Abstract
Hairy vetch (Vicia villosa Roth) is a leguminous cover crop that is generally used as a green manure to sustain soil health in arable land. Molybdenum (Mo) acts as a cofactor for the nitrogenase (NA) and nitrate reductase (NR) enzymes, which are important for nitrogen (N) fixation, nitrate reduction and N transport in plants. In this study, we applied various doses of Mo to soil to evaluate their efficacy on nodulation, nodule characteristics and biomass production of hairy vetch. Mo application increased the number and size of nodules and NA and NR enzyme activity in hairy vetch. This increase in enzyme activity increased N assimilation and led to higher biomass yield. Plants grown in soil that received 0.5 mg Mo kg−1 showed optimal physical and biochemical properties in nodules, and these properties may explain the increased N fixation in hairy vetch. Higher Mo doses (1.0 mg kg−1) led to the deterioration of nodule structure and, hence, reduced enzymatic activity in plants. The 16S rRNA gene sequencing and cluster analysis showed that the bacterial isolates found in the nodules of hairy vetch roots belonged to the Rhizobiaceae family and shared high sequence similarity with Rhizobium leguminosarum and Agrobacterium tumefaciens. Application of 0.63 mg Mo kg−1 to soil was the optimum dose to maximize the biomass yield of hairy vetch.
1. INTRODUCTION
Molybdenum (Mo), an essential micronutrient, plays an important role in nitrogen (N) metabolism and protein synthesis in plants. During symbiotic N fixation, Mo acts as a cofactor for nitrogenase enzymes to catalyze the redox reaction to convert elemental N into ammonium (NH4+) ions (Mendel and Hänsch Citation2002), and nitrate reductase enzymes required for the assimilation of soil nitrates. Therefore, plant N metabolism is closely related to the Mo concentration in soil, especially for leguminous plants (Mendel and Hänsch Citation2002). Due to this relationship, Mo-deficient legumes show an unusual proliferation of nodules, which in turn leads to N deficiency (Marschner Citation2011).
Cultivation of winter cover crops to agricultural soils as a source of organic matter is a popular practice in several countries, especially in temperate regions. Hairy vetch (Vicia villosa Roth), a leguminous cover crop, is used as a green manure in South Korea for maintaining soil fertility and to reduce the need for chemical fertilizers (Pramanik et al. Citation2013). Like other leguminous plants, hairy vetch has rhizobium bacteria in root nodules that require Mo to fix atmospheric N as a plant-available form (Campo et al. Citation2000). Molybdenum is involved in the biochemical processes catalyzed by nitrogenase (NA) and nitrate reductase (NR), and indirectly affects crop biomass production (Ingle Citation1966).
N is the major nutrient for plant growth, and biological N fixation in soil may reduce chemical fertilizer load for crop production (Islam et al. Citation2013). Many environmental factors such as soil conditions (Sylvia et al. Citation2005), N and phosphorus (P) levels (Palmer and Young Citation2000), soil types (Groffman et al. Citation1996) and soil management practices (Drew and Ballard Citation2010) influence the efficiency of biological nitrogen fixation (BNF). Solaiman (Citation1999) found that application of Mo 1.5 kg ha−1 with Bradyrhizobium inoculant increased nodule numbers, nodule weights and yields of soybean. Malla et al. (Citation2007) reported that application of Mo 1.5 kg ha−1 increased the yield of pigeon pea (Cajanus cajan). Seed priming with Mo (0.5 g L–1 solution of sodium molybdate) for 8 h increased yield of chickpea (Cicer arietinum) 27% in pot study and 20% in field study (Farook et al. Citation2012). Previous studies showed large variations in BNF efficiency both between (Anugroho et al. Citation2010) and within (Drew and Ballard Citation2010) cover crop species. Among different rhizobial genotypes, some strains have higher BNF ability in leguminous cover crops than in the others (Drew and Ballard Citation2010).
Pramanik et al. (Citation2013) found that changes in nodule characteristics influence hairy vetch growth, and Haque et al. (Citation2013) observed that hairy vetch growth determines the amount of N assimilated by cover crop biomass. Unlike the previous studies, we focused on the effect of Mo on nodule characteristics, enzyme activity and hairy vetch growth. We hypothesized that a small quantity of Mo added to the soil influences the chemistry and biochemistry in nodules by increasing rhizobial activity which, in turn, affects the growth of hairy vetch. We grew hairy vetch in soil treated with various doses of Mo fertilizer to study its effect on nodulation characteristics and biomass production. The objectives of this study were (1) to determine the optimum Mo application level with respect to nodule formation, NA, NR, and ultimately nitrogen fixation and biomass production, and (2) to isolate and identify the rhizobial community associated with hairy vetch.
2. MATERIALS AND METHODS
2.1. Experimental setup
The pot experiment was conducted in the greenhouse of Gyeongsang National University (36°50´N and 128°26´E), Jinju, South Korea, in 2012. Mo-deficient soil was collected from a university farm that had a previous history of vetch–barley (Hordeum vulgare L.) cultivation and was Haplaquents type (Hong et al. Citation2010; Pramanik and Kim Citation2014). Initial chemical properties of the soil, used for this study, were as follows: pH, 6.0 ± 03 [soil: water = 1:5, weight/volume (w/v) basis]; electrical conductivity, 34.6 ± 1.2 (μS cm−1); organic matter, 13.4 ± 0.4 (g kg−1); total N, 1.2 ± 0.02 (g kg−1); carbon (C)/N ratio, 11.6; available Mo, 0.04 (mg kg−1); total Mo, 0.12 (mg kg−1); available phosphorus pentoxide (P2O5), 65.3 ± 2.1 (mg kg−1); exchangeable cations, potassium (K) 0.32 ± 0.1, calcium (Ca) 2.48 ± 0.13 and magnesium (Mg) 0.48 ± 0.14 cmol+ kg−1; and a silt loam texture (silt 60%, clay 30% and sand 10%). The initial rhizobium population in the soil was 1.2 × 104 CFU g−1 soil, as estimated by the serial dilution method. Soil was passed through a 3-mm sieve and 4 kg of that sieved soil was placed into a plastic pot (1/5000 A size; A = 100 m2) and packed to a bulk density of 1.2 g cm−3. Mo derived from sodium molybdate (Na2MoO4.2H2O, Samchun Pure Chemical Co., Ltd, Pyeongtaek, Korea) was applied at a rate of 0, 0.13, 0.25, 0.5 and 1.0 mg kg−1 soil, with six replications per treatment. A total of 30 pots were filled with soil 1 week before the transplanting of hairy vetch seedlings, and Mo was applied on the soil surface in each pot by dilution with distilled water. Hairy vetch seeds were surface-sterilized with ethanol for 3 min, rinsed several times with sterile distilled water and germinated for 4 d at 25°C in the dark. The germinated seedlings, selected uniformly, were grown at a density of three per pot. The trial was performed at the Helmholtz Zentrum greenhouse at a 15 h day/9 h night cycle, at a temperature of 24°C/18°C (day/night), and a relative humidity of 65%. The plants were treated with 5 mM N in the form of potassium nitrate (KNO3) as an initial N source for seedling emergence and nodule initiation. Each pot received 100 mL N-free nutrient solution (Hoagland and Arnon Citation1950) at 7-d intervals along with irrigation with distilled water (400 mL pot−1 day−1) to maintain 60% water-holding capacity. The applied nutrient solution contained 235 ppm K, 200 ppm Ca, 31 ppm P, 64 ppm sulfur (S), 48 ppm Mg, 0.5 ppm boron (B), 3 ppm iron (Fe), 0.5 ppm manganese (Mn), 0.05 ppm zinc (Zn) and 0.02 ppm copper (Cu). The plant-containing pots were placed in drip trays to prevent leachate loss, and all leachate collected was returned to the respective experimental pot. The plants were grown for 3 months and no pesticide was applied during the study period.
2.2. Growth characteristics of hairy vetch
During harvesting, three hairy vetch plants per pot were carefully collected without damaging roots and biomass. The fresh total, above-ground and root biomasses were measured after separating pot soils and washing in tap water to remove adhered soil particles. The nodules were collected aseptically and nodule properties were studied. Fresh samples were dried at 70°C for 72 h for dry weight measurements.
2.3. Soil and plant analysis
Soil samples were collected after hairy vetch harvesting, air-dried and ground (2-mm sieve) prior to analyses for pH (soil: water = 1:5, w/v basis, glass electrode pH meter), electrical conductivity (conductivity meter) and total N (Kjeldahl digestion method) and organic C (Walkley and Black method; Allison Citation1965) contents. Soil and hairy vetch plant samples were digested with a di-acid mixture (Nitric acid (HNO3): Perchloric acid (HClO4) = 3:1, volume/volume) and extracts were analyzed at the Central Lab, Gyeongsang National University, using inductively coupled plasma-mass spectroscopy (ICP-MS, Perkin Elmer Optima 3300 DV) to determine the total concentrations of Mo, K, Ca and Mg. The available nitrate-nitrogen (NO3-N) content in soil samples was analyzed according to the method of Jackson (Citation1973), and the available Mo content was determined by the ammonium bicarbonate-diethylenetriaminepentaacetic acid (AB-DTPA) method (Boon and Soltanpour Citation1983). The total N content in hairy vetch was determined from undigested dry samples using an elemental analyzer (Leco CNS-932, Madrid, Spain).
2.4. Nitrogenase assay of root nodules
Nitrogenase enzyme (NA), responsible for N-fixation, also reduces acetylene (C2H2) into ethylene (C2H4). Therefore, NA in nodules was measured using the acetylene reduction method (Somasegaran and Hoben Citation1994). Two hairy vetch whole root systems were placed into an incubation bottle (500 mL capacity) with a sealed cap, and 10% of the air was replaced with acetylene gas. The bottles were incubated at 30°C for 30–40 min with periodic shaking. After incubation, 1 mL of the gas sample was injected into the gas chromatograph (GC). Ethylene produced by the nodules was measured by GC (Shimadzu, GC-2010, Kyoto, Japan) packed with a Porapak Q Column (Q 80–100 mesh) and a flame ionization detector. Helium and hydrogen (H2) gases were used as the carrier and burning gases, respectively. The algorithm used for calculating NA from acetylene reduction data was as follows:
Total amount of C2H4 produce from root samples during incubation (ΔC) =
Conc. of gas sample measured by GC × density of gas × volume of incubation bottle
Nitrogenase activity (NA, μmol C2H4 plant−1 h−1) =
ΔC (μmol)/[plant numbers × times (h)]
2.5. Nitrate reductase assay in leaf and root of hairy vetch
NR activities in leaves and roots were analyzed at the early flowering stage of hairy vetch, following the method of Konnerup and Brix (Citation2010). Leaf and root samples collected from each treatment were immediately frozen in liquid nitrogen (N2) and ground to a powder with a mortar and pestle. To analyze NR activity, 250 mg of the ground sample was added to a 2-mL Eppendorf tube containing 1 mL of pre-chilled 100 mM Di-potassium phosphate (K2HPO4)/Ethylenediaminetetraacetic acid (EDTA) (extraction buffer, pH 7.3). The extraction mixtures were shaken gently and the suspension was centrifuged at 18,000 g for 10 minutes. The supernatant (200 μL) was transferred to a sterile clean test tube containing 1.0 mL of the extraction buffer. Then, 1.6 mL of 30 mM KNO3 and 200 μL of 2.5 mM nicotinamide adenine dinucleotide (NADH) were added to the test tube, and the assay mixture was incubated for 30 min in the dark with mild shaking. Immediately after incubation, the reaction was stopped by adding 1 mL of 58 mM sulphanilamide followed by 1 mL of 0.77 mM N-(1-naphthyl)ethylenediamine dihydrochloride (NEDDH), and the color intensity was measured in a spectrophotometer at 540 nm. A standard curve was prepared using potassium nitrite, with gradient concentrations of 0, 20, 40, 60, 80, 100 and 200 μM.
2.6. Processing of nodules and genomic DNA extraction
Nodules from hairy vetch root were collected, washed in tap water, dried with tissue paper and preserved on silica gel until further use (Somasegaran and Hoben Citation1994). A single nodule was crushed in 100 μL of sterile distilled water using a homogenizer, and a loop full of suspension was streaked on yeast extract mannitol agar plates and incubated at 28°C for 3–5 days. Thirty bacterial isolates (six bacteria collected from six plants per treatment) were randomly selected from the nodules of 30 hairy vetch plants for genomic DNA isolation. Isolated bacteria were cultured in test tube (Pyrex®, USA) containing 3 mL of yeast extract mannitol liquid broth by shaking in a rotary shaker (KSI-100 L shaking incubator, Koencon, Hanam, Korea) at 180 rpm, 30°C for 24 h, and the cultures were centrifuged at 18,000 g for 5 min at 4°C. The pellet was subjected to genomic DNA extraction using the FastDNA SPIN Kit (MP Biomedicals, Santa Ana, CA, USA) and quantified using a spectrophotometer (NanoDrop 2000C, Thermo Scientific, Waltham, MA, USA). The extracted DNA was used as a template for polymerase chain reaction (PCR) to amplify 16S rDNA.
2.7. PCR amplification of 16S rDNA and cloning
Amplification of 16S rDNA fragments of extracted bacterial genomic DNA were conducted by PCR amplification. Bacterial 16S rDNA was amplified by PCR using the forward primer fD1 (5´-AGAGTTTGATCCTGGCTCAG-3´) and reverse primer rD1 (5´-AAGGAGGTGATCCAGC-3´). PCR amplifications were performed with approximately 50 ng of template DNA. The PCR conditions and primer sequences used for sequencing are shown in Table S1 in the supporting online information. The PCR products were gel purified with the QIAEX II Gel Extraction Kit (Qiagen GmbH, Hilden, Germany) and ligated into the pGEM-T Easy Vector (Promega, Madison, WI, USA) via TA cloning. The resulting ligation products were subsequently transformed into Escherichia coli DH5α competent cells (Tiangen, Beijing, China) following the protocol of He et al. (Citation2008).
2.8. Sequencing, phylogenetics and cluster analysis
DNA sequencing was performed using an Applied Biosystems 3730 automated sequencer and the M13 primer to obtain nearly full-length bacterial 16S rDNA sequences (approximately 1500 bp). The bidirectional gene sequences were compiled using DNAMAN software (DNAMAN version 4.11, Lynnon Biosoft, San, Ramon, CA, USA) and the sequences were analyzed with MEGA 5.2 software. The sequences were searched in the National Center for Biotechnology Information (NCBI) GenBank database using Basic Local Alignment Search Tool (BLAST), and a cluster analysis was performed using CD-HIT Suite for biological sequence clustering and comparison (Huang et al. Citation2010). Phylogenetic analysis was conducted using MEGA version 5.2, and a neighbor-joining tree was constructed using Kimura two-parameter distances with 1000 replicates to estimate bootstrap support. The sequences in this study were deposited in the GenBank database and assigned accession numbers KF996538 through KF996567.
2.9. Statistical analysis
Statistical analyses were conducted using standard statistical procedures (Gomez and Gomez Citation1984) implemented in SAS (SAS Institute Citation2003). The data were examined by analysis of variance (ANOVA) and regression. Differences between the treatments were determined by ANOVA, and Fisher’s protected least significant difference (LSD) was calculated at the 0.05 probability level for treatment mean comparisons.
3. RESULTS
3.1. Nodulation in hairy vetch
We observed poor nodulation in the control samples; however, Mo application increased nodule formation in the roots of hairy vetch. Mo application to the soil significantly (P < 0.05) increased both the number and weight of nodules in hairy vetch roots. Plants treated with 0.5 mg Mo kg−1 had 62.4% more nodules than the controls, and significantly more nodules (P ≤ 0.05) than the other treatment groups, except 1.0 mg Mo kg−1 (). Similarly, we observed a 53.9% increase in nodule weight after treatment with 0.5 mg Mo kg−1, and this weight was significantly (P ≤ 0.05) higher than those of the control and other Mo-treated plants ().
Figure 1 (a) Root nodule numbers, (b) nodule dry weight, (c) aboveground biomass, (d) root biomass production in plants, (e) available Mo and (f) nitrate-nitrogen (NO3-N) content in soil for various levels of molybdenum (Mo) in hairy vetch (Vicia villosa Roth) plants. Error bars indicate the standard error of the mean (n = 3, mean ± SE). Bars with different letters differ significantly [least significant difference (LSD) test, P ≤ 0.05].
![Figure 1 (a) Root nodule numbers, (b) nodule dry weight, (c) aboveground biomass, (d) root biomass production in plants, (e) available Mo and (f) nitrate-nitrogen (NO3-N) content in soil for various levels of molybdenum (Mo) in hairy vetch (Vicia villosa Roth) plants. Error bars indicate the standard error of the mean (n = 3, mean ± SE). Bars with different letters differ significantly [least significant difference (LSD) test, P ≤ 0.05].](/cms/asset/f7015472-cedd-40fc-ba8e-fb1141566988/tssp_a_1030690_f0001_b.gif)
3.2. Above-ground and root biomass of hairy vetch
The growth of hairy vetch above ground and root biomass were significantly higher after culture in Mo-treated soil than after culture in control soil (P ≤ 0.05) (, and ). Therefore, the total biomass of Mo-treated plants was higher than that of control plants. We observed significantly greater above-ground dry biomass (5 ± 0.34 g plant−1) in plants treated with 0.5 mg Mo kg−1 than in control and other Mo-treated plants. We observed the highest root dry biomass (0.35 ± 0.04 g plant−1) in plants treated with 0.5 mg Mo kg−1, though it was not statistically different from that of plants treated with 1.0 mg Mo kg−1. In the control hairy vetch, the above-ground and root biomass contributed 94.8 and 5.2% of the total biomass production, respectively. Mo application decreased the contribution of above-ground biomass to 93.3% (for plants treated with 1.0 mg Mo kg−1).
3.3. Available and total Mo in the soil, and total Mo content and uptake in plants
Mo application led to a proportionate increase in total Mo content in the soil (). Application of Mo at 1 mg kg−1 resulted in the highest total soil Mo content, which was 64.8% higher than that observed for the control treatment. We observed a similar pattern for Mo availability. Mo availability in the soil was significantly (P ≤ 0.05) higher for samples treated with Mo than for the control; specifically, treatment with 1 mg Mo per kg soil resulted in a 66.7% increase in Mo availability in the soil relative to the control (). We also observed that Mo application was proportionately related to Mo accumulation in hairy vetch biomass. Treatment with 1 mg Mo kg−1 resulted in a 96.9% increase in total Mo content and a 93.5% increase in Mo uptake in hairy vetch relative to that in control plants ().
Table 1 Total molybdenum (Mo) content in soil and total Mo content, Mo uptake, nitrogen (N) content, and N uptake of the aboveground portion of Vicia villosa Roth [n = 3, mean ± standard error (SE)]
3.4. Soil N content and hairy vetch N uptake
NO3-N content in the soil depended on the amount of Mo applied. We observed the highest NO3-N content in the soil for 0.5 mg Mo kg−1; this content was significantly (P ≤ 0.05) higher than that of other treatments, except 1 mg Mo kg−1 (). Also, the total N content of Mo-treated plants was significantly higher than that of control plants, and treatment with 0.5 mg Mo kg−1 resulted in increased total N content (41.1%) and uptake in the above-ground portion of hairy vetch plants (41.2%) relative to that in the control.
3.5. Nitrogenase activity in hairy vetch roots
Mo application influenced NA activity in vetch roots by affecting the biochemical characteristics of nodules. We observed the lowest NA activity (517.9 μmol C2H4 plant−1 h−1) in control roots, and the observed activity was positively related to the amount of Mo applied to the soil (up to 0.5 mg Mo kg−1 soil; ). Treatment with 1.0 mg Mo kg−1 reduced the NA activity in hairy vetch roots, but it did not differ significantly from that observed after treatment with 0.5 mg Mo kg−1. The NA activity after treatment with 0.5 mg Mo kg−1 was significantly higher than that in the control and that after other Mo treatments.
Figure 2 (a) Nitrogenase activity in roots, (b) nitrate reductase activity in leaves and (c) nitrate reductase activity in roots of hairy vetch (Vicia villosa Roth) at various levels of molybdenum (Mo). Error bars indicate the standard error of the mean (n = 3, mean ± SE). Bars with different letters differ significantly [least significant difference (LSD) test, P ≤ 0.05]. FW: fresh weight.
![Figure 2 (a) Nitrogenase activity in roots, (b) nitrate reductase activity in leaves and (c) nitrate reductase activity in roots of hairy vetch (Vicia villosa Roth) at various levels of molybdenum (Mo). Error bars indicate the standard error of the mean (n = 3, mean ± SE). Bars with different letters differ significantly [least significant difference (LSD) test, P ≤ 0.05]. FW: fresh weight.](/cms/asset/fe08051a-7b80-4173-8429-df6772dc2ca8/tssp_a_1030690_f0002_b.gif)
3.6. Nitrate reductase activity of hairy vetch
We observed higher NR activity in the leaves and roots of Mo-treated hairy vetch than in control plants ( and ). Irrespective of treatment, NR activity was higher in leaves than in roots. The NR activity gradually increased in leaves and roots of hairy vetch after Mo treatment. We recorded the highest NR activity in leaves and roots of plants treated with 0.5 mg Mo kg−1, although it did not differ significantly from that of plants treated with 1.0 mg Mo kg−1. The total NR activity in Mo-treated plants was significantly higher than that in control plants [1.40 ± 0.11 μmol nitrite (NO2-) g−1 fresh weight h−1; P ≤ 0.05]. We found that the total NR activity in plants treated with 0.5 mg Mo kg−1 (2.81 ± 0.14 μmol NO2- g−1 fresh weight h−1) was significantly higher than that in other Mo-treated plants (P < 0.05; ).
Figure 3 Phylogenetic relationships among rhizobia species based on 16 S rDNA sequences of clones recovered from molybdenum (Mo)-treated soil. Neighbor-joining tree with bootstrap values (1000 replicates) shown at branch points. Escherichia coli was used as the outgroup. FYRM11–FYRM15, FYRM21–FYRM25, FYRM31–FYRM35, FYRM41–FYRM45 and FYRM51–FYRM55 strains were collected from 0, 0.13, 0.25, 0.50 and 1.0 mg kg−1 Mo-treated hairy vetch root nodules, respectively.
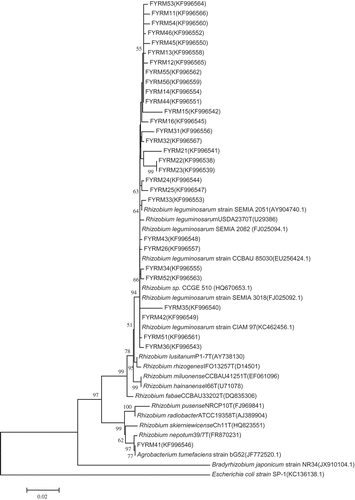
Figure 4 (a) Total biomass yield and nitrogenase activity in roots, and (b) total nitrate reductase activity in hairy vetch (Vicia villosa Roth) plants. Error bars indicate the standard error of the mean (n = 3, mean ± SE). Bars with different letters differed significantly [least significant difference (LSD) test, P < 0.05]. FW: fresh weight.
![Figure 4 (a) Total biomass yield and nitrogenase activity in roots, and (b) total nitrate reductase activity in hairy vetch (Vicia villosa Roth) plants. Error bars indicate the standard error of the mean (n = 3, mean ± SE). Bars with different letters differed significantly [least significant difference (LSD) test, P < 0.05]. FW: fresh weight.](/cms/asset/7b724ba9-2561-4cee-90bf-431d0b0edd0e/tssp_a_1030690_f0004_b.gif)
3.7. Rhizobial community study
We randomly selected 30 bacterial isolates for preliminary morphological and physiological characterization. The colonies of the selected strains varied in shape and appearance, and had diameters ranging from 0.31 to 2.85 mm. Most of the isolates produced mucilage after 5 d of incubation. All strains showed normal growth under laboratory conditions (20 ± 0.5°C and 30°C, data not shown). We collected Rhizobium isolates FYRM11–15, FYRM21–25, FYRM31–35, FYRM41–45 and FYRM51–55 from the nodules from hairy vetch plants treated with 0, 0.13, 0.25, 0.50 and 1.0 mg Mo kg−1, respectively.
PCR amplification of the 16S rRNA genes from the bacterial isolates yielded genetic material of approximately 1161–1436 bp in length (). The phylogenetic tree constructed using the maximum likelihood method showed that the bacterial sequences formed three clusters (; ). The majority of isolates (sequences from 18 strains) belonged to one cluster (cluster #1), and a representative FYRM34 nucleotide sequence belonging to the cluster showed 98% similarity with Rhizobium leguminosarum CIAM 97. The representative FYRM36 nucleotide sequence of the second group of isolates (including sequences from 11 strains, cluster #2) showed 98% sequence similarity with R. leguminosarum SEMIA 3018. FYRM41, isolated from 0.5 mg Mo kg−1-treated plants, formed cluster #3 and showed 99% sequence similarity with A. tumefaciens bG52. The rhizobial strains isolated in this study belonged to the bacterial order Rhizobiales and the family Rhizobiaceae. The isolated strains were similar to Rhizobium leguminosarum and Agrobacterium tumefaciens.
Table 2 Cluster analyses of 16 S rDNA obtained from rhizobia isolates from hairy vetch root nodules. A total of 30 clones were examined
4. DISCUSSION
Mo, an important micronutrient for biological N-fixation in soil, acts as the central metal ion in the cofactors for both NA and NR enzymes (Mendel and Hänsch Citation2002). In this study, Mo application proportionately increased both the number and weight of nodules in hairy vetch roots; however, we observed decreases in nodule characteristics in plants treated with 1.0 mg kg−1 Mo. This observation might be attributed to the fact that plants require minute quantities of micronutrients, and higher quantities inhibit plant growth (Gungula and Garjila Citation2006; Jabbar and Saud Citation2012).
Symbiotic N-fixing bacteria such as Rhizobium species convert atmospheric N2 into ammonium-N and nitrate-N forms involving NA enzymes in the nodules of leguminous plants, and Mo plays an important role in this biochemical redox reaction. In this experiment, the initial soil samples had low Mo and N contents; hence, we observed low NA activity in control plants. Mo treatments significantly (P ≤ 0.05) increased NA activity in hairy vetch, which in turn proportionately increased N-fixation by Mo-treated plants. Vieira et al. (Citation1998) revealed that higher Mo availability in the soil increases N-fixing potential of nodules, possibly by influencing physical and biochemical characteristics of nodules. Consistent with this, we found that the Mo quantity had highly positive correlation with N uptake and biomass of hairy vetch plants (, ). Pramanik et al. (Citation2013) revealed that changes in the number and weight of nodules in hairy vetch and its NA activity were directly related to the nitrate-N content in soil.
Table 3 Correlation of nitrogenase enzyme activity and nitrate reductase enzyme activity with nodules and biomass yield parameters in plants, and nitrogen (N) and molybdenum (Mo) availability, content and uptake in soil and plants after harvesting (n = 15)
Nitrate reductase activity was dependent on the total root biomass of hairy vetch and available nitrate-N content in the soil (). Nitrate reductase activity in Mo-treated plants showed a significantly (P ≤ 0.05) positive correlation with the number and weight of nodules, total biomass yield, Mo content and N content (). The final product (nitrate-N) of NA is the precursor for the NR reaction and, therefore, NA and NR activities were significantly correlated (r = 0.903) in hairy vetch. However, Nautiyal and Chatterjee (Citation2004) and Men and Li (Citation2005) proposed that decreases in NR activity might be explained by differences in the NO3-N to ammonium-nitrogen (NH4-N) ratios in plants or distortions in root cells owing to high rates of Mo application. Liu (Citation2002) also observed changes in cell morphology and a reluctance of leguminous plants to uptake Mo in high-Mo treatments. The relative NA and NR activities determine the enrichment of nitrate-N content in rhizosphere soil (Pollock et al. Citation2002). We observed the highest N content and N uptake after treatment with 0.5 mg Mo kg−1 owing to higher N assimilation, although we also recorded the highest NR in those plants. Therefore, injudicious application of Mo might cause plant biomass loss by retarding NA and NR enzyme activity in hairy vetch (Jones Citation1998; Raven et al. Citation1999), and application of 0.63 mg Mo kg−1 soil is expected to maximize the crop yield (5.16 g plant−1) by improving the biochemical properties of hairy vetch (see Supplementary Material).
The NA enzyme in N-fixing bacteria consists of the Mo–Fe protein responsible for the reduction of atmospheric N during the N fixation process (Lambers et al. Citation1998), and symbiotic Rhizobium species require approximately 10 times more Mo for N2 fixation than do host plants for protein synthesis (Thibaund Citation2005). The 16S rRNA genes are phylogenetic markers characterized by functional diversity, evolutionary information and variable structural elements (Ludwig and Klenk Citation2001). We detected Rhizobium strains such as R. leguminosarum (strain CIAM 97), R. leguminosarum (strain SEMIA 3018) and A. tumefaciens (strain bG52) in plants treated with all Mo doses, including the control. Hou et al. (Citation2009) and Tian et al. (Citation2010) also observed a large abundance of R. leguminosarum in nodules of leguminous plants. Optimum Mo application improved nodule characteristics in hairy vetch plants, and these characteristics may have enabled the presence of Rhizobium species similar to A. tumefaciens. The bacterial species may have played a role in improving enzymatic activity in nodules, which in turn enhanced the N uptake and increased the biomass of hairy vetch. Therefore, the optimum Mo application increased biomass productivity by improving nodule characteristics and enhancing N uptake by leguminous plants.
5. CONCLUSION
Mo application increased the number and weight of nodules in hairy vetch, which in turn increased the nitrate-N content in the rhizospheric soil. The improved nodule characteristics resulted in higher NA activity and increased N-fixing ability in nodules, owing to the higher total N content and N uptake. Genetic analysis indicated that Mo application might also influence microbial diversity in the Rhizobium community in the nodules of hairy vetch root. Isolates similar to A. tumefaciens were only present in optimum growth conditions. Therefore, optimum Mo application is required to increase biomass productivity of leguminous plants. For hairy vetch, 0.63 mg Mo kg−1 is the optimum dose to maximize biomass production and to improve nodule characteristics to increase N uptake.
SUPPLEMENTARY MATERIAL
The supplementary material for this article is available online from: http://dx.doi.org/10.1080/00380768.2015.1030690
Supplemental Materials
Download MS Word (21.9 KB)ACKNOWLEDGEMENTS
This work was supported by the Rural Development Administration (RDA), Republic of Korea (Project No. PJ01122702). Faridul Alam was supported by scholarships from the BK21 plus program of the Ministry of Education and Human Resources Development, Korea.
REFERENCES
- Allison LE 1965: Organic carbon. In Methods of Soil Analysis. Part II, Ed. Black CA, pp. 1367–1376. American Society of Agronomy, Madison, WI.
- Anugroho F, Kitou M, Nagumo F, Kinjo K, Jayasinghe G 2010: Potential growth of hairy vetch as a winter legume cover crops in subtropical soil conditions. Soil Sci. Plant Nutr., 56, 254–262. doi:10.1111/j.1747-0765.2010.00445.x
- Boon D, Soltanpour P 1983: The ammonium bicarbonate-DTPA soil test for determination of plant available lead, cadmium, and molybdenum in mine tailings and contaminated soils. Agronomy Abstracts. American Society of Agronomy, Madison, WI. p. 29.
- Campo RJ, Albino UB, Hungria M 2000: Importance of molybdenum and cobalt to the biological nitrogen fixation. In Nitrogen Fixation: From Molecules to Crop Productivity, Eds. Pedrosa FO, Hungria M, Yates G, Newton WE, pp. 597–598. Springer, Netherlands.
- Drew E, Ballard R 2010: Improving N2 fixation from the plant down: compatibility of Trifolium subterraneum L. cultivars with soil rhizobia can influence symbiotic performance. Plant Soil., 327, 261–277. doi:10.1007/s11104-009-0052-8
- Farooq M, Wahid A, Siddique KHM 2012: Micronutrient application through seed treatments: a review. J. Soil Sci. Plant Nutr., 12(1), 125–142. doi:10.4067/S0718-95162012000100011
- Gomez KA, Gomez AA 1984: Statistical Procedures for Agricultural Research, second ed. John Wiley and Sons, New York.
- Groffman PM, Eagan P, Sullivan W, Lemunyon JL 1996: Grass species and soil type effects on microbial biomass and activity. Plant Soil, 183, 61–67. doi:10.1007/BF02185565
- Gungula D, Garjila Y 2006: The effects of Molybdenum application on growth and yield of cowpea in Yola, Nigeria. Am- Eurasian J. Agric. Environ. Sci., 1, 96–101.
- Haque MM, Kim SY, Pramanik P, Kim G-Y, Kim PJ 2013: Optimum application level of winter cover crop biomass as green manure under considering methane emission and rice productivity in paddy soil. Biol. Fertil. Soils, 49, 487–493. doi:10.1007/s00374-012-0766-2
- He J, Zhang L, Jin S, Zhu Y, Liu F 2008: Bacterial communities inside and surrounding soil iron-manganese nodules. Geomicrobiol. J., 25(1), 14–24. doi:10.1080/01490450701829014
- Hoagland DR, Arnon DI 1950: The Water Culture Method for Growing Plants without Plants without Soil. California Agricultural Experimental Station, Circular No. 347. University of California, Berkeley, CA, USA.
- Hong SY, Minasny B, Zhang YS, Kim YH, Jung KH 2010: Digital soil mapping using legacy soil data in Korea. 19th World Congress of Soil Science, Soil Solutions for a Changing World, 1–6th August 2010, Brisbane, Australia.
- Hou BC, Wang ET, Li Y, Jia RZ, Chen WF, Man CX, Sui XH, Chen WX 2009: Rhizobial resource associated with epidemic legumes in Tibet. Microb. Ecol., 57, 69–81. doi:10.1007/s00248-008-9397-4
- Huang Y, Niu B, Gao Y, Fu L, Li W 2010: CD-HIT Suite: a web server for clustering and comparing biological sequences. Bioinformatics, 26, 680–682. doi:10.1093/bioinformatics/btq003
- Ingle J 1966: The regulation of the activity of the enzymes involved in the assimilation of nitrate by higher plants. Biochem. J., 100, 577–588.
- Islam MR, Sultana T, Joe MM, Yim W, Cho J-C, Sa T 2013: Nitrogen-fixing bacteria with multiple plant growth-promoting activities enhance growth of tomato and red pepper. J. Basic Microbiol., 53, 1004–1015. doi:10.1002/jobm.201200141
- Jabbar BKA, Saud HM 2012: Effects of Molybdenum on biological nitrogen fixation by combination of Rhizobium and Azospirillium in soybean under drip irrigation system. Int. J. Life Sci. Bt. Pharm. Res., 1, 63–37.
- Jackson ML 1973: Soil Chemical Analysis, Prentice Hall Private Ltd, New Delhi, India.
- Jones JBJ 1998: Plant Nutrition Manual, 1st, CRC Press, Boca Raton, Florida. p. 149.
- Konnerup D, Brix H 2010: Nitrogen nutrition of Canna indica: effects of ammonium versus nitrate on growth, biomass allocation, photosynthesis, nitrate reductase activity and N uptake rates. Aquat. Bot., 92, 142–148. doi:10.1016/j.aquabot.2009.11.004
- Lambers H, Chapin FS, Pons TL 1998: Plant Physiology and Ecology, 540. Springer, New York, NY.
- Liu P 2002: Effects of the stress of molybdenum on plants and the interaction between molybdenum and other element. Agri. Environ. Protect., 21, 276–278.
- Ludwig W, Klenk HP 2001: Overview: a Phylogenetic Backbone and Taxonomic Framework for Procaryotic Systematics. In Bergey’s Manual of Systematic Bacteriology, pp. 49–65. Springer New York.
- Malla RM, Padmaja B, Malathi S, Jalapathi RL 2007: Effects of micronutrients on growth and yield of pigeonpea. J. Semi-Arid Trop. Agric. Res., 5, 1–3.
- Marschner H 2011: Marschner’s Mineral Nutrition of Higher Plants, Academic press, London.
- Men ZH, Li SX 2005: Effects of molybdenum on nitrate metabolism of winter wheat. J. Plant Nutr. Fert., 11, 205–210.
- Mendel RR, Hänsch R 2002: Molybdoenzymes and molybdenum cofactor in plants. J. Exp. Bot., 53, 1689–1698. doi:10.1093/jxb/erf038
- Nautiyal N, Chatterjee C 2004: Molybdenum stress-induced changes in growth and yield of chickpea. J. Plant Nutr., 27(1), 173–181. doi:10.1081/PLN-120027554
- Palmer K, Young J 2000: Higher diversity of Rhizobium leguminosarum biovar viciae populations in arable soils than in grass soils. Appl. Environ. Microb., 66, 2445–2450. doi:10.1128/AEM.66.6.2445-2450.2000
- Pollock VV, Conover RC, Johnson MK, Barber MJ 2002: Bacterial expression of the molybdenum domain of assimilatory nitrate reductase: production of both the functional molybdenum-containing domain and the nonfunctional tungsten analog. Arch. Biochem. Biophys., 403, 237–248. doi:10.1016/S0003-9861(02)00215-1
- Pramanik P, Haque MM, Kim PJ 2013: Effect of nodule formation in roots of hairy vetch (Vicia villosa) on methane and nitrous oxide emissions during succeeding rice cultivation. Agric. Ecosyst. Environ., 178, 51–56. doi:10.1016/j.agee.2013.06.021
- Pramanik P, Kim PJ 2014: Mitigate CH4 emission by suppressing methanogen activity in rice paddy soilsusing ethylenediaminetetraacetic acid (EDTA). Geoderma, 219-220, 58–62. doi:10.1016/j.geoderma.2013.12.024
- Raven PH, Evert RF, Eichhorn SE 1999: Biology of Plants, 6th, W. H. Freeman and Company, Worth Publishers, New York, p. 686.
- SAS Institute 2003: System for windows release 9.1. SAS Institute, Cary, NC.
- Solaiman ARM 1999: Effect of Bradyrhizobium japanicum inoculation and molybdenum on soybean. Bangladesh J. Bot., 28(2), 181–183.
- Somasegaran P, Hoben HJ 1994: Handbook for Rhizobia: Methods in Legume Rhizobium Technology, Springer-Verlag, New York, NY.
- Sylvia DM, Fuhrmann JJ, Hartel P, Zuberer DA 2005: Principles and Applications of Soil Microbiology, Upper Saddle River, Pearson Prentice Hall, NJ.
- Thibaund GR 2005: Molybdenum relationships in soils and plants. KwaZulu-Natal Department of Agriculture and Environmental Affairs, Cedara College, Private Bag X, 9059.
- Tian CF, Young JPW, Wang ET, Tamimi SM, Chen WX 2010: Population mixing of Rhizobium leguminosarum bv. viciae nodulating Vicia faba: the role of recombination and lateral gene transfer. FEMS Microbiol. Ecol., 73, 563–576.
- Vieira RF, Cardoso EJBN, Vieira C, Cassini STA 1998: Foliar application of molybdenum in common beans. I. Nitrogenase and reductase activities in a soil of high fertility. J. Plant Nutr., 21(1), 169–180. doi:10.1080/01904169809365391
