Abstract
Effects of solarization on microbial communities in soil were investigated with an incubation experiment and in a greenhouse experiment with isolated bed culture of tomato (Solanum lycopersicum L.) by molecular techniques. Microbial biomass carbon in soil was decreased by about half in the incubation experiment treated with 45°C for 14 days and the greenhouse experiment with solarization for 45 days. Bacterial and fungal communities in soil were affected by the heat and solarization treatments in both the experiments and greatly different from the communities in the unheated soil or the soil before solarization. Copy number of amoA gene of ammonia-oxidizing bacteria in soil was decreased to one-tenth or less by the treatments in both the experiments. This study revealed that the solarization made a great impact on the abundance and composition of microbial communities in soil.
INTRODUCTION
Solarization is a cost-effective and environment-friendly alternative soil disinfection technique to soil fumigation with methyl bromide that was prohibited to use since 2005. The solarization is conducted by covering moist soil with clear polyethylene film during hot summer season to achieve high soil temperature for the disinfestation of plant pathogens and pests (Katan Citation1981; Stapleton Citation2000). Numerous studies have been conducted on effects of solarization on soil pathogens and pests so far.
Heating soil may make a great impact on soil microorganisms besides pathogenic microorganisms. Effect of solarization on populations of soil microorganisms was first reported by Stapleton and DeVay (Citation1982) and afterwards the effects were investigated mainly by many workers by using cultivation methods. However, a relatively few studies have been conducted on the effect of solarization on soil microbial community by molecular methods. In these studies, effects of solarization were investigated mainly on bacterial communities in soil by Schönfeld et al. (Citation2003), Gelsomino and Cacco (Citation2006), Culman et al. (Citation2006), Wada et al. (Citation2008), Bonanomi et al. (Citation2008) and Simmons et al. (Citation2014), and also on fungal communities by only two studies by Culman et al. (Citation2006) and Bonanomi et al. (Citation2008). Compositions of the bacterial and fungal communities in soil were generally influenced and altered by solarization in the reports, but the degrees of the influences were not consistent among them, indicating a need for further studies.
Ammonia-oxidizing bacteria play an important role through nitrification of ammonia that provides nitrate-N to plants. It is well known that nitrification is conspicuously inhibited and the viable counts of ammonia-oxidizing bacteria are greatly decreased by soil solarization (e.g., Nishihara Citation2008; Wada et al. Citation2008; Ihara et al. Citation2014). However, effect of soil solarization on ammonia-oxidizing bacteria has not yet been investigated by molecular methods so far.
The objective of this study was to elucidate effects of solarization on the abundance and composition of soil microbial communities comprehensively for further understanding of its impact on soil microorganisms by using molecular techniques. We conducted an incubation experiment and a greenhouse experiment and determined microbial biomass carbon by the fumigation-extraction method and compositions of both bacterial and fungal communities by the denaturing gradient gel electrophoresis (DGGE) of 16S/18S rRNA genes. Copy number of amoA gene of ammonia-oxidizing bacteria was also measured by the quantitative real-time PCR method.
MATERIALS AND METHODS
Experiment 1: incubation experiment
The incubation experiment was conducted by using upland and paddy field soils. In this model experiment, a paddy field soil as well as an upland soil was used because some of the fields converted from paddy fields are cropped with vegetables under upland conditions in Japan (Takahashi et al. Citation2013). The upland soil sample was collected from plow layer (0–10 cm depth) from a plot applied with chemical fertilizers and farmyard manure at an upland field under long-term fertilizer treatments (Katayama et al. Citation1998; Islam and Toyota Citation2004) (Hapludults; Light Clay texture [Soil Survey Staff Citation1999a]) in the Nagoya University Farm, Togo, Aichi, Japan (35º6ʹN, 137º4ʹE) on 27 April in 2013. The paddy field soil sample was from plow layer (0–10 cm depth) at a paddy field (Oxyaquic Dystrudepts with the Light Clay texture [Soil Survey Staff Citation1999b]) located at the Aichi-ken Anjo Research and Extension Center, Anjo, Aichi, Japan (34º58ʹN, 137º4ʹE) on 8 May in 2013. Some properties of the soils are shown in . The soil samples were passed through a 2-mm mesh sieve. The 30 g of soil were placed in a 70-mL polypropylene container (70 mm inner diameter × 115 mm height; Nakaya Kagaku Sangyo, Higashiosaka, Japan) and mixed with 0.06 g (0.2% w/w) of rice straw powder (<2 mm). Then, the moisture content was adjusted to 60% of maximum water holding capacity with distilled water. The container was closed with a polyethylene lid and kept at 45 or 25°C (control) under dark conditions as a heat treatment which mimicked solarization because the temperature elevation is the most important factor of the solarization treatment. The heating condition at 45°C for longer than 6 days was reported to be lethal to most of soil-borne pathogens in soil solarization treatments (Kodama and Fukui Citation1979; Kodama et al. Citation1979; Fukui et al. Citation1981). Sixteen containers were prepared for each treatment and the soil samples in two replicate containers were collected on 0, 1, 2, 4, 6, 8, 11 and 14 days for the microbial analyses. Microbial biomass for the samples on day 0, 2, 8 and 14 was determined immediately on the same day of sampling. The soil samples for molecular analyses were stored at −20°C until use.
Table 1 Selected soil properties used in the experiments
Experiment 2: greenhouse experiment
The greenhouse experiment was conducted in a greenhouse with isolated bed culture of tomato (Solanum lycopersicum L.) in the Nagoya University Farm, Togo, Aichi, Japan. The greenhouse consisted of three beds (0.85 m × 26 m × 0.3 m depth; Kumiai Drain Bed, Katakura Chikkarin, Tokyo, Japan) with application (2 × 104 kg ha−1 [fresh weight]) of cattle manure compost produced in the University Farm (bed A), compost of sewage sludge from food industry (UT Compo, Kinuura Utility, Hekinan, Japan; bed B) and “bokashi” compost of animal and plant wastes (Aging, Chlorella Industry, Tokyo, Japan; bed C) as a basal fertilizer for tomato. Tomato plants were cultivated twice a year from February to June and from August to December. The soil was Hapludults with the Light Clay texture (Soil Survey Staff Citation1999a) and some properties are shown in .
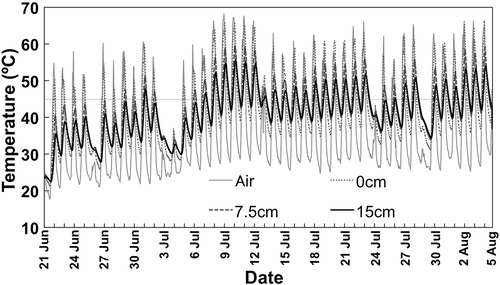
The soil solarization has been performed in the greenhouse annually since 2004. In 2013, the soil in the beds was puddled with pouring irrigation water and covered with a transparent polyethylene film (Sekisui Film, Tokyo, Japan) on 21 June. Then, the greenhouse was obturated for 45 days until 5 August. The soil was fertilized and plowed on 5 August and tomato seedlings were planted on 13 August. Air temperature at 1.5 m above the ground and soil temperatures at 0, 7.5 and 15 cm (at the bottom of the bed) depths were measured hourly throughout the solarization period at the center of the greenhouse (bed B) with a thermo recorder (Ondotori Jr. TR-52i, T&D Corporation, Matsumoto, Japan).
Soil samples were collected at three points in each bed (located about 6 m apart) from the depths of 0–5, 5–10 and 10–15 cm using three sampling cores (100 mL stainless sampling tube; Daiki Rika Kogyo, Tokyo, Japan) on 20 June (before solarization), 13 July (midpoint of the solarization period), 5 August (termination of solarization), 17 September (5 weeks after planting of tomato seedlings) and 18 November (14 weeks after planting of tomato seedlings). The three soil samples from each depth were mixed for each bed, sieved through a 2-mm mesh screen and subjected to the microbial analyses.
Determination of microbial biomass C
Microbial biomass was determined by the chloroform-fumigation extraction method (Vance et al. Citation1987). The 10 g portion of the fumigated or non-fumigated soil was extracted with 50 mL of 0.5 M potassium sulfate according to the protocols described by Sakamoto (Citation1997, Citation2003). Total organic C in the extracts was quantified with a TOC analyzer (TOC-VCPH, Shimadzu, Kyoto, Japan). All the measurements were performed in duplicates. Value for the conversion factor kC was 0.45, for the calculation of biomass C (Joergensen Citation1996; Urashima Citation2013).
DNA extraction from soil
DNA for PCR-DGGE analysis and real-time quantitative PCR was extracted from 0.5 g of soil samples using the ISOIL for Beads Beating Kit for soil (NIPPON GENE, Tokyo, Japan) by the bead-beating method according to the manufacture’s instruction with two replicates. The extracted DNA was dissolved in 100 µL of TE buffer (10 mM Tris–HCl, 1 mM EDTA; pH 8.0).
PCR amplification and DGGE analysis
PCR amplification and DGGE analysis of bacterial 16S rRNA and fungal 18S rRNA genes were conducted based on the procedures described by Morimoto and Hoshino (Citation2008). Bacterial 16S rRNA and fungal 18S rRNA gene fragments were amplified with primer pairs 357f-GC/517r (Muyzer et al. Citation1993) and NS1/GCFung (May et al. Citation2001) for bacteria and fungi, respectively. The 50 µL of reaction mixture for bacterial 16S rRNA gene amplification contained 5 µL of 10 × Ex Taq buffer (TaKaRa, Otsu, Japan), 4 µL of deoxyribonucleotide triphosphate (dNTP) mixture (2.5 mM each), each 0.5 μL of primers (50 μM each), 0.25 μL of Ex Taq polymerase (5 U μL–1), 1 µL of template DNA and 38.75 µL of sterilized water. The PCR program had an initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1.5 min, and a final extension at 72°C for 10 min. The 50 µL of reaction mixture for fungal 18S rRNA gene amplification consisted of 5 µL of 10 × buffer for KOD-Plus- (TOYOBO, Osaka, Japan), 5 µL of deoxyribonucleotide triphosphate (dNTP) mixture, (2 mM each), each 1.5 μL of primers (10 μM each), 1 μL of KOD-Plus- polymerase (1 U μL–1), 1 µL of template DNA and 33 µL of sterilized water. The PCR program had an initial denaturation at 94°C for 2 min, followed by 30 cycles of denaturation at 94°C for 0.25 min, annealing at 50°C for 0.5 min and extension at 68°C for 0.5 min. The PCR products were purified with a NucleoSpin® Gel and PCR Clean-up (MACHEREY-NAGEL, Düren, Germany) following to the manufacturer’s instruction.
DGGE was carried out with a Dcode Universal Mutation Detection System (Bio-Rad Laboratories, Hercules, CA, USA) with 1 × TAE buffer (40 mM Tris-acetate, 1 mM EDTA) at 60°C and 100 V for 14 h for bacterial community or at 60°C and 50 V for 20 h for fungal community. Approximately 200 or 150 ng of the purified PCR amplicons were applied onto 8 or 7% (w/v) polyacryamide gel for bacteria or fungi, respectively, in 1 × TAE buffer. A denaturing gradient range of the gel was from 30% to 70% and from 20% to 45% for the analysis of bacterial and fungal communities, respectively, in which 100% denaturant contained 7 M urea and 40% (v/v) formamide. The gel was stained with SYBR® Green I nucleic acid gel stain (Lonza, Rockland, ME, USA) for 30 min and photographed with a Polaroid photo equipment (RB67, Mamiya, Tokyo, Japan) attached with a SYBR Green gel stain photographic filter under UV light at 302 nm on a transilluminator (Vilber Lourmat, Marne-la-Vallée, France). One of the replicated DNA extracts was subjected to the PCR-DGGE for bacterial communities because the preliminary test showed that the band patterns were identical between the replicates, whereas both of the replicated DNA extracts were subjected to the analysis for fungal communities because slight differences were observed between the patterns from the replicates.
The DGGE bands were classified into four categories depending on the relative intensities (0 = no band; 1 = weak; 2 = moderate; 3 = strong; 4 = very strong) by visual observation. The matrix data was then analyzed by non-metric multi-dimensional scaling (NMDS) based on the Euclidean distances and the Bray-Curtis similarity index between the treatments and between the different dates was calculated with the PAST program (Hammer et al. Citation2001).
Quantification of amoA of ammonia-oxidizing bacteria
amoA genes of ammonia-oxidizing bacteria were quantified using the primer pair amoA1F/amoA2IR according to the method by Morimoto et al. (Citation2011) with a Thermal Cycler Dice Real Time System (TaKaRa). The 25 µL of reaction mixture contained 12.5 µL of SYBR Premix Ex Taq (TaKaRa), 0.5 µL of each primer (50 µM each), 0.25 µL of bovine serum albumin (0.2 mg mL−1; TaKaRa), 0.5 µL of template DNA and 10.75 µL of sterilized water. The PCR program had an initial denaturation at 94°C for 2 min, followed by 40 cycles of denaturation at 94°C for 0.5 min, annealing at 54°C for 0.5 min and extension at 72°C for 0.5 min. A standard curve was generated using ten-fold serial dilutions of pGEM-T easy (Promega, Madison, WI, USA) containing an amoA fragment derived from Nitrosospira multiformis ATCC25196 (GenBank accession number U91603) as described by Morimoto et al. (Citation2011).
RESULTS AND DISCUSSION
Elevation of temperature by solarization
The air and soil temperatures were elevated during the solarization period in the experiment 2 (). Maximum temperature was 68.2, 66.2, 59.5 and 55.7°C at 1.5 m above from the ground, 0, 7.5 and 15 cm soil depths, respectively. The maximum temperatures above 45°C were recorded for totally 196 h in consecutive 17 days from 7 July to 23 July 2013 even at the bottom of the bed (15 cm depth). Kodama and Fukui (Citation1979) showed that a heat treatment at 45°C for 144 h (6 days) was lethal to Fusarium oxysporum f. sp. fragariae and the lethal condition was generally applicable to most of other soil-borne pathogens (Kodama et al. Citation1979; Fukui et al. Citation1981). Therefore, the solarization treatment in this experiment seemed to be almost sufficient for controlling soil-borne diseases. Indeed, no incidence of tomato bacterial wilt by Ralstonia solanacearum and a very few tomato plants with wilting symptoms only for the susceptible cultivar were observed in the beds for the crops from August 2013 and subsequently from February 2014, respectively (M. Maesaka, unpublished results).
Microbial biomass C
Microbial biomass C was drastically decreased by the heat treatment in the experiment 1 and the solarization in the experiment 2 (). The amount was decreased to less than half of that in the control soils after 8 days by the treatment at 45°C in the experiment 1 () and also to less than half, much less in most cases, of the initial amount (before solarization) after 23 days of the solarization treatment in the experiment 2 (). The conspicuous decrease, reduced by < about 50%, in microbial biomass C was also reported in previous studies in open fields (Gelsomino et al. Citation2006; Baptista et al. Citation2007) and greenhouses (Scopa and Dumontet Citation2007; Scopa et al. Citation2008). The findings in this study confirmed that solarization has severe decreasing effect on abundance of soil microbial community in accordance with the former findings.
Figure 2 Contents of soil microbial biomass C in experiments 1 (a) and 2 (b). Error bars indicate ranges of two replicate measurements. Arrows show the solarization period from 21 June to 5 August 2013.
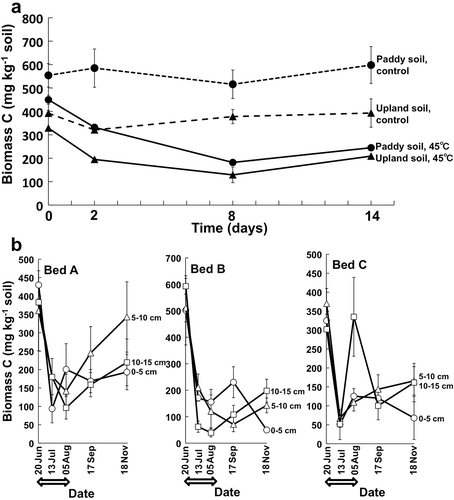
In experiment 2, microbial biomass C seemed to increase gradually in the tomato cultivation period after the solarization, but did not recover to the initial amount before solarization even after 15 weeks, although the data had great fluctuation and also variations in the measurement (). This partial recovery of the microbial biomass C may have been caused by growth of microorganisms utilizing available substrates derived from killed biomass by solarization and also from organic fertilizers applied to soil just after solarization. The finding that the microbial biomass C did not yet show full recovery in three months after the termination of solarization indicates severity of the treatment. Slight increase in soil microbial biomass C was also found 60 days after solarization in the study by Baptista et al. (Citation2007). Further investigation in long-term is necessary to monitor dynamics of microbial biomass in soil during crop cultivation followed by solarization, which could provide important information related to overall activity of soil including nutrient cycling.
Compositional changes in bacterial and fungal communities
DGGE band patterns of bacterial and fungal communities in soil were conspicuously changed by the heat treatment in the experiment 1 () and the solarization in the experiment 2 (). The bacterial and fungal communities after 14 days of the heat treatment at 45°C in the experiment 1 or just after solarization on 5 August in the experiment 2 were located distantly from those before the heat treatment (day 0) or the solarization (20 June) in the NMDS scatter plots of the patterns ( and ). These results indicate that compositions of the bacterial and fungal communities were greatly affected by the treatments.
Figure 3 DGGE band patterns of bacterial (a) and fungal (b) communities of upland and paddy field soils in the experiment 1. Number of bands is shown at the bottom of each lane.
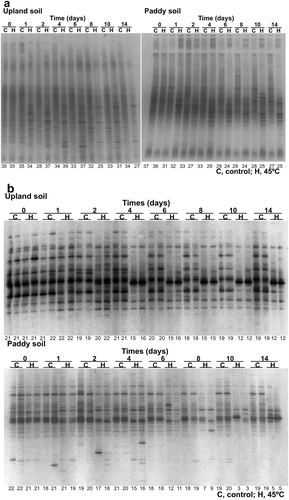
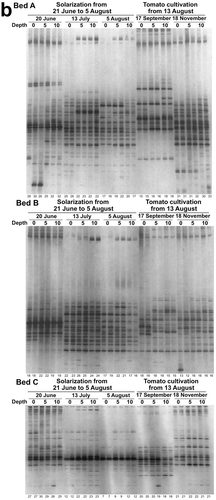
Figure 4 DGGE band patterns of bacterial (a) and fungal (b) communities in soil under solarization treatment and subsequent tomato cultivation in the experiment 2. The solarization was conducted from 21 June to 5 August 2013 and tomato seedlings were planted on 13 August 2013. Depth of soil: 0, 0–5 cm; 5, 5–10 cm; 10, 10–15 cm. Number of bands is shown at the bottom of each lane.
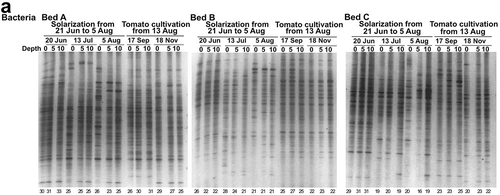
Figure 5 Non-metric multi-dimensional scaling of DGGE band patterns of bacterial (a) and fungal (b) communities of upland and paddy field soils in the experiment 1. Control, control at 25°C; 45°C, heat treatment at 45°C. The figure shows the incubation time (day).
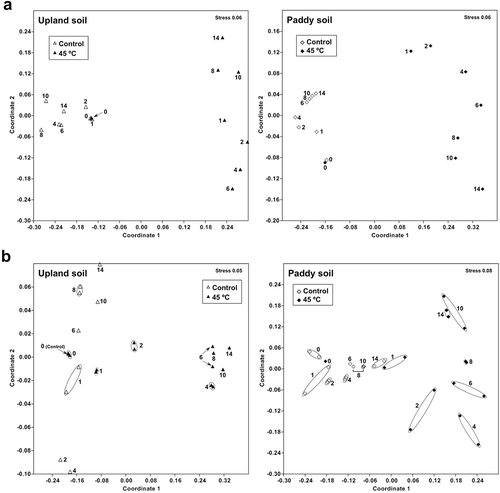
Figure 6 Non-metric multi-dimensional scaling of DGGE band patterns of bacterial (a) and fungal (b) communities in soils of the three beds in the experiment 2. 0, 0–5 cm depth soil; 5, 5–10 cm depth soil; 10, 10–15 cm depth soil. The solarization was treated from 21 June to 5 August 2013.
The heat treatment at 45°C in the experiment 1 decreased the number of the DGGE bands by 37% (upland soil) to 85% (paddy soil) of that in the control soils for fungi after 10 days of the treatment, while only by 18% (paddy soil after 2 days) to 22% (upland soil after 8 days) of that in the control soils for bacteria at most (). The degree of decreases in the number of bands was significantly greater for fungi than for bacteria (P < 0.05, Wilcoxon signed-rank test) when it was compared at each day during the heat treatment; it was significantly greater in paddy soil than in upland soil for fungi (P < 0.05, Wilcoxon signed-rank test), but was not significantly different between the soils for bacteria. Values of the Bray-Curtis similarity index between the band patterns of control and heat treatments were decreased to be lower than 0.5 after 10 and 14 days of the treatment both for bacteria and fungi in paddy and upland soils (). The value was not significantly different between bacteria and fungi both in paddy and upland soils during the heat treatment, but was significantly lower in paddy soil than in upland soil both for bacteria and fungi (P < 0.01, Wilcoxon signed-rank test). These findings as a whole indicate that the effect of the heat treatment at 45°C on microbial community composition tended to be greater in the paddy soil than in the upland soil. Narrower ranges of diurnal soil temperature in summer have been expected in paddy fields probably due to high specific heat capacity of water (Kasubuchi Citation1977) by flooding than in upland fields, which might have been related in part to the differences of the effect between the paddy soil and the upland soil.
Table 2 Effect of heat treatment at 45°C in experiment 1 or solarization in experiment 2 on Bray-Curtis similarity index of the DGGE band pattern
The solarization in the experiment 2 also decreased the number of the DGGE bands, but the decreases were variable among the beds, which may have been caused in part by the fluctuation of soil temperature () in contrast to the experiment 1 under the constant temperature treatment. The number was decreased by 0–74% (0–5 cm), 2–69% (5–10 cm) and 13–53% (10–15 cm) after 45 days of the solarization treatment for fungi in comparison with the number before the treatment, while by 13–31% (0–5 cm), 5–48% (5–10 cm) and 5–39% (10–15 cm) for bacteria (). The degree of decreases in the number of bands was significantly greater for fungi than for bacteria (P < 0.05, Wilcoxon signed-rank test) when it was compared at each depth on the day at the termination of the solarization, but was not significantly different between fungi and bacteria after 22 days of the treatment at the midpoint of solarization. The number of the DGGE bands in 14 weeks after planting of tomato seedlings was recovered up to 69–107% and 69–97% (0–5 cm), 75–124% and 74–105% (5–10 cm), and 82–98% and 71–100% (10–15 cm) of those before solarization for fungi and bacteria, respectively (); the reduction in the number was significantly smaller in 14 weeks after tomato planting than just after solarization (P < 0.05 for fungi and P < 0.01 for bacteria, Wilcoxon signed-rank test). Values of the Bray-Curtis similarity index between the band patterns before and after solarization were decreased to about 0.6 for bacteria or 0.4 for fungi on the day at the termination of the solarization (). The value was significantly lower for fungi than for bacteria at each depth on the day (P < 0.01, Wilcoxon signed-rank test), but was not significantly different among the depths of soil both for bacteria and fungi (P > 0.05, Friedman test). The similarity value after 14 weeks of tomato planting was slightly increased from that on the day at the termination of solarization with variations depending on the depths of soil and beds () and the difference was significant both for bacteria and fungi (P < 0.05, Wilcoxon signed-rank test). These findings indicate that the solarization in experiment 2 altered the microbial community composition in soil, greatly that of fungal community in particular, and seemed to be recovered gradually, but not fully, during the tomato cultivation subsequent to the solarization.
Reduction in number of DGGE bands by solarization was reported for bacteria by Gelsomino and Cacco (Citation2006) and Bonanomi et al. (Citation2008), and for fungi by Bonanomi et al. (Citation2008). Bonanomi et al. (Citation2008) found a greater reduction for bacteria than for fungi in contrast to the finding in this study. In addition, Schönfeld et al. (Citation2003) observed no significant difference in the bacterial DGGE band patterns between the solarized and control soils. Wada et al. (Citation2008) investigated the recovery of the bacterial community composition in the crop cultivation period subsequent to solarizaiton and reported that the DGGE band patterns were recovered and almost same as that without solarization in 17 weeks after termination of the solarization, which was also in contrast to the findings in this study. These variations in the influences of solarization on the microbial community composition perhaps seemed to be caused by the differences in the soil conditions, especially soil temperatures during the solarization. In the field and greenhouse experiments, soil temperature is influenced by weather and fluctuated diurnally. In this study, the soil temperature was monitored in the bed B in the experiment 2 () and an accumulated temperature can be estimated from the data in . The accumulated temperature ≥ 45°C was 845°C·day (0–5 cm), 781°C·day (5–10 cm), and 624°C·day (10–15 cm) at the termination (after 45 days) of the solarization. The temperature even at the depth of 10–15 cm was much greater than the value (450°C·day) after 10 days of the heat treatment in the experiment 1. The similarity index value of the DGGE band patterns in the bed B in the experiment 2 was as small as the value in the experiment 1 for fungi, but it was not the case for bacteria (). The diurnal fluctuation in temperature and/or differences in physiological diversity between bacteria and fungi may have influenced the discrepancy. Recently effect of solarization on the taxonomic composition of bacterial communities in soil was investigated by pyro-sequencing of 16S rRNA gene and enrichment of potential thermophilic members was observed (Simmons et al. Citation2014). Effects of solarization on microbial community composition need to be further investigated in relation to the accumulated temperature caused by the treatment.
Change in abundance of ammonia-oxidizing bacterial community
Copy number of ammonia-oxidizing bacterial amoA gene was conspicuously decreased to one-tenth or less of that in control soil and before the solarization by the heat treatment at 45°C in experiment 1 and by the solarization in experiment 2, respectively (). Many investigations have been conducted on the effect of soil disinfestation on ammonia-oxidizing bacteria by the cultivation (MPN) method and fatal influences on the viable counts with the decrease to less than 1/100–1/1000 were reported also for the solarization by Nishihara (Citation2008) and Wada et al. (Citation2008). This study showed the conspicuous decrease to less than 1/10–1/100 in the abundance of ammonia-oxidizing bacterial community by the heat treatment or solarization based on qPCR analysis of amoA gene for the first time as far as we know.
Figure 7 Number of amoA gene of ammonia-oxidizing bacteria in soil in experiments 1 (a) and 2 (b). Error bars indicate ranges of two replicate measurements. Arrows show the solarization period from 21 June to 5 August 2013.
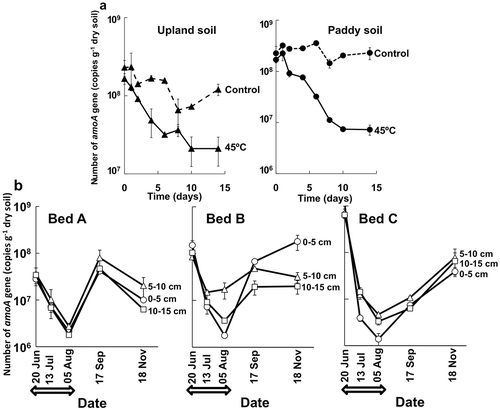
In the experiment 2, the copy number of amoA gene was recovered during the tomato cultivation subsequent to the solarization, indicating proliferation of ammonia-oxidizing bacteria in the soils, although the abundance did not reach to that before the solarization in bed C (). Recovery of ammonia-oxidizing bacterial community in the crop cultivation subsequent to solarization is important to prevent growth retardation of the crop by ammonium accumulation in the disinfested soil (Morikuni et al. Citation1999; Nishihara Citation2008). The qPCR estimation of the amoA gene of ammonia-oxidizing bacteria is possibly a quick and useful diagnosis of the recovery of the abundance because enumeration of ammonia-oxidizing bacteria by the MPN method takes about one month to obtain the viable counts. Further investigation is necessary on the behavior of ammonia-oxidizing bacteria and inorganic nitrogen contents in the solarized soil to establish the diagnostic technique by the qPCR method.
This study showed that the heat treatment at 45°C or solarization made a great impact on the abundance and composition of microbial communities, including ammonia-oxidizing bacteria, in soil by molecular methods. Evaluation of the recovery of these microbial communities and their activities and functions is important for the crop cultivation subsequent to the solarization and the molecular techniques could be a useful diagnostic tool for estimating the abundance and composition of the microbial communities in the soil.
ACKNOWLEDGMENTS
We thank Professor Takashi Tsuge of the Graduate School of Bioagricultural Sciences, Nagoya University for helpful advices on the study. This study was supported in part by Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry. This article is dedicated to the late Dr. Subrata Mowlick.
REFERENCES
- Baptista MJ, dos Reis Jr FB, Xavier GR, de Alcântara C, de Oliveira AR, Souza RB, Lopes CA 2007: Efficiency of solarization and biofumigation on tomato bacterial wilt control in the field. Pesquisa Agropecuária Brasileira, 42, 933–938. (in Portuguese with English summary). doi:10.1590/S0100-204X2007000700004
- Bonanomi G, Chiurazzi M, Caporaso S, Del Sorbo G, Moschetti G, Felice S 2008: Soil solarization with biodegradable materials and its impact on soil microbial communities. Soil Biol. Biochem., 40, 1989–1998. doi:10.1016/j.soilbio.2008.02.009
- Culman SW, Duxbury JM, Lauren JG, Thies JE 2006: Microbial community response to soil solarization in Nepal’s rice–wheat cropping system. Soil Biol. Biochem., 38, 3359–3371. doi:10.1016/j.soilbio.2006.04.053
- Fukui T, Kodama T, Nakanishi Y 1981: Solar heating sterilization in the closed vinyl house against soil-borne diseases. IV. Solar heating sterilization by polyethylene mulching in the open-field. Bull. Nara Pref. Agric. Exp. Stn., 12, 109–119. (in Japanese with English summary).
- Gelsomino A, Badalucco L, Landi L, Cacco G 2006: Soil carbon, nitrogen and phosphorus dynamics as affected by solarization alone or combined with organic amendment. Plant Soil, 279, 307–325. doi:10.1007/s11104-005-2155-1
- Gelsomino A, Cacco G 2006: Compositional shifts of bacterial groups in a solarized and amended soil as determined by denaturing gradient gel electrophoresis. Soil Biol. Biochem., 38, 91–102. doi:10.1016/j.soilbio.2005.04.021
- Hammer Ø, Harper DAT, Ryan PD 2001: PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron., 4, 9pp.
- Ihara H, Kato N, Takahashi S, Nagaoka K 2014: Effect of soil solarization on subsequent nitrification activity at elevated temperatures. Soil Sci. Plant Nutr., 60, 824–831. doi:10.1080/00380768.2014.947233
- Islam TMD, Toyota K 2004: Susceptibility of soils with or without repeated application of farmyard manure to bacterial wilt of tomato caused by Ralstonia solanacearum. Soil Microorganisms, 58, 33–42.
- Joergensen RG 1996: The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol. Biochem., 28, 25–31. doi:10.1016/0038-0717(95)00102-6
- Kasubuchi T 1977: Thermal properties of soil. Soil Phys. Cond. Plant Growth, Jpn, 35, 29–33. (in Japanese).
- Katan J 1981: Solar heating (solarization) of soil for control of soil-borne pests. Annu. Rev. Phytopathol., 19, 211–236. doi:10.1146/annurev.py.19.090181.001235
- Katayama A, Hu H-Y, Nozawa M, Yamakawa H, Fujie K 1998: Long-term changes in microbial community structure in soils subjected to different fertilizing practices revealed by quinone profile analysis. Soil Sci. Plant Nutr, 44, 559–569. doi:10.1080/00380768.1998.10414479
- Kodama T, Fukui T 1979: Solar heating sterilization in the closed vinyl house against soil-borne diseases. I. The movements of soil temperature and determination of thermal lethal conditions for some soil-borne pathogens. Bull. Nara Pref. Agric. Exp. Stn., 10, 71–82. (in Japanese with English summary).
- Kodama T, Fukui T, Nakanishi Y 1979: Solar heating sterilization in the closed vinyl house against soil-borne diseases. II. Effects of solar heating sterilization in commercial vinyl-house and basic methods for estimating the effects obtain by the treatment. Bull. Nara Pref. Agric. Exp. Stn., 10, 83–92. (in Japanese with English summary).
- May LA, Smiley B, Schmidt MG 2001: Comparative denaturing gradient gel electrophoresis analysis of fungal communities associated with whole plant corn silage. Can. J. Microbiol., 47, 829–841. doi:10.1139/w01-086
- Morikuni H, Niizuma S, Shimada N 1999: Recovery of nitrifying bacteria after sterilization and the method of nitrogen application to decrease blossom end rot of tomatoes grown in isolated bed. Jpn. J. Soil Sci. Plant Nutr., 70, 542–549. (in Japanese with English summary).
- Morimoto S, Hayatsu M, Takada-Hoshino Y, Nagaoka K, Yamazaki M, Karasawa T, Takenaka M, Akiyama H 2011: Quantitative analyses of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in fields with different soil types. Microbes Environ, 26, 248–253. doi:10.1264/jsme2.ME11127
- Morimoto S, Hoshino TY 2008: Methods for analysis of soil communities by PCR-DGGE (1) Bacterial and fungal communities. Soil Microorganisms, 62, 63–68. (in Japanese).
- Muyzer G, de Waal EC, Uitterlinden AG 1993: Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol., 59, 695–700.
- Nishihara M 2008: The effect of soil disinfection by improved Miyazaki model solar disinfection. Jpn. J. Soil Sci. Plant Nutr.,79, 393–396. (in Japanese).
- Sakamoto K 1997: Soil biomass. In Analytical Methods for Soil and Environments, Ed. Konno T, pp. 146–156. Hakuyusha, Tokyo. (in Japanese).
- Sakamoto K 2003: Measurement of soil microbial biomass. In Manual of Microbiological Experiments for Beginners, 2nd ed. Ed.Ando A, pp. 58–62. Gihodo, Tokyo.(in Japanese).
- Schönfeld J, Gelsomino A, van Overbeek LS, Gorissen A, Smalla K, van Elsas JD 2003: Effects of compost addition and simulated solarisation on the fate of Ralstonia solanacearum biovar 2 and indigenous bacteria in soil. FEMS Microbiol. Ecol., 42, 63–74. doi:10.1111/j.1574-6941.2003.tb01046.x
- Scopa A, Candido V, Dumontet S, Miccolis V 2008: Greenhouse solarization: effects on soil microbiological parameters and agronomic aspects. Sci. Hort., 116, 98–103. doi:10.1016/j.scienta.2007.11.008
- Scopa A, Dumontet S 2007: Soil solarization: effects on soil microbiological parameters. J. Plant Nutr., 30, 537–547. doi:10.1080/01904160701209212
- Simmons CW, Claypool JT, Marshall MN, Jabusch LK, Reddy AP, Simmons BA, Singer SW, Stapleton JJ, van der Gheynst JS 2014: Characterization of bacterial communities in solarized soil amended with lignocellulosic organic matter. Appl. Soil Ecol., 73, 97–104. doi:10.1016/j.apsoil.2013.08.014
- Soil Survey Staff 1999a: Chapter 19 Ultisols. In Soil Taxonomy, A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed, pp. 750–754. United States Department of Agriculture Natural Resources Conservation Service, Washington.
- Soil Survey Staff 1999b: Chapter 15 Inceptisols. In Soil Taxonomy, A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed, pp. 518–524. United States Department of Agriculture Natural Resources Conservation Service, Washington.
- Stapleton JJ 2000: Soil solarization in various agricultural production systems. Crop. Prot., 19, 837–841. doi:10.1016/S0261-2194(00)00111-3
- Stapleton JJ, DeVay JE 1982: Effect of solarization on populations of selected soilborne microorganisms and growth of deciduous fruit tree seedlings. Phytopathology, 72, 323–326.
- Takahashi T, Sumida H, Nira R 2013: A new framework for study of irrigated paddy rice and upland crops rotation farming and its relation to soil and plant nutrition science. 1. Advances and perspectives in irrigated paddy rice and upland crops rotation farming. Jpn. J. Soil Sci. Plant Nutr., 84, 202–207. (in Japanese).
- Urashima Y 2013: Chloroform-fumigation method. In Experimental Methods for Soil Microorganisms, 3rd ed, Ed. Japanese Society of Soil Microbiology, pp. 87–90. Yokendo, Tokyo. (in Japanese).
- Vance ED, Brookes PC, Jenkinson DS 1987: An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem., 19, 703–707. doi:10.1016/0038-0717(87)90052-6
- Wada S, Fujino C, Toyota K, Okamoto T 2008: Effect of soil disinfection with 1,3-dichrolopropene, chloropicrin and solarization on cellulose-decomposing ability and nitrifying bacteria. Soil Microorganisms, 62, 21–31. (in Japanese with English summary).