri M 1998: Storage of foliar-absorbed nitrogen and remobilization for spring growth in young nectarine (Prunus persica var. nectarina) trees. Tree Physiol., 18, 203–207. doi:10.1093/treephys/18.3.203
- Tea I, Genter T, Naulet N, Marie LM, Kleiber D 2007: Interaction between nitrogen and sulfur by foliar application and its effects on flour bread-making quality. J. Sci. Food Agric., 87, 2853–2859. doi:10.1002/jsfa.3044
- Tokuda S, Hayatsu M 2004: Nitrous oxide flux from a tea field amended with a large amount of nitrogen fertilizer and soil environmental factors controlling the flux. Soil Sci. Plant Nutr., 50, 365–374. doi:10.1080/00380768.2004.10408490
- Uscola M, Villar-Salvador P, Oliet J, Warren CR 2014: Foliar absorption and root translocation of nitrogen from different chemical forms in seedlings of two Mediterranean trees. Environ. Exp. Bot., 104, 34–43. doi:10.1016/j.envexpbot.2014.03.004
- Venkatesan SS, Murugesan Ganapathy MNK, Verma DP 2004: Long-term impact of nitrogen and potassium fertilizers on yield, soil nutrients and biochemical parameters of tea. J. Sci. Food Agric., 84, 1939–1944. doi:10.1002/jsfa.1897
- Witte CP, Tiller SA, Taylor MA, Davies HV 2002: Leaf urea metabolism in potato. Urease activity profile and patterns of recovery and distribution of 15N after foliar urea application in wild-type and urease-antisense transgenics. Plant Physiol., 128, 1129–1136. doi:10.1104/pp.010506
- Xia GH, Cheng LL 2004: Foliar urea application in the fall affects both nitrogen and carbon storage in young ‘Concord’ grapevines grown under a wide range of nitrogen supply. J. Am. Soc. Hort. Sci., 129, 653–659.

![Figure 2 Nitrogen derived from foliar applied urea (Ndff) in (a) leaves and (b) other organs of tea (Camellia sinensis L.) plants [means ± standard deviation (SD), solution experiment]. Bars of least significant difference (LSD) value indicate significant difference at p < 0.05.](/cms/asset/e4d5cfed-3471-4256-8e80-72334137d7f5/tssp_a_1027134_f0002_b.gif)
![Figure 3 Amount of foliar applied urea isotope nitrogen-15 (15N) in tea (Camellia sinensis L.) plants [means ± standard deviation (SD), solution experiment]. Bars of least significant difference (LSD) value and NS indicate significant and insignificant difference at p < 0.05, respectively.](/cms/asset/548f1ba7-4b1c-4e41-ad26-2707ecc4e8e7/tssp_a_1027134_f0003_b.gif)