ABSTRACT
Our previous study revealed the high diversity of the major capsid gene (g23) of T4-type phages that existed in the paddy field soils in Northeast China. In this study, the phylogeny and genetic diversity of the g23 gene in the paddy floodwater samples collected from five sampling sites at three sampling times during the rice (Oryza sativa L.) growth season in Northeast China are reported. In total, 104 different g23 clones were isolated, among which 50% of the clones exhibited the highest identities with the clones retrieved in paddy soils and upland black soils. The remaining clones had the highest identities with lake origins. Phylogenetic analysis revealed that 43% of the g23 clones grouped into three novel subgroups which included the clones unique to paddy floodwater, and no g23 sequences obtained in paddy floodwater fell into the paddy soil groups II, III, IV, V, VI, VII and NPC-A. UniFrac analysis of g23 clone assemblages demonstrated that T4-type phage communities in paddy floodwater were changed spatially and temporally, and the communities were different from those in paddy soils. Further comparison of the g23 clone assemblages from different environments demonstrated that T4-type phages were biogeographically distributed, and the distribution was both affected by geographical separation and ecological processes across the biomes.
1. Introduction
Bacteriophages (phages) are viruses that infect protokaryotic microorganisms of bacteria and archaea. Although phages are ubiquitous, with an estimated population size of > 1030 in the ocean alone (Breitbart and Rohwer Citation2005; Suttle Citation2005), information on their genetic diversity and community distribution is still limited compared with that of their hosts (Weinbauer and Rassoulzadegan Citation2004; Filée et al. Citation2005; Paul and Sullivan Citation2005).
Tailed phages consist of the three families Myoviridae, Siphoviridae and Podoviridae (Fauquet et al. Citation2005). Among these families, the genetic diversity of T4-type phages of the family Myoviridae was widely studied using culture-dependent and culture-independent methods. For example, isolated T4-type phages were divided into T-evens, PseudoT-evens, SchizoT-evens and ExoT-evens base on the comparison of the sequences of the tail and capsid genes of g18, g19 and g23 (Desplats and Krisch Citation2003); By using cultured-independent molecular methods targeting the conserved g23 gene which encodes the major capsid protein, many regional or specific groups of T4-type phages were observed in marine waters (Filée et al. Citation2005), lake waters (López-Bueno et al. Citation2009; Butina et al. Citation2010, Citation2013; Huang et al. Citation2011), wetlands (Zheng et al. Citation2013), arctic glaciers (Bellas and Anesio Citation2013), paddy field soils (Jia et al. Citation2007; Fujii et al. Citation2008; Wang et al. Citation2009a, Citation2009b, Citation2009c; Liu et al. Citation2012) and upland black soils (Liu et al. Citation2011; Wang et al. Citation2011).
Rice (Oryza sativa L.) fields are a unique agro-ecosystem characterized by several agricultural practices, and are maintained under flooded conditions during most periods of rice cultivation (Okabe et al. Citation2000; Nakayama et al. Citation2007). Flooding the field results in several specific habitats for microbiota where microorganisms promote different catabolic processes compared with paddy field soils and/or other natural aquatic environments (Nakayama et al. Citation2007; Kimura et al. Citation2008). Jia et al. (Citation2007) first reported that the distribution of g23 genes of T4-type phages in a Japanese paddy field was quite distinctly different from those in marine environments. Subsequently, Fujii et al. (Citation2008) and Wang et al. (Citation2009a, Citation2009b) grouped the g23 clones obtained from paddy field soils of Japan and Northeast (NE) China into five and nine paddy groups, respectively. Recently, we discovered that T4-type phages were highly diversified in paddy field soils of NE China during the rice growth season, and approximately 50% of the clones formed seven new paddy clusters (NPC-A~-G) that contained clones exclusively from Chinese paddy field soils (Liu et al. Citation2012). However, in that paper, we did not report the distribution of g23 genes in paddy floodwaters, given that previous studies indicated that the distributions of the g23 sequences in Japanese paddy floodwaters were phylogenetically distant from and more diversified than those sequences isolated from the corresponding soils in the same sampling sites (Nakayama et al. Citation2009b). We speculated that some new findings would be discovered through surveying the g23 genes in paddy floodwaters of NE China. Therefore, in this study, upon surveying the g23 sequences in five paddy floodwaters in NE China, we aimed (1) to isolate the novel g23 sequences in paddy floodwaters; (2) to assess the phylogenetic distribution of the obtained g23 sequences compared with those from paddy soils reported previously; and (3) to compare the T4-type phage community distribution in paddy fields with those in other environments.
2. Materials and methods
2.1 Paddy floodwater sampling
The paddy floodwater samples were collected at the same time with the paddy soil samples as reported previously (Liu et al. Citation2012). Briefly, we collected paddy floodwater and soil samples synchronously from five paddy fields in NE China during the rice growth season on 28 May, 25 June and 3 August in 2012. Approximately 1 L of the floodwater sample was collected from several sites in the middle part of each field. The water samples were kept in a container with an ice bag and transported to the laboratory for DNA extraction. The details of sample sites, soil properties, and the numbers of g23 clones isolated from each individual floodwater sample are shown in the Supplementary material, Table S1.
2.2 Viral particle collection
After the water samples arrived at the laboratory, the waters were centrifuged immediately at 8000 g for 30 min at 4°C to remove soil particles, plankton and partial bacteria. Next, the supernatants were filtrated through 0.4-μm and 0.2-μm cellulose filters in sequence to completely remove the bacteria. Virus-size particles were collected on 0.02-μm filter membrane (Nuclepore Track-Etch Membrane, Whatman, UK) using vacuum filtration. The filters were then put into a 2-mL centrifuge tube with 700 μL of 10 mM Tris-HCl buffer (pH 7.5).
2.3 DNA extraction and polymerase chain reaction amplification
The viral DNA was extracted from the filters according the methods described previously (Wang et al. Citation2010). The extracted DNA was dissolved in 50 μL of TE (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) buffer and stored at −20°C until use. The capsid assembly protein gene g23 was amplified with the primers MZIA1bis (5ʹ-GAT ATT TGI GGI GTT CAG CCI ATG A-3ʹ) and MZIA6 (5ʹ-CGC GGT TGA TTT CCA GCA TGA TTT C-3ʹ) (Filée et al. Citation2005) according to the methods described previously (Liu et al. Citation2012; Zheng et al. Citation2013). The polymerase chain reaction (PCR) products were later checked on a 2% agarose gel with 1 × TAE buffer (40 mM Tris-HCl, 40 mM Acetate, 1.0 mM EDTA) and ethidium bromide (0.5 µg mL−1) under ultraviolet light.
2.4 Cloning, denaturing gradient gel electrophoresis and sequencing
As the g23 PCR products are widely variable in length (Nakayama et al. Citation2009b; Wang et al. Citation2009a; Liu et al. Citation2012), the PCR products between 300 and 700 bp in length were cut from a 2% agarose gel and purified with the QIAquick Gel Extraction kit (QIAGEN, Crawley, UK). The purified DNA was cloned into the pMD18-T plasmid vector (TaKaRa, Dalian, China) and transformed into the competent cells of Escherichia coli DH5α according to the manufacturer’s instructions. About 50 positive clones from each transformation were chosen from white colonies and checked for the correct insertion of the g23 fragment by PCR with the primers MZIA1bis and MZIA6.
To efficiently isolate the different g23 clone, polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) was conducted to separate the g23 sequences according to the method of Fujii et al. (Citation2008). Briefly, 5 μL of the PCR product of each positive clone were used for DGGE according to the previously described method (Wang et al. Citation2009a, Citation2009b; Liu et al. Citation2012). The bands with the same mobility on a DGGE gel were considered the same clones. The plasmid DNA from the different clones was harvested from an overnight culture of E. coli DH5a and submitted to a commercial company (BGI, Shenzhen, China) for sequencing.
2.5 Sequence analysis
The clone sequences were translated to deduced amino acid sequences using the EMBOSS Transeq program at European Bioinformatics Institute (EBI) website (http://www.ebi.ac.uk/). The closest relatives of the respective g23 clones were examined using the Basic Local Alignment Search Tool (BLAST) search program within the National Center for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/) at the amino acid level. The identities of the deduced amino acid sequences among the clones obtained in this study were analyzed using the ClustalW program available at the DNA Data Bank of Japan (DDBJ) website (http://www.ddbj.nig.ac.jp/).
The phylogenetic distribution of the g23 clones obtained in this study was first compared with the clones obtained from paddy field soils in China and Japan (Fujii et al. Citation2008; Wang et al. Citation2009a, Citation2009b; Liu et al. Citation2012). Then the g23 assemblages, which indicate the communities of the T4-type phages, were compared with the UniFrac statistical analysis tool available at http://bmf.colorado.edu/unifrac/ within this study and among the different environments across the global scale (Lozupone and Knight Citation2005), including marine waters (Filée et al. Citation2005), lake waters (López-Bueno et al. Citation2009; Butina et al. Citation2010, Citation2013; Huang et al. Citation2011), wetlands (Zheng et al. Citation2013), arctic glaciers (Bellas and Anesio Citation2013), paddy field soils and waters (Jia et al. Citation2007; Fujii et al. Citation2008; Nakayama et al. Citation2009b; Wang et al. Citation2009a, Citation2009b, Citation2009c; Liu et al. Citation2012), and upland black soils (Liu et al. Citation2011; Wang et al. Citation2011). The amino acid sequences were aligned using ClustalX 1.81 (Thompson et al. Citation1997), and the neighbor-joining tree was constructed by Molecular Evolutionary Genetic Analysis software (MEGA 4.0) with 1000-fold bootstrap support (Tamura et al. Citation2007). Furthermore, to view the relationships of the T4-type phage communities in different environments, the g23 sequences obtained from the different biomes were defined by the number of operational taxonomic units (OTUs) at sequence divergences of 3% (Goldsmith et al. Citation2015), and the cluster analysis of the T4-type phage community (OTU data) based on the non-metric multi-dimensional scaling (NMDS) of a Bray-Curtis distance matrix was performed using the ‘picante’ and ‘vegan’ packages in the R environment (R Development Core Team Citation2006).
The DNA sequences of the g23 genes in this study have been deposited in the DDBJ under accession numbers from KR066798 to KR066901.
3. Results
3.1 PCR amplification and the closest relatives of g23 clones
In total, we obtained 104 clones of the g23 sequences in this study. The lengths of the deduced amino acid sequences of the g23 fragments (between primers) ranged from 117 to 148, and the identities within the g23 sequences ranged from 37 to 100% at the amino acid level, which indicated the high diversity of the g23 clones obtained in this study (Table S2).
The BLAST search at the amino acid level revealed that 52 clones showed the highest identities from 73 to 100% with the clones retrieved from the paddy field soils in NE China and Japan, and the upland black soils in NE China (Wang et al. Citation2009a, Citation2009b; Liu et al. Citation2011, Citation2012) (Table S2). Among these, four clones, PW-AC-Jun25-9, −13, PW-DA-Aug03-5 and PW-QF-May28-7, were 100% identical to the upland black soil clones BLSoil-HB-10 (BAJ61636), BLSoil-DH-15 (BAJ61623), BLSoil-DH-11 (BAJ61619) and BLSoil-N2-2 (BAJ61685), respectively (Liu et al. Citation2011). Six clones, PW-DA-May28-6, −11, PW-QF-Jun25-1 and PW-SH-May28-1, −5 and −6, were 100% identical to the paddy soil clones PS-AC-Aug03-4 (BAK52101), PS-NA-May28-1 (BAK52054) and PS-SH-May28-5 (BAK52125) obtained from NE China (Liu et al. Citation2012). The remaining 52 clones showed the highest identities from 53 to 94% with the clones obtained from the lakes of Donghu, Kotokel, Antarctic and Proglacial and wetland environments (López-Bueno et al. Citation2009; Butina et al. Citation2010, Citation2013; Huang et al. Citation2011; Zheng et al. Citation2013). Among these clones, 41 had the highest similarity to the clones obtained from lake Donghu in China.
3.2 Phylogenetic analysis of the g23 clones in paddy fields
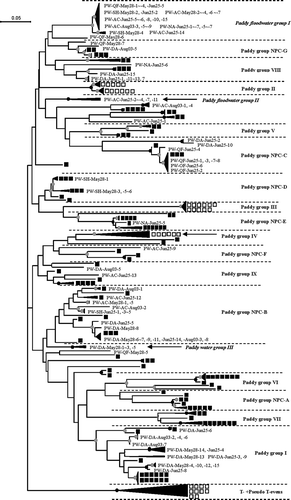
A neighbor-joining tree was constructed with the g23 clones obtained in this study, 121 representative clones obtained from paddy field soils in China (Wang et al. Citation2009b; Liu et al. Citation2012) and 38 representative clones from paddy field soils in Japan (Fujii et al. Citation2008; Wang et al. Citation2009a). The phylogenetic tree showed that none of the clones belonged to the T- and PseudoT-evens groups which contained 10 clones from Japanese paddy soils. Although the majority of the clones fell into formerly known paddy groups, three clusters formed with 36, five and four clones exclusively from this study; thus, these three clusters were denominated as new paddy floodwater groups I, II and III, respectively ().
Figure 1. Neighbor-joining phylogenetic tree of the g23 sequences showing the relationships of g23 amino acid sequences obtained in this study with those obtained from paddy field soils in Japan (Fujii et al. Citation2008; Wang et al. Citation2009a) and Northeast China (Wang et al. Citation2009b; Liu et al. Citation2012). The black and gray circles indicate internal nodes with at least 90% and 50% bootstrap support, respectively. The black and white squares represent clones obtained from Northeast China and Japan, respectively. The new designated paddy floodwater groups are shown in italic letters.
3.3 UniFrac analysis of g23 clone assemblages
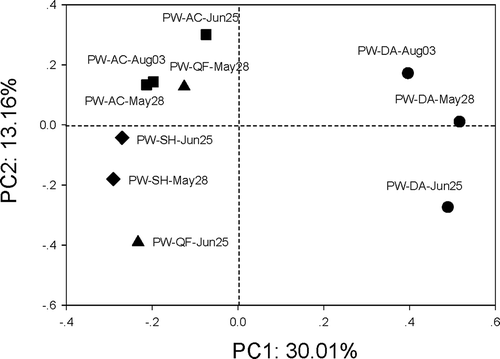
UniFrac analysis of the g23 assemblages was conducted to evaluate whether the T4-type phage communities changed with location or sampling time in this study (Lozupone and Knight Citation2005). Considering there was only one sample from the Ning-An (NA)-generated g23 clones, the location NA was excluded from the UniFrac analysis. The plot of the principal coordinate analysis (PCoA) clearly showed that the T4-type phage communities significantly changed with sampling location and sampling time (p < 0.01/0.05; Table S3; ). However, several samples had high p-test values; for example, PW-SH-May was similar to PW-AC-Aug (p = 0.08). Additionally, PW-QF-May had a p-test value of 0.5 and 0.32 with PW-AC-Aug and PW-AC-May, respectively, and PW-AC-May had values of 0.47 and 0.07 with PW-AC-Aug and PW-AC-Jun, respectively (Table S3).
Figure 2. Principal coordinate (PC) analysis of g23 assemblages obtained in this study by the UniFrac method. Samples PW-AC, -QF, -SH and -DA represent the paddy floodwater samples obtained from A-Cheng, Qian-Feng, Sui-Hua and Da-An, respectively. The percentages in the axis labels represent the percentages of variation explained by the principal coordinates.
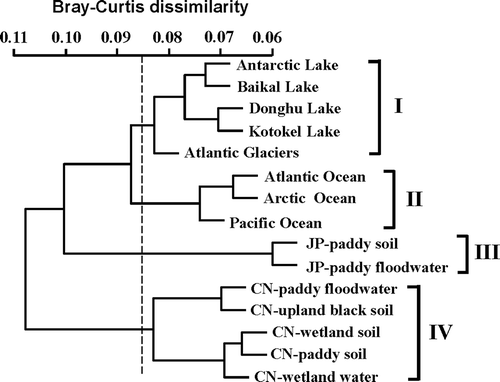
The g23 sequence assemblages from paddy floodwater in this study were further compared with the clones retrieved from other environments by UniFrac analysis (). The PCoA plot showed that the spots of paddy floodwaters in this study were located near the paddy field soils, upland soils and wetland soils in NE China in the first and fourth quadrants. The g23 assemblages from lake waters of Donghu, Baikal, Antarctic and Kotokel, and from wetland freshwaters as well as Arctic glaciers, were located relatively closely in the third quadrant. Interestingly, the spots of the Japanese paddy floodwaters and two sampling sites of Japanese paddy soils were located close to the spots of marine waters in the second quadrant, and the other two spots of the Japanese paddy soils were located close to the samples obtained in NE China. In addition, the g23 assemblages of the wetland environment were more divergent from the spots of fresh water near the assemblages of lake water, and the wetland soil spots close to the paddy field water or soil samples obtained in NE China.
Figure 3. Principal coordinate (PC) analysis of g23 assemblages obtained in this study compared with those obtained from marine waters (Filée et al. Citation2005), lake waters (López-Bueno et al. Citation2009; Butina et al. Citation2010, Citation2013; Huang et al. Citation2011), wetlands (Zheng et al. Citation2013), arctic glaciers (Bellas and Anesio Citation2013), paddy field soils (Jia et al. Citation2007; Fujii et al. Citation2008; Wang et al. Citation2009a, Citation2009b; Liu et al. Citation2012) and upland black soils (Liu et al. Citation2011) by the UniFrac method.
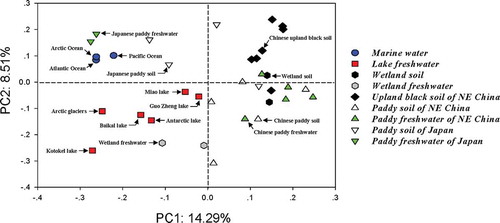
3.4 NMDS analysis of T4-type phage community
The OTUs at sequence divergences of 3% were defined for comparing the difference or similarity of the T4-type phage communities in different biomes. Based on the NMDS dissimilarity matrix, the T4-type phage communities in different biomes were roughly clustered into four groups, which corresponded to different environments and sampling locations (). Groups I and II contained samples from lake fresh waters and marine waters, respectively. Group III contained paddy soil and water samples from Japan, and group IV included the samples collected from NE China, irrespective of being sampled from paddy field soils and waters, wetland soils and waters, or upland black soils.
4. Discussion
4.1 Diverse origins and movement of T4-type phages between biomes
Comparison of amino acid sequences of g23 clones from paddy floodwater showed that about 22% of clones (22 out of 104) had an identity of less than 85% with those in currently known databases (Table S2), which suggests that novel g23 sequences of T4-type phages exist in paddy floodwaters in NE China. Although many clones grouped and overlapped with known g23 sequences from paddy soils (), approximately 43% (45 out 104) of the g23 clones from paddy floodwater grouped into three novel paddy floodwater groups, and none of the g23 clones from paddy floodwaters fell into paddy groups II, III, IV, V, VI, VII and NPC-A, which contain g23 clones exclusively from paddy soils (), suggesting that the distribution patterns of the g23 sequences in the paddy floodwater were different from those in paddy field soils.
Previous studies revealed that several g23 sequences collected from Japanese paddy fields far away (Cahyani et al. Citation2009; Nakayama et al. Citation2009a, Citation2009b) and even between rice field soils in NE China and Japan were identical to each other (Wang et al. Citation2009a, Citation2009b). In this study, several clones in the paddy floodwater were identical to the g23 sequences detected not only in the paddy field soils but also in the remote upland black soils in NE China. Furthermore, Bellas and Anesio (Citation2013) reported that a g23 clone of VB1 collected from cryoconite hole sediment was identical to marine clone KF86. To date, the detection of identical g23 clones across biomes should not be unexpected due to virus diversity, and further proves that horizontal gene transfer occurs among phage communities in distant/different environments (Short and Suttle Citation2005). Although phages have broad host ranges and the ability to infect hosts from different biomes (Sano et al. Citation2004; Bonilla-Findji et al. Citation2009), the possibility that an identical virus infects a diverse range of hosts or that phages prefer similar or identical hosts between biomes still remains open for future study.
4.2 Dynamics of T4-type phage communities
Spatial or/and temporal changes of cyanophage communities were reported in coastal waters (Marston and Sallee Citation2003), in the Chesapeake Bay (Wommack et al. Citation1999), in three inlets of British Columbia, Canada (Frederickson et al. Citation2003), in Lake Bourget (Dorigo et al. Citation2004) and also in paddy floodwaters (Wang et al. Citation2010). Filée et al. (Citation2005) indicated a heterogeneous geographic distribution of T4-type phages in marine environments, similar to that of the cyanophages. In addition, the distribution of the g23 clones was different between Northern and Southern Baikal Lake (Butina et al. Citation2010), and between lakes Annecy and Bourget (Zhong and Jacquet Citation2014). Those findings suggest that the spatial changes of T4-type phage communities exist in aquatic environments. In paddy field soils, several reports indicate that the sampling sites and sampling date are not the major factors in determining T4-type bacteriophage distribution (Fujii et al. Citation2008; Wang et al. Citation2009b; Fujihara et al. Citation2010), even with different soil textures (Wang et al. Citation2009c); however, those studies only focused on the distribution of g23 sequences at the group level and did not consider the g23 assemblage at the community level. UniFrac is a powerful tool for comparing the differences between microbial communities based on phylogenetic information (Lozupone and Knight Citation2005). The PCoA plot of g23 assemblages observed in this study clearly shows that T4-type phage communities in paddy floodwater in NE China varied with sampling location and sampling time (Table S3; ); this finding suggests that the phage community in paddy floodwater may sort by spatial and temporal changes. The change of the phage community is recognized to correlate with their corresponding bacterial hosts (Jessup and Forde Citation2008; Sandaa Citation2008). Although the bacterial hosts of the T4-type phages cannot be examined using current techniques, several reports indicated that the cyanobacterial communities changed seasonally and spatially in the paddy fields (Song et al. Citation2005; Kim and Lee Citation2006; Asari et al. Citation2008). Thus, an understanding of the relationship between the T4-type phage and their hosts warrants further study in the paddy floodwater.
4.3 Driving factors of g23 distribution in the biosphere
The T4-type phage communities were predominantly affected by geographic separation because the g23 assemblages varied with different environments (Liu et al. Citation2012); however, other studies indicated that the T4-type phage communities were more similar in the same ecological environments, although those sites were geographically separated (Bellas and Anesio Citation2013; Zheng et al. Citation2013). To date, the primary factors in determining the distribution patterns of viral communities in aquatic and other ecosystems are still unclear (Butina et al. Citation2013).
In this study, we utilized UniFrac and NMDS methods to analyze g23 assemblages in different environments ( and ). The PCoA plot showed that the spots of g23 assemblages from NE China were located relatively closely irrespectively of where they were collected, including paddy fields, wetlands and upland soils, which are roughly far away from spots collected from marine waters, lake fresh waters and paddy soils and waters of Japan. This finding strongly suggests that geographical separation is one of the major factors in determining the T4-type phage community. It should be pointed out that, although three marine water samples and five lake fresh water samples were collected from a very large scale globally, those samples were located relatively closely according to their sampling environments. Furthermore, even the paddy floodwater samples were collected synchronously with soil samples at the same fields in NE China, and the position spots of g23 assemblages in floodwaters are generally located in different areas from those in soils. These findings suggest that ecological process is another important factor in determining the T4-type phage community. Therefore, T4-type phage community compositions might resemble each other in similar environments across the biosphere.
5. Conclusions
This study indicated that g23 genes in paddy floodwater were highly diverse and their distribution was different from those in paddy soils. We observed three novel paddy floodwater groups containing g23 clones exclusively in this study. None of the g23 clones from paddy floodwaters fell into paddy soil groups II, III, IV, V, VI, VII, and NPC-A. UniFrac analysis of g23 assemblages demonstrated that T4-type phage communities in paddy floodwater changed with sampling time and sampling location. Both UniFrac and NMDS analyses demonstrated that geographical separation and ecological process are the two major factors driving T4-type phage communities in the biosphere.
TSSP_A_1163507_supplementary_material.pdf
Download PDF (151.8 KB)Acknowledgments
We appreciate the editor and two anonymous reviewers for their insightful and constructive comments on this paper.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Asari N, Ishihara R, Nakajima Y, Kimura M, Asakawa S 2008: Cyanobacterial communities of rice straw left on the soil surface of a paddy field. Biol. Fert. Soils, 44, 605–612. doi:10.1007/s00374-007-0244-4
- Bellas CM, Anesio AM 2013: High diversity and potential origins of T4-type bacteriophages on the surface of Arctic glaciers. Extremophiles, 17, 861–870. doi:10.1007/s00792-013-0569-x
- Bonilla-Findji O, Rochelle-Newall E, Weinbauer MG, Pizay MD, Kerros ME, Gattuso JP 2009: Effect of seawater–freshwater cross-transplantations on viral dynamics and bacterial diversity and production. Aquat. Microb. Ecol., 54, 1–11. doi:10.3354/ame01256
- Breitbart M, Rohwer F 2005: Here a virus, there a virus, everywhere the same virus? Trends Microbiol., 13, 278–284. doi:10.1016/j.tim.2005.04.003
- Butina TV, Belykh OI, Maksimenko SY, Belikov SI 2010: Phylogenetic diversity of T4-like bacteriophages in Lake Baikal, East Siberia. FEMS Microbiol. Lett., 309, 122–129.
- Butina TV, Belykh OI, Potapov SA, Sorokovikova EG 2013: Diversity of the major capsid genes (g23) of T4-like bacteriophages in the eutrophic Lake Kotokel in East Siberia, Russia. Arch. Microbiol., 195, 513–520. doi:10.1007/s00203-013-0884-8
- Cahyani VR, Murase J, Ishibashi E, Asakawa S, Kimura M 2009: T4-type bacteriophage communities estimated from the major capsid genes (g23) in manganese nodules in Japanese paddy fields. Soil Sci. Plant Nutr., 55, 264–270. doi:10.1111/j.1747-0765.2009.00363.x
- Desplats C, Krisch HM 2003: The diversity and evolution of the T4-type bacteriophages. Res. Microbiol., 154, 259–267. doi:10.1016/S0923-2508(03)00069-X
- Dorigo U, Jacquet S, Humbert J-F 2004: Cyanophage diversity, inferred from g20 gene analyses, in the largest natural lake in France, Lake Bourget. Appl. Environ. Microbiol., 70, 1017–1022. doi:10.1128/AEM.70.2.1017-1022.2004
- Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, Eds. 2005: Virus Taxonomy: eighth Report of the International Committeee on Taxonomy of Viruses. Elsevier Academic Press, London.
- Filee J, Tetart F, Suttle CA, Krisch HM 2005: Marine T4 type bacteriophages, a ubiquitous component of the dark matter of the biosphere. Proc. Natl. Acad. Sci. USA, 102, 12471–12476. doi:10.1073/pnas.0503404102
- Frederickson CM, Short SM, Suttle CA 2003: The physical environment affects cyanophage communities in British Columbia inlets. Microb. Ecol., 46, 348–357. doi:10.1007/s00248-003-1010-2
- Fujihara S, Murase J, Tun CC, Matsuyama T, Ikenaga M, Asakawa S, Kimura M 2010: Low diversity of T4-type bacteriophages in applied rice straw, plant residues and rice roots in Japanese rice soils: Estimation from major capsid gene (g23) composition. Soil Sci. Plant Nutr., 56, 800–812. doi:10.1111/j.1747-0765.2010.00513.x
- Fujii T, Nakayama N, Nishida M, Sekiya H, Kato N, Asakawa S, Kimura M 2008: Novel capsid genes (g23) of T4-type bacteriophages in a Japanese paddy field. Soil Biol. Biochem., 40, 1049–1058. doi:10.1016/j.soilbio.2007.11.025
- Goldsmith DB, Brum JR, Hopkins M, Carlson CA, Breitbart M 2015: Water column stratification structures viral community composition in the Sargasso Sea. Aquat. Microb. Ecol., 76, 85–94.
- Huang HZ, Cheng K, Xu M, Zhao YJ 2011: Genetic diversity of T4 virioplankton, inferred from g23 gene, in Wuhan Donghu Lake. China Environ. Sci., 31, 44–47. (in Chinese with English abstract)
- Jessup CM, Forde SE 2008: Ecology and evolution in microbial systems: the generation and maintenance of diversity in phage-host interactions. Res. Microbiol., 159, 382–389. doi:10.1016/j.resmic.2008.05.006
- Jia Z, Ishihara R, Nakajima Y, Asakawa S, Kimura M 2007: Molecular characterization of T4-type bacteriophages in a rice field. Environ. Microbiol., 9, 1091–1096. doi:10.1111/emi.2007.9.issue-4
- Kim JD, Lee CG 2006: Diversity of heterocystous filamentous cyanobacteria (blue-green algae) from rice paddy fields and their differential susceptibility to ten fungicides used in Korea. J. Microbiol. Biotechn., 16, 240–246.
- Kimura M, Jia Z, Nakayama N, Asakawa S 2008: Ecology of viruses in soils: past, present and future perspectives. Soil Sci. Plant Nutr., 54, 1–32. doi:10.1111/j.1747-0765.2007.00197.x
- Liu J, Wang G, Wang Q, Liu J, Jin J, Liu X 2012: Phylogenetic diversity and assemblage of major capsid genes (g23) of T4-type bacteriophages in paddy field soils during rice growth season in Northeast China. Soil Sci. Plant Nutr., 58, 435–444. doi:10.1080/00380768.2012.703610
- Liu J, Wang G, Zheng C, Yuan X, Jin J, Liu X 2011: Specific assemblages of major capsid genes (g23) of T4-type bacteriophages isolated from upland black soils in Northeast China. Soil Biol. Biochem., 43, 1980–1984. doi:10.1016/j.soilbio.2011.05.005
- Lopez-Bueno A, Tamames J, Velazquez D, Moya A, Quesada A, Alcami A 2009: High diversity of the viral community from an Antarctic lake. Science, 326, 858–861. doi:10.1126/science.1179287
- Lozupone C, Knight R 2005: UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol., 71, 8228–8235. doi:10.1128/AEM.71.12.8228-8235.2005
- Marston M, Sallee J 2003: Genetic diversity and temporal variation in the cyanophage community infecting marine Synechococcus species in Rhode island’s coastal waters. Appl. Environ. Microbiol., 69, 4639–4647. doi:10.1128/AEM.69.8.4639-4647.2003
- Nakayama N, Asakawa S, Kimura M 2009b: Comparison of g23 gene sequence diversity between Novosphingobium and Sphingomonas phages and phage communities in the floodwater of a Japanese paddy field. Soil Biol. Biochem., 41, 179–185. doi:10.1016/j.soilbio.2008.06.008
- Nakayama N, Okumura M, Inoue K, Asakawa S, Kimura M 2007: Abundance of bacteriophages of common heterotrophic bacteria in the floodwater of a Japanese paddy field. Soil Sci. Plant Nutr., 53, 595–605. doi:10.1111/j.1747-0765.2007.00189.x
- Nakayama N, Tsuge T, Asakawa S, Kimura M 2009a: Morphology, host range and phylogenetic diversity of Sphingomonas phages in the floodwater of a Japanese paddy field. Soil Sci. Plant Nutr., 55, 53–64. doi:10.1111/j.1747-0765.2008.00332.x
- Okabe A, Oike H, Toyota K, Kimura M 2000: Comparison of phospholipid fatty acid composition in floodwater and plow layer soil during the rice cultivation period in a Japanese paddy field. Soil Sci. Plant Nutr., 46, 893–904. doi:10.1080/00380768.2000.10409155
- Paul JH, Sullivan MB 2005: Marine phage genomics: what have we learned? Curr. Opin. Biotech., 16, 299–307. doi:10.1016/j.copbio.2005.03.007
- R Development Core Team 2006: R, a Language and Environment for Statistical Computing. R 21. Foundation for Statistical Computing. Vienna, Austria.
- Sandaa R-A 2008: Burden or benefit? Virus–host interactions in the marine environment. Res. Microbiol., 159, 374–381. doi:10.1016/j.resmic.2008.04.013
- Sano E, Carlson S, Wegley L, Rohwer F 2004: Movement of viruses between biomes. Appl. Environ. Microbiol., 70, 5842–5846. doi:10.1128/AEM.70.10.5842-5846.2004
- Short CM, Suttle CA 2005: Nearly identical bacteriophage structural gene sequences are widely distributed in both marine and freshwater environments. Appl. Environ. Microbiol., 71, 480–486. doi:10.1128/AEM.71.1.480-486.2005
- Song T, Martensson L, Eriksson T, Zheng W, Rasmessen U 2005: Biodiversity and seasonal variation of the cyanobacterial assemblage in rice field in Fujian, China. FEMS Microbiol. Ecol., 54, 131–140. doi:10.1016/j.femsec.2005.03.008
- Suttle CA 2005: Viruses in the sea. Nature, 437, 356–361. doi:10.1038/nature04160
- Tamura K, Dudley J, Nei M, Kumar S 2007: MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol., 24, 1596–1599. doi:10.1093/molbev/msm092
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG 1997: The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res., 24, 4876–4882. doi:10.1093/nar/25.24.4876
- Wang G, Hayashi M, Saito M, Tsuchiya K, Asakawa S, Kimura M 2009a: Survey of major capsid genes (g23) of T4-type bacteriophages in Japanese paddy field soils. Soil Biol. Biochem., 41, 13–20. doi:10.1016/j.soilbio.2008.07.008
- Wang G, Jin J, Asakawa S, Kimura M 2009b: Survey of major capsid genes (g23) of T4-type bacteriophages in rice fields in Northeast China. Soil Biol. Biochem., 41, 423–427. doi:10.1016/j.soilbio.2008.11.012
- Wang G, Murase J, Asakawa S, Kimura M 2010: Unique viral capsid assembly protein gene (g20) of cyanophages in the floodwater of a Japanese paddy field. Biol. Fert. Soils, 46, 93–102. doi:10.1007/s00374-009-0410-y
- Wang G, Murase J, Taki K, Ohashi Y, Yoshikawa N, Asakawa S, Kimura M 2009c: Changes in major capsid genes (g23) of T4-type bacteriophages with soil depth in two Japanese rice fields. Biol. Fert. Soils, 45, 521–529. doi:10.1007/s00374-009-0362-2
- Wang G, Yu Z, Liu J, Jin J, Liu X, Kimura M 2011: Molecular analysis of the major capsid genes (g23) of T4-type bacteriophages in an upland black soil in Northeast China. Biol. Fert. Soils, 47, 273–282. doi:10.1007/s00374-010-0533-1
- Weinbauer MG, Rassoulzadegan F 2004: Are viruses driving microbial diversification and diversity? Environ. Microbiol., 6, 1–11. doi:10.1046/j.1462-2920.2003.00539.x
- Wommack KE, Ravel J, Hill RT, Chun JS, Colwell RR 1999: Population dynamics of Chesapeake Bay virioplankton: total community analysis by pulsed-field gel electrophoresis. Appl. Environ. Microbiol., 65, 131–140.
- Zheng C, Wang G, Liu J, Song C, Gao H, Liu X 2013: Characterization of the major capsid genes (g23) of T4-type bacteriophages in the wetlands of northeast China. Microb. Ecol., 65, 616–625. doi:10.1007/s00248-012-0158-z
- Zhong X, Jacquet S 2014: Differing assemblage composition and dynamics in T4-like myophages of two neighbouring sub-alpine lakes. Freshwater Boil., 59, 1577–1595. doi:10.1111/fwb.2014.59.issue-8