Abstract
Cluster roots are structures formed by many plants adapted to phosphorus (P)-deficient soils. We investigated the combined influence of spatial heterogeneity in soil water and P distribution on the allocation of cluster root formation in white lupin (Lupinus albus L.). In this study, single plants were grown at a low or a high rate of water supply in containers filled with a P-poor sand to which either no P was added or which was fertilized homogeneously or heterogeneously. Furthermore, heterogeneous soil water distribution was established in half of the containers by using a finer instead of a coarser sand in a lateral third of the containers. Plant growth increased with water supply rate, but P fertilization had no influence on shoot biomass production. Although overall cluster root production decreased with increasing homogeneous P supply, localized P fertilization had no effect on cluster root allocation. However, cluster roots were preferentially allocated in the soil sections with lower water availability when overall water supply rate was low. The results suggest that overall cluster root production was a systemic response to initial plant P status, while cluster root growth was stimulated locally in drier patches when overall water supply was limiting plant growth.
INTRODUCTION
Phosphorus (P) is the most limiting nutrient for plant growth in many agricultural and natural ecosystems (Vance et al. Citation2003; Hu and Schmidhalter Citation2005). The solubility and mobility of P is generally low in soil, as compared to nitrogen (Hinsinger et al. Citation2011). Phosphorus in soil solution is the only source of P directly available to the roots and is rapidly absorbed by the roots. Resupply of P from the bulk soil to the rhizosphere is determined primarily by diffusive but also by mass flow. Limited availability of water is a further constraint on plant growth in many ecosystems (Vance et al. Citation2003; Hu and Schmidhalter Citation2005). As not only mass flow but also diffusion coefficients of solutes decrease with decreasing soil water content, dry soil conditions can also cause or aggravate P limitation (Bhadoria et al. Citation1991).
To increase P uptake from soil solution, roots often enhance P solubility in the rhizosphere by exuding organic acids such as citric acid (Gardner et al. Citation1983; Lamont Citation2003; Shane and Lambers Citation2005). Many plant species, particularly of the families Proteaceae and Fabaceae, but also others, have specialized in this strategy by developing so-called cluster roots for the acquisition of soil P fractions that are not available to “normal” roots (Lamont Citation2003). Cluster roots are bottlebrush-like structures of densely haired rootlets clustered in specific sections along the axis of growing roots (Purnell Citation1960; Shane and Lambers Citation2005; Lambers et al. Citation2006). As not only P but also metals are solubilized by organic acids, cluster roots may also be beneficial for the acquisition of metal micronutrients such as zinc (Zn) and copper (Cu). On the other hand, cluster roots are very expensive structures in terms of carbon costs, and therefore reduced production is usually found in soils with increased P availability (Neumann and Martinoia Citation2002; Lamont Citation2003; Shane et al. Citation2003; Shen et al. Citation2005; Shu et al. Citation2005, Citation2007; Lambers et al. Citation2006).
As for other soil resources, there is often remarkable heterogeneity in the spatial distribution of P and water in soil, even within the domain of a single root system (Jackson and Caldwell Citation1993; Farley and Fitter Citation1999). Preferential allocation of roots in soil patches or zones with increased P and water availability has been reported for many plant species (Kume et al. Citation2006; Ma et al. Citation2007; Weligama et al. Citation2007; Ma and Rengel Citation2008). Little is known about growth responses to heterogeneous soil water distribution for plants developing cluster roots, while contrasting findings have been reported for responses of cluster root formation to P heterogeneity. Some authors found that localized P supply stimulated cluster root production (Shen et al. Citation2005; Shu et al. Citation2007) in white lupin (Lupinus albus L.), while others found no stimulation (Shane et al. Citation2003; Shane and Lambers Citation2005). Shane et al. (Citation2003) even found that cluster root production was inhibited by locally increased P supply in white lupin. If the availability of soil P increases with water content, and cluster root growth responds to locally increased P supply, it can be expected that localized water supply will also affect cluster root production. In two studies it was found that cluster and non-cluster root growth occurred during the wet season in Hakea and Banksia species, anticipating shoot growth (Lamont Citation1976, Citation2003). In the Hakea species, cluster root growth was induced by irrigation during the dry season (Lamont Citation1976).
These results suggest that the localized supply of water has a stimulating effect on cluster root growth. However, effects of heterogeneities in soil water distribution on spatial cluster root allocation patterns apparently have not been studied so far. This is surprising as water is often considered the main driver of root allocation (Hodge Citation2010). Difficulties in establishing and maintaining sufficiently well-defined heterogeneities in soil water distribution over an adequate period of root growth may be a major reason for this lack of experimental data. In comparison to P, water is highly mobile in soil and will quite rapidly redistribute if it is added locally. In addition, the storage capacity of soil for water is generally small in comparison to the consumption of water by growing plants making frequent replenishment necessary during a root growth experiment. Recently, neutron radiography (NR) was shown to provide an elegant way to cope with these difficulties in climate chamber experiments (Menon et al. Citation2007; Oswald et al. Citation2008; Moradi et al. Citation2009; Carminati et al. Citation2010). This imaging technique is based on the absorption and scattering of neutrons by hydrogen as the main attenuation agent in soil. It is non-invasive and allows simultaneous imaging of roots and soil water distributions, provided that there is sufficient contrast in water content between soil and roots. A plant species highly suitable to investigate interactions between roots and soil water by means of NR is white lupin (Menon et al. Citation2007). It is also a model plant species for cluster root studies, having the advantage that it forms no mycorrhizal associations that could confound cluster root effects on P uptake (Shane and Lambers Citation2005; Lambers et al. Citation2006). Being a legume, it can form an association with nitrogen-fixing rhizobia and then does not depend on soil nitrogen status.
Taking advantage of the potential of NR imaging, the objective of this study was to investigate the effects of spatially heterogeneous soil water and P availability on the initiation of cluster root formation in white lupin under different water supply conditions. Plants grown in sand-filled containers with constructed heterogeneity in soil P and/or water availability were subjected to different combinations of the following experimental treatments: (i) heterogeneous versus homogeneous P application and no P addition, (ii) heterogeneous versus homogeneous soil water distribution; (iii) low versus high rate of irrigation. Root growth and soil water distribution were monitored over time by repeated NR imaging. At the end of the experiment, plants were harvested and the partitioning of normal roots and cluster roots between soil sections of different water and P availability was determined.
MATERIALS AND METHODS
Experimental substrates
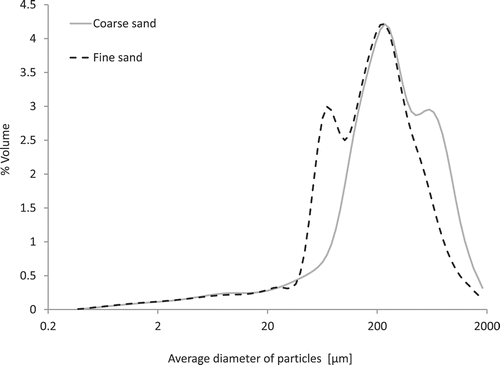
The two experimental substrates used in this study both contained 85% [weight/weight (w/w)] P-poor Pleistocene sediment extracted from the forefield of an open cast mine near Cottbus (Welzow Süd, Germany) and 15% (w/w) of either a coarse grained (300–900 µm) or of a fine grained (40–70 µm) silica sand. The particle size distributions of the two substrates, briefly referred to as fine sand and coarse sand in the following, are given in , and chemical properties are given in . The water retention curves of the two experimental substrates (see supplementary material S1) show that water storage capacity (the change in water content per unit change in soil water potential) was higher in the coarse sand than in the fine sand between 0 and 80 hPa and between 160 and 220 hPa soil water tension, but lower between 80 and 160 hPa and between 220 and 690 hPa. To establish the P treatments, three levels of P fertilization were applied as calcium-monophosphate (Ca(H2PO4)2.H2O) to the coarse sand (0, 10 and 30 mg P per kg sand) and 2 P levels to the fine sand (0 and 10 mg P per kg sand).
Table 1 Chemical properties of the two experimental substrates.
Container filling
The aluminum (Al) containers had an internal size of 27 × 27 × 1.2 cm (L × H × W). The inner sides of the walls were coated with Teflon to prevent Al toxicity. During filling the containers were laid down onto one side, while the wall of the opposite, upward-looking lateral side was removed. Then the substrate was filled in three vertical sections of 9 cm width each, using for each section the sand mixture assigned to it according to the respective treatment. No barriers separated the sections. Depending on their position when looking from above at the open container during filling, the sections will be referred to as left (LS), middle (MS) and right (RS) in the following. After filling was completed, the lateral wall was replaced, and the container was put back into the upright position. Care was taken to avoid pressing of the soil and to achieve a dry soil bulk density of approximately 1.7 g cm−3 in all containers. The net dry weight of the fillings varied between 1.33 and 1.45 kg.
Experimental setup
Three P treatments were established in combination with homogeneous and heterogeneous water distribution as well as high and low overall water supply rate. In the homogeneous P treatments, sand to which 10 mg P per kg sand (P hom) or no P (P no) had been added was used for all three sections. In the heterogeneous P treatments (P het), sand fertilized with 30 mg P per kg was filled into the right section and sand with no P fertilization into the other two sections, resulting in a total of 14 mg fertilized P per container, which is the same total as in the treatment with homogeneous P fertilization. For the treatments with heterogeneous water (W het) distribution, fine sand was filled into the left section and coarse sand into the other two sections. Only coarse sand was used for treatments with (horizontally) homogeneous water distribution (W hom). Treatments with high (+) and low water (–) supply were established as described below. The plant containers were arranged in four blocks in the climate chamber, with all treatments represented at randomly assigned positions in each block. A few plants showed atypical rooting patterns, e.g., due to tap root injury during transplanting, in the NR images. These plants were excluded from analysis.
Plant growth conditions
Seeds of white lupin var. Amiga were germinated on filter paper. Single seedlings were transplanted to the middle of each Al container (equidistant to the LS and RS sections) 2 days after germination (DAG), when the tap root was approximately 3 cm long. Except for the times when the containers were moved to the Paul Scherrer Institute (PSI) for NR imaging, the plants were kept in a climate chamber at 60% humidity, a 16/8 h day/night cycle and 21/16°C day/night temperature, respectively. During the day, the photon flux was 450 µmol m−2 s−1. Plants were watered from above with high or low water supply rates. In the treatments with low water supply, an initial water content of 16.3–17.6% was established on DAG 2 and subsequently no water was applied until DAG 12. From DAG 12 on, equal irrigation rates were supplied to each plant container with low water supply 2 to 3 times a week. The water supply rate between the irrigation dates ranged from 5–30 mL. In the treatments with high water supply, plants were irrigated 2–3 times a week with equal water supply rates ranging from 17–60 mL depending on irrigation date. The total amount of water supplied over 5 weeks of growth was 148 ± 5 mm in the treatments with high water supply, and 84 ± 4 mm in the treatments with low water supply.
Neutron radiography imaging
All containers were NR imaged at 12, 19 and 25 DAG. For this purpose, the containers were transported to PSI at Villigen, Switzerland, and carried back to ETH (Eidgenössische technische Hochschule) at Zürich on the day after imaging. In the treatments with high water supply, the removable walls on the side through which the containers had been filled were temporarily taken off on the day before NR imaging to increase NR contrast between roots and soil by enhancing evaporation. Neutron radiography imaging was performed at the thermal neutron facility NEUTRA at PSI in Villigen (Switzerland). The NR set-up has been described in detail by Moradi et al. (Citation2009). An Lithium-6 scintillator was used as a neutron detector and a charge-coupled device (CCD) - camera with an array of 1024 × 1024 pixel. The nominal resolution was 0.01765 cm per pixel.
Plant harvest and analysis
After 35 days of growth, shoots were harvested by clipping them at the soil surface. After weighing, drying and milling, the shoot samples were digested in 15 mL of 69% nitric acid (HNO3) in a heating block at 120°C. Phosphorus was analyzed in the experimental solutions by means of inductive coupled plasma - atomic emission spectroscopy (ICP-AES, Vista-MPX from Varian).
For the analysis of root parameters, the following root washing technique was applied: the front wall of the container was removed and a rectangular metal grid (27 × 27 cm, mesh size 4 mm) was placed onto the soil on the upward-looking open side of the Al container. The Al container was carefully flipped over, so that the open front site was facing downwards with the roots lying on the metal grid, which were then loosened from the soil and removed. Then the soil was thoroughly washed through the metal grid with a shower. The roots were transferred onto a Plexiglas screen from the grid with minimal displacement from their original position in the container. Finally, roots were scanned with a conventional scanner.
Image analysis
Neutron radiography images were processed using the method of Menon et al. (Citation2007) to correct for beam variation and root segmentation, and to produce a bi-color image of the root system. Both the corrected NR images and the root scans were analyzed for total root length. In addition, we determined the length of root sections carrying cluster roots in the bi-color images after tracing these sections in the images by hand. All root length measurements were performed separately for the left (LS), middle (MS) and right (RS) sections, using Winrhizo. The length of root axes carrying cluster roots will be referred to as cluster length in the following.
Assessment of water availability in heterogeneous water distribution treatments
For logistical reasons, the dynamics of short-term changes in soil moisture distribution in containers with homogeneous and heterogeneous sand packings were determined in a separate experiment. A description of this supplementary experiment and its results is given in the supplementary material section.
Data analysis
To quantify deviations from horizontal symmetry in root and cluster root allocation, we calculated the (normalized) precision of cluster root allocation relative to root length as the difference between RS and LS in cluster length per root length divided by the average ratio between cluster length and total root length of the respective container. A value significantly different from zero indicates preferential root allocation in either RS (> 0) or LS (< 0). Significance of precision of root allocation was tested with a Bonferroni-corrected 95% confidence interval for each treatment combination.
Treatment effects on shoot dry-weight, shoot P concentrations, root length and cluster length were analyzed using a linear mixed model. The fixed factors were P fertilization, water distribution and overall water supply rate, as well as their interactions. The block was taken as random factor. Significance of effects was tested using analysis of variance (ANOVA). The statistical software package R was used for all statistical analyses (Team RDC Citation2008).
RESULTS
Water availability and distribution
In the treatments with low water supply, water availability for root uptake rapidly dropped in the second week after germination and, after some further slight decrease, remained low until harvest, as indicated by the low water contents (Fig. S2). By contrast, water potentials never reached levels that would limit plant water uptake in the treatments with high overall water supply. Furthermore, aeration was sufficient at all times at both water supply rates, as the air-filled porosity was always larger than 10% of the bulk soil volume (see Supplementary material).
In the treatments with low overall water supply and heterogeneous water distribution, water availability was higher in the finer sand than in the coarser sand during the time when the lateral sections were colonized by roots, whereas at high overall water supply there was no obvious difference in water availability between the coarse and the fine sand.
Shoot growth and shoot P concentrations
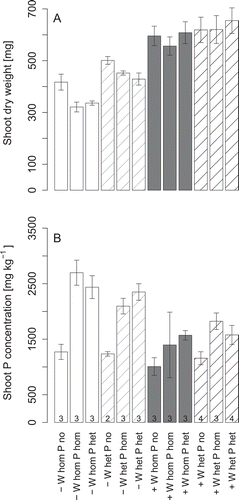
Water distribution and water supply rate had a significant influence on shoot biomass, whereas P had no effect (ANOVA, p < 0.05, Table S1; ). Shoot biomass was not only reduced by low water supply, but was also lower in the homogeneous than in the heterogeneous water distribution treatments. The latter effect appeared to be stronger at low than at high water supply, but the interaction effect of these two factors was not significant. The lack of a P effect on shoot growth was not related to a lack of plant response in P accumulation: P fertilization led to a significant increase in shoot P concentrations (ANOVA, p < 0.05, Table S2). Shoot P concentrations decreased inversely to shoot biomass with increasing water supply rate (ANOVA, p < 0.05). It did not differ between treatments with heterogeneous or homogeneous P supply. The P fertilizer effect on shoot P concentration was less pronounced at high than at low overall water supply rate (ANOVA, p < 0.05).
Figure 2 Shoot dry weights (A) and phosphorus (P) concentrations (B) of white lupin (Lupinus albus L.) grown at low (–) or high water supply (+), in homogeneous (W hom) or heterogeneous (W het) substrate with no additional P supply (P no), homogeneous P fertilization (P hom) or heterogeneous P fertilization (P het). Bars represent averages of all plants with herringbone root system. The numbers of replicates is given at the bottom of each bar in the lower graph. The error bars give the standard errors of the means.
Root growth
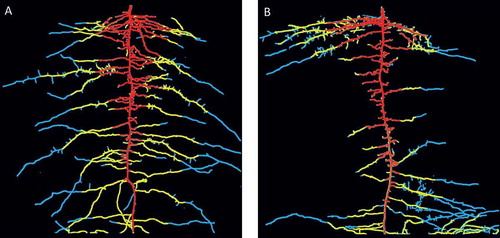
shows the typical herringbone root system of white lupin, consisting of a central tap root from which first-order laterals “with determined growth” and first-order laterals “with undetermined growth” are branching off. Laterals with determined growth ceased growth at 12 DAG. The lateral sections were colonized only by first-order laterals with undetermined growth (including cluster roots) and laterals of higher order. Only few first order laterals had reached the lateral sections by 12 DAG in the treatments with low water supply. At this time, the soil water content was low enough to limit plant growth.
Figure 3 Examples of root system development at low (A) and high (B) overall water supply. The colors indicate root growth from germination to 12 (red) days after germination (DAG), DAG 12 to 19 (yellow) and DAG 19 to 25 (blue). The two examples were not selected to represent average cases but to highlight the differences between the two water supply treatments. Quantitative analysis of root length distributions over depth is given in the supporting information (S1).
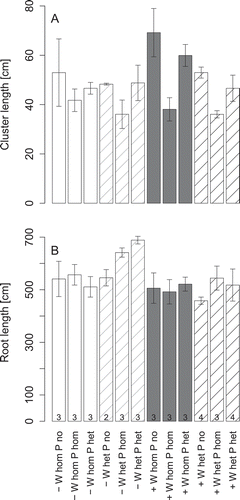
Only the rate of water supply had a significant effect on root length growth (ANOVA, p < 0.05, Table S3; ). In the treatments with low water supply, root length was on average slightly greater than in the treatments with high water supply. Also, the vertical allocation of root length growth differed between these treatments. At high water supply more root length was produced at the top and the bottom of the containers than at intermediate depths, while the opposite was the case at low overall water supply (; Fig. S4). The rate of root length increment was almost constant over time, while most of the cluster length was produced toward the end of the experiment between 25 and 35 DAG (Fig. S5). While P fertilization had no effect on root length production, cluster length showed a strong negative influence of P fertilization (ANOVA, p < 0.05, Table S3; ), which was more pronounced at high than low water supply, and stronger for homogeneous than heterogeneous P fertilization. At low water supply, the effect of heterogeneous P fertilization on cluster length (compared to no fertilization) was not significant. Heterogeneous water distribution had a slight negative influence on cluster length (ANOVA, p < 0.05), while there was no significant influence of the water supply rate ().
Figure 4 Cluster length (A) and root length (B) of white lupin (Lupinus albus L.) grown at low (–) or high water supply (+) in homogeneous (W hom) or heterogeneous (W het) substrate with no additional phosphorus (P) supply (P no), homogeneous P fertilization (P hom) or heterogeneous P fertilization (P het). The numbers at the bottom of the bars in the lower graph give the number of replicates. The error bars give the standard errors of the means.
Spatial allocation of root growth
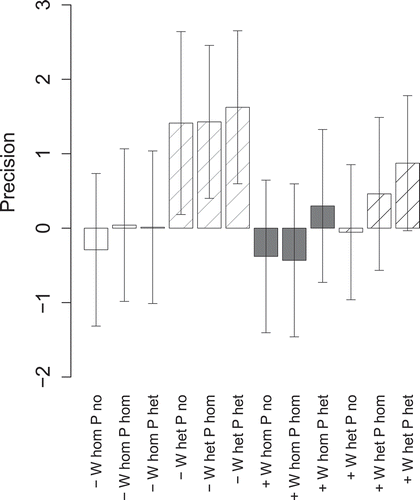
None of the treatments led to a significant imbalance in root length allocation to one side of the containers (ANOVA, p < 0.05, ). In contrast, there was a strong preference of cluster roots to grow in the right section (RS) of the containers, i.e. opposite to the side of increased water availability, in the treatments with heterogeneous water distribution and low water supply ( and ). This effect was associated with a reduced cluster length in the left section of the containers (LS), and it was independent of P application. A similar trend occurred at high water supply only in the treatment with heterogeneous P and water distribution.
Figure 5 Cluster length (A) and root length (B) of white lupin (Lupinus albus L.) in the left (LS, light gray) and right container section (RS, dark gray bar) at low (–) or high water supply (+) in homogeneous (W hom) or heterogeneous (W het) substrate with no additional phosphorus (P) supply (P no), homogeneous P fertilization (P hom) or heterogeneous P fertilization (P het). The error bars give the standard errors of the means.
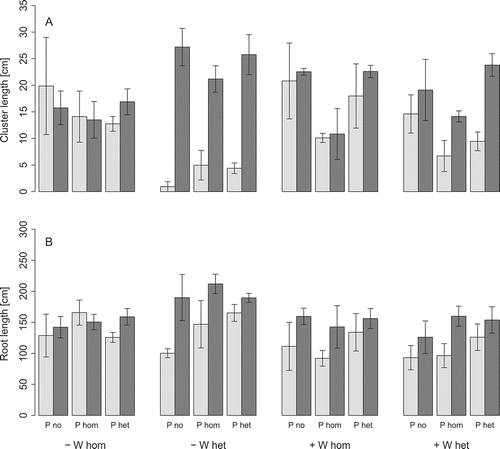
Figure 6 Precision of cluster length allocation (relative to total root length) of white lupin (Lupinus albus L.) at low (–) or high overall water supply (+) in homogeneous (W hom) or heterogeneous (W het) substrate with no additional phosphorus (P) supply (P no), homogeneous P fertilization (P hom) or heterogeneous P fertilization (P het). The precision of cluster root allocation relative to root length was calculated as the difference in cluster length per root length between the right (RS) and the left container sections (LS) divided by the average ratio between clustered and total length of the roots in the respective container. A value significantly different from zero indicates preferential root allocation in either RS (> 0) or LS (< 0). The error bars represent the Bonferroni-corrected 95% confidence interval of the mean.
DISCUSSION
Effects of water and P on shoot, root and cluster root growth
The results suggest that growth was not limited by P, even in the unfertilized soil at low water supply, whereas water availability was a limiting factor at the low supply rate. Limitation by water supply was reflected not only in the shoot biomass response to the treatments, but also in the increased allocation of growth to the root system with water shortage. Furthermore, the difference in root distribution profiles between treatments with high and low water supply showed a close correspondence to the different dynamics in water availability. As the soil started to dry out again from the surface in the treatments with low water supply while the wetting fronts had not yet reached the container bottoms, soil water was available for most of the time at intermediate depths in the treatments with low water supply. On the other hand, the much more rapid movement of the wetting fronts in the treatments with high water supply made it probably advantageous for roots to forage at the bottom of the containers for nutrients carried with the infiltrating water. The observed root growth patterns can thus be understood as an adaptation to optimize the acquisition of a limiting soil resource.
The lack of a P fertilization effect on shoot biomass production was in contrast to P-limited growth observed on the same soil in Lotus corniculatus L. (Felderer et al. Citation2013). It may have been due to a higher P acquisition efficiency of white lupin and/or to a larger P supply from the seeds. Until a functional root is formed, plants must rely on P stored in the seed. With growth, initial P stocks get progressively diluted and plants become more and more dependent on uptake of soil-P. Such a dilution effect is indicated by the observed inverse relationship between shoot P concentration and shoot biomass. To find out whether seed P storage overrode a potential limitation due to low soil P availability, a longer duration of our experiment would have been required. Although P had no effect on biomass production for the duration of our experiment, the increased P availability in the fertilized soil induced a decrease in cluster root production. As cluster roots are costly for a plant in terms of assimilates, it would seem advantageous for a plant to limit their production to a number that is really needed to secure sufficient P acquisition. Given that fewer cluster roots than in the unfertilized controls appeared to be sufficient to avoid sub-critical plant P levels in the treatments with homogeneous P fertilization, the question arises why cluster root production was not reduced also in the treatments with heterogeneous P fertilization, although shoot P concentrations were increased to similar levels as in the homogeneous P fertilization treatment. This finding means that cluster root growth was neither determined by the actual soil P level to which the roots developing cluster roots were exposed, nor by the shoot P status at that time. Nonetheless, the soil P level seemed to play some role. An explanation could come from the fact that no P was applied in the middle section of the containers with heterogeneous P fertilization. Thus, the plants were exposed to P-poor soil during the initial stage of root system development in these containers, like in the containers with no P application and in contrast to those with homogenous P fertilization. This suggests that cluster root growth at later stages responded to P supply from the soil during early root system development, “in preparation,” so to speak, for later stages of development when, with exhausted P supply from the seed, the plant would become fully dependent on P acquisition from the soil.
Effect of horizontal heterogeneities on root allocation patterns
The fact that the allocation of cluster root length was not affected by heterogeneous P application, while homogeneous P supply led to a reduction in overall cluster root production, provides further evidence that the P effect on root growth was a systemic response to plant P status and not the result of a local response to soil P availability. These results are in line with the hypothesis of Shane et al. (Citation2003) that cluster root formation depends primarily on shoot P status and much less on the exposure of cluster-forming roots to local soil P concentrations. While some studies showed that localized P availability can influence cluster root allocation, the effects that have been reported are very diverse. In a split-root pot experiment, (Shu et al. Citation2007) found that white lupin allocated more cluster roots in P-enriched soil than in unfertilized P-poor soil. Apart from a higher level of P enrichment (60–80 mg P kg−1) than in our experiment, the difference in responses may also be due to differences in the genotypes and experimental systems used. Studying cluster root responses of white lupin to heterogeneous P exposure in hydroponics supplying P exclusively to one half of a split root system and essentially no P at all to the other half, Shane et al. (Citation2003) found no difference in cluster root production between the two root system halves at low to moderate contrasts in P supply, and even a slight reduction in cluster root formation in root halves that were exposed to very high P levels compared to the roots exposed to nutrient solution without P. On the other hand, using a similar system, Shen et al. (Citation2005) found that cluster root production of white lupin was enhanced in P-containing solution at P concentrations that were even higher than in the study of Shane et al. (Citation2003). Differences of cluster root growth response to localized P supply between the hydroponic experimental system and experiments with real soil are probably co-determined by interactions between soil microbes and white lupin roots (Wasaki et al. Citation2005).
The lack of a cluster root allocation effect in the heterogeneous P treatment was in striking contrast to the strong stimulation of cluster root growth on the sides with lower water availability in the containers with heterogeneous soil texture and low water supply. This stimulation was associated with a reduction of cluster root formation to a density in cluster length that was lower than found in any lateral container section in any other treatment, including those with similar local P and water conditions. Thus, it cannot be explained as a local root response to water or associated P availability, but it appears that also a systemic effect played a role in the regulation of cluster root formation. The fact that total cluster length remained unaffected by the heterogeneity in soil texture and water availability indicates that the total amount of resource allocation to cluster root production was under systemic control, while its spatial allocation was governed to a large extent by local soil conditions.
Although in our experimental system heterogeneity in soil moisture availability was associated with heterogeneity in soil texture, the fact that the effect of this heterogeneity on cluster root allocation was limited to the treatments with low water supply indicates that it was directly or indirectly related to the resulting difference in soil moisture conditions and not just due to other soil texture effects. Otherwise, we should have expected some effect also in the treatments with high water supply, in which there was essentially no difference in soil water availability between the two substrates.
Two factors that showed clear differences between the two substrates at low water supply rate, but did not do so at high water supply, were water availability for plant uptake and mechanical resistance to root penetration. When we sampled and cleaned the roots, we found that with the lower moisture content the coarse sand was also firmer than the fine sand in these treatments. It has been found in some plants that mechanical inhibition of lower order root growth can promote branching off of higher order laterals (Goss Citation1977; Bingham and Bengough Citation2003). Considering that cluster roots are modified laterals, mechanical inhibition of first-order lateral root growth may by analogy have promoted cluster root growth more in the coarse than in the fine sand in the treatment with low water supply.
However, preferential cluster root allocation to the coarser textured soil at low water supply could also have been an active response to the difference in water availability. If this was the case, then of course the question arises: what is the advantage for a plant to boost cluster root growth under water-limited conditions in dry zones of soil, where the mobility of water, P and other nutrients is reduced, and not in the wetter zones? A plausible explanation relies on the assumption that horizontal water redistribution via the roots was involved, supplying the cluster roots in the dry zones with the moisture required to solubilize phosphate in the surrounding soil. The plausibility of such redistribution is based on the notion following from soil hydraulic principles that during nights, in the absence of substantial water extraction for transpiration, the gradient in total soil water potential drove soil water from the wetter to the drier parts of the soil and that this flow preferentially passed through the roots, as these are known to be much more conductive to water flow than dry soil. No metabolic energy is needed to drive this process. During the days, the water released during the night would have been taken up again in response to plant water demand for transpiration, explaining why the NR images did not reveal a clear net effect of soil moistening around the roots in the dry soil zones. Root-mediated transfer of moisture from wetter to drier soil zones allows roots to grow and mine for nutrients even in extremely dry patches of soil (Burgess et al. Citation2000; Lambers et al. Citation2006). Phosphorus solubilization by cluster roots is probably most effective if the roots can operate with their own, local water supply. The diffusion of solutes is strongly reduced in dry soil (Bhadoria et al. Citation1991; Jungk and Claassen Citation1997), and as exudates thus remain more concentrated for a longer time around roots in dry rather than in moist soil, more P can be solubilized and less solubilized P is lost from the rhizosphere into the bulk soil.
In conclusion, the main finding of this study is that under conditions of water limitation, cluster root growth of white lupin was stimulated in patches of reduced water availability resulting from textural heterogeneity, whereas it showed no response to heterogeneity in soil P. The only clear P effect was that total resource allocation to cluster root growth was related to the level of soil P to which the roots were exposed at the initial stages of growth. These results suggest that the control of cluster root growth depends on both systemic regulation and local soil conditions. Although P supply influenced the systemic response, water distribution – and not P distribution – was responsible for the spatial allocation pattern.
SUPPLEMENTARY MATERIAL
The supplementary material for this article is available online from: http://dx.doi.org/10.1080/00380768.2015.1081076
TSSP_A_1081076_Supplementary_material.pdf
Download PDF (700.1 KB)ACKNOWLEDGMENTS
This study was part of the German Transregional Collaborative Research Centre 38 (SFB/TRR 38). We gratefully acknowledge financial support by the German Research Foundation (DFG) and the Ministry of Science, Research and Culture of Brandenburg (MWFK, Potsdam).
References
- Bhadoria PBS, Kaselowsky J, Claassen N, Jungk A 1991: Phosphate diffusion- coefficient in soil as affected by bulk density and water-content. Z Pflanzen Bodenk, 154, 53–57. doi:10.1002/jpln.19911540111
- Bingham IJ, Bengough AG 2003: Morphological plasticity of wheat and barley roots in response to spatial variation in soil strength. Plant Soil, 250, 273–282. doi:10.1023/A:1022891519039
- Burgess SSO, Pate JS, Adams MA, Dawson TE 2000: Seasonal water acquisition and redistribution in the Australian woody Phreatophyte, Banksia prionotes. Ann. Bot., 85, 215–224. doi:10.1006/anbo.1999.1019
- Carminati A, Moradi AB, Vetterlein D, Vontobel P, Lehmann E, Weller U, Vogel H-J, Oswald SE 2010: Dynamics of soil water content in the rhizosphere. Plant Soil, 332, 163–176. doi:10.1007/s11104-010-0283-8
- FAL, RAC, FAW 1996a: Determination of Ca, Mg and Ca in a 1:10 Soil Water Suspension. Swiss Reference Methods of the Federal Agricultural Research Stations. Zurich, Swiss Federal Research Station FAL, RAC, FAW, Zurich, Switzerland.
- FAL, RAC, FAW 1996b: Ph in Water Suspension (1:2.5) and Ph in Cacl2 Suspension (1:2.5). Swiss Reference Methods of the Federal Agricultural Research Stations. Zurich, Swiss Federal Research Station FAL, RAC, FAW, Zurich, Switzerland.
- Farley RA, Fitter AH 1999: Temporal and spatial variation in soil resources in a deciduous woodland. J. Ecol., 87, 688–696. doi:10.1046/j.1365-2745.1999.00390.x
- Felderer B, Boldt-Burisch KM, Schneider BU, Hüttl RFJ, Schulin R 2013: Interaction between root growth of Lotus corniculatus and heterogeneous P distribution in soil during initial stages of ecosystem development. Biogeosciences, 10, 1737–1749. doi:10.5194/bg-10-1737-2013
- Gardner WK, Parbery DG, Barber DA, Swinden L 1983: The Acquisition of phosphorus by Lupinus albus L. 5. The diffusion of exudates away fro roots - a computer-simulation. Plant Soil, 72, 13–29. doi:10.1007/BF02185090
- Goss MJ 1977: Effect of mechanical impedance on root-growth in barley (Hordeum vulgare L.). 1. Effects on elongation and branching of seminal roots axes. J. Exp. Bot., 28, 96–111. doi:10.1093/jxb/28.1.96
- Hinsinger P, Brauman A, Devau N, Gérard F, Jourdan C, Laclau J-P, Le Cadre E, Jaillard B, Plassard C 2011: Acquisition of phosphorus and other poorly mobile nutrients by roots. Where do plant nutrition models fail? Plant Soil, 348, 29–61. doi:10.1007/s11104-011-0903-y
- Hodge A 2010: Roots: the acquisition of water and nutrients from the heterogeneous soil environment. In Progress in Botany 71, Eds. Luttge, U, Beyschlag, W, Budel, B, Francis, D, pp. 307–337. Springer-Verlag Berlin, Berlin.
- Hu YC, Schmidhalter U 2005: Drought and salinity: a comparison of their effects on mineral nutrition of plants. Z Pflanzen Bodenk, 168, 541–549. doi:10.1002/jpln.200420516
- Jackson RB, Caldwell MM 1993: Geostatistical patterns of soil heterogeneity around individual perennial plants. J. Ecol., 81, 683–692. doi:10.2307/2261666
- Jungk A, Claassen N 1997: Ion diffusion in the soil-root system. In Advances in Agronomy, Ed. Sparks, Elsevier, Vol. 61, pp. 53–110.
- Kume T, Sekiya N, Yano K 2006: Heterogeneity in spatial P-distribution and foraging capability by Zea mays: effects of patch size and barriers to restrict root proliferation within a patch. Ann. Bot., 98, 1271–1277. doi:10.1093/aob/mcl216
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ 2006: Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann. Bot., 98, 693–713. doi:10.1093/aob/mcl114
- Lamont B 1976: Effects of seasonality and waterlogging on root system of a number of Hakea species. Aust. J. Bot., 24, 691–702. doi:10.1071/BT9760691
- Lamont BB 2003: Structure, ecology and physiology of root clusters - a review. Plant Soil, 248, 1–19. doi:10.1023/A:1022314613217
- Ma Q, Rengel Z 2008: Phosphorus acquisition and wheat growth are influenced by shoot phosphorus status and soil phosphorus distribution in a split-root system. Z Pflanzen Bodenk, 171, 266–271. doi:10.1002/jpln.200700183
- Ma QF, Rengel Z, Bowden B 2007: Heterogeneous distribution of phosphorus and potassium in soil influences wheat growth and nutrient uptake. Plant Soil, 291, 301–309. doi:10.1007/s11104-007-9197-5
- Menon M, Robinson B, Oswald SE, Kaestner A, Abbaspour KC, Lehmann E, Schulin R 2007: Visualization of root growth in heterogeneously contaminated soil using neutron radiography. Eur. J. Soil Sci., 58, 802–810. doi:10.1111/ejs.2007.58.issue-3
- Moradi AB, Conesa HM, Robinson B, Lehmann E, Kuehne G, Kaestner A, Oswald S, Schulin R 2009: Neutron radiography as a tool for revealing root development in soil: capabilities and limitations. Plant Soil, 318, 243–255. doi:10.1007/s11104-008-9834-7
- Neumann G, Martinoia E 2002: Cluster roots - an underground adaptation for survival in extreme environments. Trends Plant Sci., 7, 162–167. doi:10.1016/S1360-1385(02)02241-0
- Olsen SR, Cole CV, Watanabe FS, Dean LA 1954: Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U S Dept. Agric. Circ., 939, 1–19.
- Oswald SE, Menon M, Carminati A, Vontobel P, Lehmann E, Schulin R 2008: Quantitative imaging of infiltration, root growth, and root water uptake via neutron radiography. Vadose Zone J., 7, 1035–1047. doi:10.2136/vzj2007.0156
- Purnell HM 1960: Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Aust. J. Bot., 8, 38–50. doi:10.1071/BT9600038
- Reed ST, Martens DC 1996: Copper and Zinc. In Methods of Soil Analysis Part 3 Chemical Methods, Ed. Sparks, DL. SSSA, Madison, Wisconsin, USA.
- Shane MW, De Vos M, De Roock S, Lambers H 2003: Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant Cell Environ., 26, 265–273. doi:10.1046/j.1365-3040.2003.00957.x
- Shane MW, Lambers H 2005: Cluster roots: a curiosity in context. Plant Soil, 274, 101–125. doi:10.1007/s11104-004-2725-7
- Shen J, Li H, Neumann G, Zhang F 2005: Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Sci., 168, 837–845. doi:10.1016/j.plantsci.2004.10.017
- Shu LZ, Shen JB, Rengel Z, Tang CX, Zhang FS 2005: Growth medium and phosphorus supply affect cluster root formation and citrate exudation by Lupinus albus grown in a sand/solution split-root system. Plant Soil, 276, 85–94. doi:10.1007/s11104-005-3105-7
- Shu LZ, Shen JB, Rengel Z, Tang CX, Zhang FS, Cawthray GR 2007: Formation of cluster roots and citrate exudation by Lupinus albus in response to localized application of different phosphorus sources. Plant Sci., 172, 1017–1024. doi:10.1016/j.plantsci.2007.02.006
- Team RDC 2008: R: A Language and Environment for Statistical Computing, Austria, http://cran.at.r-project.org.
- Vance CP, Uhde-Stone C, Allan DL 2003: Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol., 157, 423–447. doi:10.1046/j.1469-8137.2003.00695.x
- Wasaki J, Rothe A, Neumann G, Römheld V, Shinano T, Osaki M, Kandeler E 2005: Root exudation, phosphorus acquisition, and microbial diversity in the rhizosphere of White Lupine as affected by phosphorus supply and atmospheric carbon dioxide concentration. J. Environ. Qual., 34, 2157–2166. doi:10.2134/jeq2004.0423
- Weligama C, Tang C, Sale PWG, Conyers MK, Liu DL 2007: Localised Nitrate and Phosphate Application Enhances Root Proliferation by Wheat and Maximises Rhizosphere Alkalisation in Acid Subsoil. 2nd International Conference on Rhizosphere. Springer, Montpellier, France.