ABSTRACT
A pot experiment was conducted to examine how soil amendment with water-treatment residue (WTR) containing polysilicate-iron affected dissolved arsenic (As) in flooded soils and As uptake by rice plants (Oryza sativa L.). The WTR was applied at a rate of 0 (control), 5, 10 or 20 t ha−1. Simple linear regression analyses showed significant negative relationships between the concentrations of dissolved As in soil solution and WTR application rates at all sampling times, probably due to adsorption of As onto ferrihydrite in the WTR. Compared to As concentrations in rice plants grown on control soil, the concentrations in plants grown on WTR-treated soils decreased by 20.1–41.6% in straw (stems and leaves), 19.8–31.7% in husk and 18.6–21.0% in grain. The regression analyses demonstrated that the concentration and content of As in rice are negatively correlated with WTR application rate. Total As content was 16.5–32.0% lower in rice shoots grown on WTR-treated soils than on control soil. The percentage of each As species in grain decreased in the following order: As(III) » dimethylarsinic acid » As(V). The application of WTR did not change the As speciation in grain. Silicon contents in shoot significantly increased with application of WTR, indicating the potency of WTR as a silicate fertilizer. Taken together, our results indicate that WTR containing polysilicate-iron promises to be a practical amendment for stabilizing As and attenuating As uptake by rice plants.
1. Introduction
Arsenic (As) is a well-known carcinogen and a cause of several diseases. Rice consumption is one of the major routes of As exposure in Japan, accounting for about 6.3% of total As intake and 60% of total inorganic As intake in Japanese people (MAFF Citation2014; Oguri et al. Citation2014). In 2014, the Codex Alimentarius Commission determined a maximum permitted concentration for inorganic As in polished rice (Oryza sativa L.) grain of 0.2 mg kg−1. In some regions of Japan, flooded cultivation for 3 weeks before and after ear emergence has been recommended to reduce cadmium (Cd) concentrations to below nationally or internationally permitted values for unpolished or polished rice grain (0.4 mg kg−1). However, in contrast to Cd uptake, As uptake by rice plants is markedly increased by flooded cultivation. Therefore, practical techniques to simultaneously attenuate As and Cd in rice grain are urgently required.
One strategy under consideration is a combination of flooded cultivation to attenuate Cd and the application of an amendment(s) to attenuate As. Many As adsorbents have been tested for removing As from the water and stabilizing As in heavily polluted soils (Goh et al. Citation2008; Kumpiene et al. Citation2008). On the whole, iron (Fe)-type adsorbents such as Fe oxides (including hydroxides and oxyhydroxides) are considered promising for stabilizing As. There are many commercial Fe-type As adsorbents; however, the use of industrial waste or by-products containing Fe is a realistic option for reducing cost. Some trials using Fe-rich wastes or by-products, such as water-treatment residue (WTR) and steel shot, to stabilize As in polluted soils have been reported (Lidelöw et al. Citation2007; Nagar et al. Citation2013). However, only a few studies have focused on As stabilization in flooded soils or attenuation of As in rice by using Fe oxides or Fe-rich materials (Xie and Huang Citation1998; Ultra et al. Citation2009). A precipitate of polysilicate-iron (PSI), which is the major component of WTR from some water purification plants in Japan, significantly decreased dissolved As during long-term flooded incubation (Suda et al. Citation2015). That study indicates that the application of WTR containing PSI might stabilize As and subsequently decrease uptake of As by rice plants.
The objective of the present study was to assess WTR containing PSI as an amendment for stabilizing As in flooded soils and attenuating As uptake by rice plants.
2. Materials and methods
2.1. Pot experiment
A pot experiment was conducted in triplicate in a greenhouse under sunlight. The soil (Aquept) had a pH of 5.51; contained 54.3 g kg−1 total carbon, 3.99 g kg−1 total nitrogen, 20.8 g kg−1 oxalate-extractable Fe, 34.1 g kg−1 dithionite-citrate-extractable Fe and 366 g kg−1 clay; and contained 7.42 mg kg−1 As extracted with 1 mol L−1 hydrochloric acid. The soil was not “polluted soil (> 15 mg kg−1 1 mol L−1 hydrochloric acid-extractable As)” as defined by the Agricultural Land-Soil Pollution Prevention Law in Japan. We mixed 3 kg (oven-dry basis) of raw soil (< 8 mm) with a compound fertilizer (0.2 g nitrogen, 0.087 g phosphorus and 0.17 g potassium) and 0, 10, 20 or 40 g of WTR (air dried). The amounts of WTR were equivalent to application rates of 0, 5, 10 and 20 t ha−1. The WTR contained 43.1 g kg−1 total carbon, 2.67 g kg−1 total nitrogen, 401 g kg−1 Fe, 94.4 g kg−1 silicon (Si) and 30.8 mg kg−1 As. X-ray diffraction analysis detected ferrihydrite. The As content was lower than the maximum permitted value (50 mg kg−1) mandated by the Fertilizer Control Act in Japan. Soil without WTR was used as a control. The mixtures were put into tall-type Wagner pots (1/5000 a) with tap water and were then homogenized well (5 March 2014). Rice seedlings (Oryza sativa L. cv. Koshihikari) were then transplanted into the pots (three seedling per pot; three pots for each soil mixture) (10 March 2014). The rice plants were grown under flooded conditions during the whole growth period. After seeds matured, each rice plant was cut at the base of the stem (5 September 2014). The harvested plant was air dried and then separated into straw (stems and leaves), husk and grain. Unfilled grain was screened out with a 1.85-mm-mesh sieve.
On days 46, 61, 69, 79, 90 and 107 after transplanting, two soil solution samples were collected with a soil solution sampler (DIK-305A; Daiki Rika Kogyo, Saitama, Japan) connected with a vacuum tube (Vacutainer; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at a depth of 5–10 cm. Soil solution pH was measured using the solution collected first. Although the pH of the solution was measured as soon as possible, the oxidation of Fe(II) in the solution more or less affected the pH. Prior to the second collection, in order to inhibit the precipitation of Fe oxides in the collected solution, 6.3 mL of 100 g kg‒1 nitric acid was added into the vacuum tube by using a needle-syringe unit. The acidified solution was left until the produced Fe oxides completely disappeared. Then the solution was filtered and diluted. The concentration of As in the diluted solution was determined by inductively coupled plasma mass spectrometry (ICP-MS: Elan DRCe; Perkin Elmer, Waltham, MA, USA), and concentrations of Fe and Si were determined by inductively coupled plasma optical emission spectrometry (ICP-OES: Agilent 700 Series; Agilent Technologies, Santa Clara, CA, USA).
Soil redox potential (Eh) was measured at a depth of 7 cm, at 45, 62, 68, 79, 89 and 107 days after transplanting, with a platinum electrode and a silver-silver chloride reference electrode (Type 4400, DKK-TOA Corporation, Tokyo, Japan).
2.2. Plant analysis
The straw and husk were oven dried at 75°C, whereas the grain was dried at room temperature to avoid any change in As speciation. Samples were ground in a ball mill to a fine powder, and the powdered grain was stored at 4°C until use. All chemical analyses below were conducted on the powdered samples.
To determine total As concentration, 0.2 g of grain powder was digested with 5 mL of nitric acid overnight and then heated for 1 h at 105°C. After the digestion mixture cooled, 1 mL of 30% hydrogen peroxide solution was added, and the mixture was heated for 1 h at 105°C. The powdered straw and husk samples were similarly digested, but 1 g of each sample was used, and 1 mL of hydrofluoric acid was also added along with hydrogen peroxide. Although the digestion of silica in straw and husk was incomplete, the values were referred to as total As for simplicity. The digested solution was diluted and filtered, and As concentration was measured by ICP-MS (Elan DRCe, Perkin Elmer). To determine As speciation in grain, 1 g of powdered grain was extracted with 4 mL of 0.15 mol L−1 nitric acid at 100°C for 2 h. The extract was diluted and passed through a filter. Arsenic speciation in the solution was determined as described by Baba et al. (Citation2014) with little modification, using high-performance liquid chromatography/ICP-MS [column: Discovery HS F5, 15 cm × 4.6 mm, 3-μm particles, Sigma-Aldrich, St. Louis, MO, USA; eluent composition (isocratic elution): 1 g kg−1 formic acid, 30 g kg−1 methanol, 1.85 g kg−1 phosphoric acid; flow rate: 1 mL min−1; spectrometer: Nexion 300X, Perkin Elmer]. The averages of the sums of measured As species [As(III), As(V), monomethylarsonic acid, and dimethylarsinic acid (DMA)] were equivalent to 70–71% of the total As. The recovery meets the control limit mandated by the US Food and Drug Administration (FDA; 65–135%; Kubachka et al. Citation2012), although our method is not identical to the US FDA method.
Silicon in straw and husk was extracted with a mixture of 1.5 mol L−1 hydrofluoric acid and 0.6 mol L−1 hydrogen chloride for 2 h as described in Saito et al. (Citation2005). After filtration and dilution, the Si concentration in the solution was determined by ICP-OES (Agilent 700 Series, Agilent Technologies).
2.3. Data analysis
All values are for grain at 15% water content, and for oven-dried (75°C) straw and husk. Significant differences of percent decreases in dissolved As in soil solution were analyzed by two-way repeated measures analysis of variance (two-way ANOVA, factors: WTR application rate and sampling day) using R software (version 3.1.1). Simple linear regression analyses were also conducted to assess the relationships between WTR application rate and dissolved As, Fe and Si in soil solution, the concentration of As in each rice tissue, and contents of As and Si in total shoot.
3. Results and discussion
3.1. Soil Eh and soil solution pH
The values of soil Eh and soil solution pH were very close among all treatments, indicating that the addition of WTR did not affect soil Eh or soil solution pH at least between days 46 and 107. The averaged Eh value in all soils at day 45 was −206 mV, and it stabilized to between −240 and −257 mV at the other sampling times. The pH values fell within the range of 6.58–6.78 for all sampling times.
3.2. Concentrations of dissolved arsenic, iron and silicon in soil solution
In all soils, dissolved As tended to increase over time until reaching a maximum at day 90 (). One explanation for the increase in dissolved As observed in flooded soils is the reduction of As(V), the dominant species under aerobic conditions, to As(III) (Takahashi et al. Citation2004). As(III) has a lower sorption distribution coefficient between solid and solution (Kd) in soils; therefore, the reduction of As(V) to As(III) causes As solubilization (Takahashi et al. Citation2004). Moreover, dissolved As and Fe in flooded soils are closely correlated, indicating that reductive dissolution of As-bearing Fe oxides is an important cause of As solubilization (Marin et al. Citation1993; Yamaguchi et al. Citation2011). In this study, however, dissolved Fe increased to around 250 μg L−1 at day 61 or 69 and then decreased to around 200 μg L−1 (), and the changes in dissolved Fe over time did not correspond well to changes in dissolved As. The dissolved Fe was not a good indicator of the reductive dissolution of As-bearing Fe oxides, probably because dissolved Fe changed with certain factors such as re-sorption onto the solid phase, re-oxidation by oxygen from roots and air, and precipitation as sulfide.
Table 1. Time course of the concentrations of dissolved arsenic (As), iron (Fe) and silicon (Si) in soil solution during the cultivation period.
The application of WTR substantially decreased dissolved As relative to the control over the whole cultivation period (). Simple linear regression analyses showed significant negative linear relationships between dissolved As concentration and application rate of WTR at all sampling times (). Moreover, the percentage decrease increased as the rate of WTR application increased. The two-way ANOVA demonstrated the significant effect of WTR application rate on the percent decrease in dissolved As (). Most dissolved As in flooded soils is in the form of reduced species, namely As(III) (Masscheleyn et al. Citation1991). Although As(III) is more soluble than As(V) in soils (Takahashi et al. Citation2003), As(III) strongly adsorbs onto Fe oxides (Dixit and Hering Citation2003). Therefore, Fe oxides in WTR, namely ferrihydrite, probably re-fixed As onto their surface from the soil solution. Percentage decreases in dissolved As relative to control at all sampling time averaged 15.0 ± 5.5% at a WTR application rate of 5 t ha−1, 27.9 ± 2.6% at 10 t ha−1, and 43.1 ± 5.7% at 20 t ha−1 (). The two-way ANOVA revealed no significant effect of time on the percent decrease of dissolved As in soil solution (). Therefore, the efficiency of WTR application for removing As from soil solution was unlikely to change during cultivation period, although the Fe oxides in WTR likely have been partly dissolved by reduction.
Table 2. Simple linear regression analyses for dissolved arsenic (As), iron (Fe) and silicon (Si) (Y) against water-treatment residue (WTR) application rate (X).
Table 3. Time course of the decrease percentage of dissolved arsenic (As) concentration by water-treatment residue (WTR) application and two-way repeated measures analysis of variance (ANOVA)
Dissolved Si concentration tended to increase with time (). The WTR application significantly raised the dissolved Si concentration at day 46 and 61 (), although the effect disappeared in the later part of the cultivation period.
3.3. Plant growth
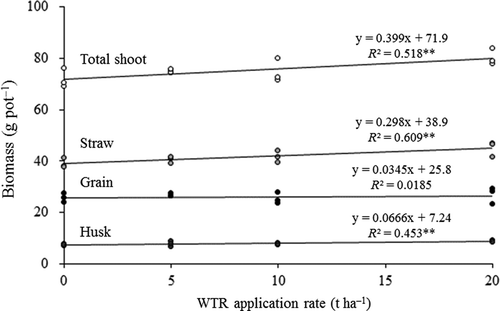
shows the effects of WTR application on biomasses of grain, husk, straw and total shoot. WTR application had no significant effect on grain biomass, whereas others are positively correlated with WTR application rate (r = 0.673 for husk, 0.780 for straw, 0.719 for total shoot; P < 0.01, n = 12). The increases of biomass are expected to be caused by improved nutrient conditions with increased nitrogen and Si, as pointed out by Horikawa et al. (Citation2007). WTR application had no significant positive or negative effect on plant height, number of stems or number of effective panicles (data not shown).
3.4. Arsenic in rice tissues
The concentrations of As in rice tissues (grain, husk and straw) are shown in . The concentrations of As in the grain of rice plants grown on soils with WTR application rates of 0 (control), 5, 10 and 20 t ha−1 were 0.941, 0.766, 0.753 and 0.743 mg kg−1, respectively. This means that WTR decreased As concentration by 18.6% relative to control at application rates of 5 t ha−1, 19.9% at 10 t ha−1, and 21.0% at 20 t ha−1 (calculated from ). Arsenic concentration in husk ranged from 3.95 to 5.78 mg kg−1. The percentage decreases with WTR application rates of 5, 10 and 20 t ha−1 were 19.8, 22.9 and 31.7%, respectively. The concentration in straw ranged from 15.3 to 26.2 mg kg−1. The applications of WTR at 5, 10 and 20 t ha−1 decreased As concentration by 20.1, 26.0 and 41.6%, respectively. The regression results showed that the concentration of As in each rice tissue is negatively correlated with the application rate of WTR (r = ‒0.567, P < 0.05 for grain; r = ‒0.776, P < 0.01 for husk; r = ‒0.858, P < 0.001 for straw; n = 12).
Table 4. Concentrations and contents of arsenic (As) and silicon (Si) in rice (Oryza sativa L.) tissues and simple linear regression analyses for As and Si in rice (Oryza sativa L.) tissues (Y) against water-treatment residue (WTR) application rate (X).
also shows total As content in rice shoots per pot. The percentage decreases with 5, 10 and 20 t ha−1 applications were 16.5, 20.9 and 32.0%, respectively. The total As content in shoot is negatively correlated with the WTR application rate (; r = ‒0.847, P < 0.001, n = 12).
These results strongly indicate the application of WTR attenuated As uptake by rice plant and reduced concentration in rice tissues via some mechanisms. The decrease in concentration of dissolved As in soil solution is one plausible cause for the decrease in As uptake by rice plants. However, Marin et al. (Citation1993) showed no correlation between dissolved As and As concentration in rice tissues. Iron plaque, the coating of Fe oxide on rice roots, may affect As uptake. Iron plaque is generally considered to be a buffer or barrier to As uptake, because of the plaque’s high affinity for As (Tripathi et al. Citation2014). Ultra et al. (Citation2009) reported that the application of hydrous ferric oxide increased Fe plaque on roots, resulting in a decrease in As uptake by rice plants. A similar phenomenon might also be the cause in the present study, due to the high content of Fe in the WTR. On the other hand, Bogdan and Schenk (Citation2008) reported a strong negative correlation (r = ‒0.9 to ‒0.99) between dissolved silicic acid in soil solution and As concentration in rice tissues. Because As(III) is transported by the same pathways as Si (Ma et al. Citation2008), dissolved Si inhibits the uptake of As in rice plants (Guo et al. Citation2005; Li et al. Citation2009). Therefore, the dissolved Si derived from WTR might inhibit As uptake by rice plants. In the present study, a significant increase in the dissolved Si by application of WTR was observed at day 46 and 61; however, it disappeared on and after day 69 (). The time course of dissolved Si might be a cause for the greater decrease in percentage of As in straw than that in grain. The increase of dissolved Si from the early to middle growth period was unlikely to attenuate As concentration in grain, because grain filling should occur in a later period, and leaf-to-grain re-translocation of As(III) is negligible (Carey et al. Citation2011). However, the increase in dissolved Si during the early to middle period might decrease As in straw and total uptake of As. Thus, the attenuation of As in rice plants could be explained by more than one mechanism, although the degree to which each contributes could not be quantified in the present study.
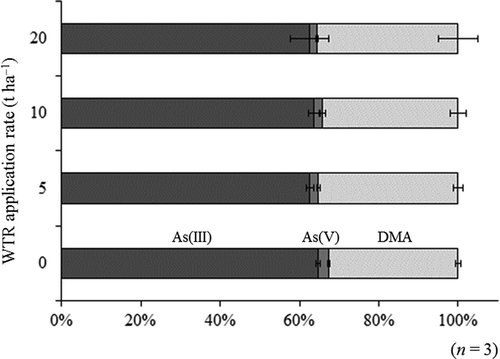
shows the proportion of each chemical species of As in grain. The averaged percentage for As species in all grain samples decreased in the following order: As(III) (63.4 ± 2.4%) > DMA (34.4 ± 2.7%) > As(V) (2.23 ± 0.45%) (the sum of detected As species is set at 100%). Li et al. (Citation2009) reported that silicate fertilizer (20 g silica gel per kg soil) increased the proportion of DMA in grain. In the present study, however, the proportion of DMA did not significantly change. Probably, even at 20 t ha−1, the application rates in the present study may be insufficient to induce an increase in the relative accumulation of DMA in grain.
Figure 2. Effects of water-treatment residue (WTR) application on arsenic (As) speciation in rice (Oryza sativa L.) grain. DMA denotes dimethylarsinic acid. The sum of As(III), As(V) and DMA was set as 100%. Monomethylarsonic acid was not detected (< 0.0006 mg As kg‒1).
Confirming the report by Horikawa et al. (Citation2007), we found that WTR is a potent silicate fertilizer. The total Si content in shoots linearly increased as WTR application rate increased (; r = 0.943, P < 0.001, n = 12). Silicon has many beneficial effects such as increasing the resistance of rice plants to disease and lodging (Guntzer et al. Citation2012). These benefits on rice growth are expected to compensate for the economic burden involved with using WTR to reduce As risk. Furthermore, because WTR is a by-product of water purification processes, the cost can be potentially much lower than commercial As adsorbents. Taken together, our results indicate that WTR containing PSI promises to be a practical soil amendment for stabilizing As and attenuating As uptake by rice plants.
Acknowledgments
The authors appreciate the work of Mr. Hiroshi Sasaki (Suido Kiko Kaisha, Ltd.) and Ken Nakamura (National Institute for Agro-Environmental Sciences) in sampling WTR containing PSI. We acknowledge Dr. Tadashi Ito and Dr. Shimpei Nakagawa (Akita Agricultural Experiment Station) for soil sampling. We deeply appreciate Dr. Tetsuhisa Miwa for helpful advice and discussions about statistical analysis (National Institute for Agro-Environmental Sciences). We also thank Dr. Tomohito Arao, Dr. Akira Kawasaki and Dr. Yuji Maejima (National Institute for Agro-Environmental Sciences) for their useful suggestions and helpful comments. This work was supported by a Grant-in-Aid from the Ministry of Agriculture, Forestry and Fisheries for the research project for improving food safety and animal health As-210.
References
- Baba K, Arao T, Yamaguchi N, Watanabe E, Eun H, Ishizaka M 2014: Chromatographic separation of arsenic species with pentafluorophenyl column and application to rice. J. Chromatogr. A, 1354, 109–116. doi:10.1016/j.chroma.2014.05.072
- Bogdan K, Schenk MK 2008: Arsenic in rice (Oryza sativa L.) related to dynamics of arsenic and silicic acid in paddy soils. Environ. Sci. Technol., 42, 7885–7890. doi:10.1021/es801194q
- Carey A-M, Norton GJ, Deacon C, et al. 2011: Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol., 192, 87–98. doi:10.1111/j.1469-8137.2011.03789.x
- Dixit S, Hering JG 2003: Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ. Sci. Technol., 37, 4182–4189. doi:10.1021/es030309t
- Goh K-H, Lim T-T, Dong Z 2008: Application of layered double hydroxides for removal of oxyanions: a review. Water Res., 42, 1343–1368. doi:10.1016/j.watres.2007.10.043
- Guntzer F, Keller C, Meunier J-D 2012: Benefits of plant silicon for crops: a review. Agron. Sustain. Dev., 32, 201–213. doi:10.1007/s13593-011-0039-8
- Guo W, Hou YL, Wang SG, Zhu YG 2005: Effect of silicate on the growth and arsenate uptake by rice (Oryza sativa L.) seedlings in solution culture. Plant Soil, 272, 173–181. doi:10.1007/s11104-004-4732-0
- Horikawa T, Ito T, Hasegawa T, Masuda Y, Arai T, Saigusa M 2007: Properties of polysilicate-iron (PSI) sludge from water purification plants: its effects on rice growth and methane emission. Jpn. J. Soil Sci. Plant Nutr., 78, 261–267 (in Japanese).
- Kubachka KM, Shockey NV, Hanley TA, Conklin SD, Heitkemper DT 2012: FDA Elemental analysis manual: Section 4.11: Arsenic speciation in rice and rice products using high performance liquid chromatography-inductively coupled plasma-mass spectrometric determination. Version 1.1. ( November 2012). http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm328363.htm (February, 2014)
- Kumpiene J, Lagerkvist A, Maurice C 2008: Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments-a review. Waste Manag., 28, 215–225. doi:10.1016/j.wasman.2006.12.012
- Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ 2009: Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ. Sci. Technol., 43, 3778–3783. doi:10.1021/es803643v
- Lidelöw S, Ragnvaldsson D, Leffler P, Tesfalidet S, Maurice C 2007: Field trials to assess the use of iron-bearing industrial by-products for stabilisation of chromated copper arsenate-contaminated soil. Sci. Total Environ., 387, 68–78. doi:10.1016/j.scitotenv.2007.07.018
- Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, Zhao F-J 2008: Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. U. S. A., 105, 9931–9935. doi:10.1073/pnas.0802361105
- MAFF (Ministry of Agriculture, Forestry and Fisheries of Japan) 2014: Arsenic intake from foodstuffs. http://www.maff.go.jp/j/syouan/nouan/kome/k_as/exposure.html. (January, 2015) (in Japanese)
- Marin A, Masscheleyn P, Patrick WH Jr 1993: Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil, 152, 245–253. doi:10.1007/BF00029094
- Masscheleyn P, Delaune RD, Patrick WH Jr 1991: Effect of redox potential and pH on arsenic speciation and solubility in a contaminated soil. Environ. Sci. Technol., 25, 1414–1419. doi:10.1021/es00020a008
- Nagar R, Sarkar D, Makris KC, Datta R 2013: Inorganic arsenic sorption by drinking-water treatment residual-amended sandy soil: effect of soil solution chemistry. Int. J. Environ. Sci. Technol., 10, 1–10. doi:10.1007/s13762-012-0106-y
- Oguri T, Yoshinaga J, Tao H, Nakazato T 2014: Inorganic arsenic in the Japanese diet: daily intake and source. Arch. Environ. Contam. Toxicol., 66, 100–112. doi:10.1007/s00244-013-9947-8
- Saito K, Yamamoto A, Sa T, Saigusa M 2005: Rapid, micro-methods to estimate plant silicon content by dilute hydrofluoric acid extraction and spectrometric molybdenum method. Soil Sci. Plant Nutr., 51, 29–36. doi:10.1111/j.1747-0765.2005.tb00003.x
- Suda A, Baba K, Yamaguchi N, Akahane I, Makino T 2015: The effects of soil amendments on arsenic concentrations in soil solutions after long-term flooded incubation. Soil Sci. Plant Nutr., 61, 592–602. 10.1080/00380768.2015.1006119
- Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K 2004: Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ. Sci. Technol., 38, 1038–1044. doi:10.1021/es034383n
- Takahashi Y, Ohtaku N, Mitsunobu S, Yuita K, Nomura M 2003: Determination of the As(III)/As(V) ratio in soil by X-ray absorption near-edge structure (XANES) and its application to the arsenic distribution between soil and water. Anal. Sci., 19, 891–896. doi:10.2116/analsci.19.891
- Tripathi RD, Tripathi P, Dwivedi S, Kumar A, Mishra A, Chauhan PS, Norton GJ, Nautiyal CS 2014: Roles for root iron plaque in sequestration and uptake of heavy metals and metalloids in aquatic and wetland plants. Metallomics, 6, 1789–1800. doi:10.1039/C4MT00111G
- Ultra VU, Nakayama A, Tanaka S, Kang Y, Sakurai K, Iwasaki K 2009: Potential for the alleviation of arsenic toxicity in paddy rice using amorphous iron-(hydr)oxide amendments. Soil Sci. Plant Nutr., 55, 160–169. doi:10.1111/j.1747-0765.2008.00341.x
- Xie ZM, Huang CY 1998: Control of arsenic toxicity in rice plants grown on an arsenic-polluted paddy soil. Commun. Soil Sci. Plant Anal., 29, 2471–2477. doi:10.1080/00103629809370125
- Yamaguchi N, Nakamura T, Dong D, Takahashi Y, Amachi S, Makino T 2011: Arsenic release from flooded paddy soils is influenced by speciation, Eh, pH, and iron dissolution. Chemosphere, 83, 925–932. doi:10.1016/j.chemosphere.2011.02.044