ABSTRACT
To determine the genotypic variation in response to salt (NaCl) stress in cotton (Gossypium hirsutum L.) seedlings, potassium (K+) and sodium (Na+) homeostasis, photochemical energy utility, reactive oxygen metabolism and the activity of antioxidant enzymes were comparatively analyzed in three cotton cultivars (CCRI 49, CCRI 35 and Z 51504) under salt constraint. The results showed that NaCl treatment significantly inhibited biomass accumulation, and the extent of inhibition was highest in CCRI 49 and lowest in Z 51504. Salinity caused an ion imbalance in plants but ion homeostasis was less pronounced in Z 51504, as it accumulated more K+ and less Na+. Experiments of salt shock treatment were tested using a non-invasive micro-test (NMT) system, which also revealed that Z 51504 had lower Na+ influx and better K+ retention. Salinity increased excess-energy dissipation [non-photochemical quenching (qN) and photorespiration rate (PR)], but depressed photochemical efficiency such as photosynthesis rate (Pn), quenching (qP), photochemical quantum yield of photosystem (ΦPSII) and electron transport rate (ETR). As a result, more electrons were driven to other sinks, for example decreasing ETR/Pn and increasing the O2− generation rate. However, the superior tolerance of Z 51504 had a better balance of photochemical energy under salt conditions, displayed higher photochemical efficiency and excess-energy dissipation. Furthermore, the antioxidant enzyme activities were also affected by salt stress and less effectively removed reactive oxygen species. The antioxidant enzyme activities of Z 51504 were higher than those of CCRI 49 and CCRI 35, which resulted in lower levels of reactive oxygen species (ROS) and mitigated the salt-induced membrane lipid peroxidation. The overall results indicated that more effective retention of ions, photochemical energy utility and ROS-removing capability were probably the main reasons for the stronger salt tolerance in Z 51504.
1. Introduction
A large part of agricultural soils are salt-affected and salinity is becoming a serious threat to modern agriculture worldwide (Greenway and Munns Citation1980; Flowers and Yeo Citation1995). More than 800 million hectares of land in the world, about 20% of irrigated land and 6% of the world’s total land area, are affected by salinity (Munns and Tester Citation2008). This problem has been aggravated by industrial modernization and the development of agricultural practices. Therefore, raising the salt tolerance of crops is a feasible way to meet the challenges of farmland salinity in the future.
Sodium (Na+) exerts toxic effects on physiological metabolism and plant growth in saline conditions; thus, the ability of a plant to exclude Na+ from the root and sustain Na+ homeostasis is an important component of plant salinity tolerance (Xu and Fujiyama Citation2013; Dai et al. Citation2014). Takahashi et al. (Citation2007) reported that the regulation of Na+ flux was highly controlled in salt-resistant plants, while the absence of such capacity was identified in salt-sensitive plants. Luo et al. (Citation2005) also revealed that wild soybean (Glycine soja L.) had higher salt resistance than cultivated soybean (G. max L.) due to less Na+ ion accumulation in tissue. However, some studies have shown that it was not Na+ but the potassium (K+)/Na+ ratio in the cytosol that determined plant performance under saline conditions (Ding et al. Citation2010; Teaklea et al. Citation2011). Shabala and Cuin (Citation2008) also indicated that maintaining an optimal K+/Na+ ratio was more important than simply maintaining a low content of Na+ in many cultivars under high salinity.
The more efficient photosynthesis characteristics in salt stress conditions could also have contributed to salt tolerance (Lima-Neto et al. Citation2014). It has been reported that salinity reduced photosynthetic pigments in plants (Chen et al. Citation2013; Duarte et al. Citation2013) and limited the utilization of absorbed light energy during carbon dioxide (CO2) fixation. Under these conditions, excess electrons from the photochemical phase accumulated in thylakoid membranes, which caused photoinhibition and resulted in the accumulation of large quantities of reactive oxygen species (ROS) in chloroplasts (Wang et al. Citation2012; Sperdouli and Moustakas Citation2014). To maintain a low ROS level and efficient photosynthesis characteristics, plants have developed a complex antioxidative defense system that protects them from salt stress (Neto et al. Citation2006; Rivas et al. Citation2013). Several studies have shown that antioxidant capacity is often used for differentiating the salt resistance in many cultivars, and higher antioxidant enzyme activity is thought to be a salt resistance mechanism (Chen et al. Citation2013; Zhang et al. Citation2014). However, the results of some scholars have also been verified to be somewhat contradictory (Abogadallah Citation2010; Miller et al. Citation2010). Such inconsistent results might be attributable to the differences in plant species, tissues and time of exposure to the salinity (Mittova et al. Citation2004; Munns and Tester Citation2008).
Although cotton (Gossypium hirsutum L.) is classified as a salt-tolerant crop, with a salinity threshold level of 7.7 ds m−1, its growth is severely reduced at high salinity levels, especially at the seedling stage (Leidi and Saiz Citation1997; Ashraf and Ahmad Citation2000). In fact, several studies attempting to research the salt resistance mechanism of cotton have been conducted (Kong et al. Citation2012; Li et al. Citation2013; Zhang et al. Citation2014), but the interactions between rates of growth, ionic homeostasis, photochemical energy utility and antioxidant enzyme activities may be complex and perhaps different between species. Thus, understanding the salt tolerance difference among cotton cultivars remains a difficult task that requires multidisciplinary efforts and detailed investigation.
To elucidate those questions, we comparatively studied the response of three cotton cultivars, CCRI 49, CCRI 35 and Z 51504, after exposure to varying salt treatments by analyzing root ion flux, photosynthetic function and reactive oxygen cultivar metabolism. Our results not only enrich the knowledge of genotypic variations in response to salt stress in cotton but also provide a framework to identify breeding targets for improving salt resistance in cultivated cotton.
2. Materials and methods
2.1. Plant material, growth conditions and treatment
The cotton cultivars CCRI 35, Z 51504 and CCRI 49 (CCRI 35 × Z 51504), developed by the Cotton Research Institute at the Chinese Academy of Agricultural Sciences, were used in the present study. The seeds were soaked in sterile deionized water for 14 h at 28°C and germinated in sand medium. Then, the sand medium was placed in a growth chamber (light/dark regime of 14/10 h at 28/20°C, relative humidity of 70–80%, photosynthetically active radiation of 500 μmol m−2 s−1). The seeds germinated after 5 d in the sand medium and the uniform seedlings were transplanted to plastic pots filled with Hoagland solution. At the one-leaf stage (8 d after transfer to Hoagland solution), a 150 mM salinity treatment was imposed on the seedlings (increasing the salt concentration by 50 mM NaCl step every 6 h until the final concentration was reached). A completely randomized design was used with five replications (pots). The solution was aerated constantly and changed twice weekly throughout the experiment.
2.2. Gas exchange and chlorophyll a fluorescence
After 9 days of 150 mM salinity treatment, the photosynthetic rate (Pn) of the third fully expanded young leaf of the main stem from the terminal end was measured in five seedlings per cultivar per treatment with an Li-6400 (Li-COR, Lincoln, USA) between 9:00 and 11:30 am at 28°C, 60% relative humidity, 400 μmol mol−1 CO2 concentration and 800 μmol m−2 s−1 quantum flux. The photorespiration rate (PR) was estimated from the difference in the photosynthesis rate between low and normal oxygen content in the air (Aliyev Citation2012). The chlorophyll fluorescence was measured with a modulated fluorometer (PAM 2100, Walz, Effeltrich, Germany), as described by Sperdouli and Moustakas (Citation2014). The electron transport rate (ETR) was estimated as ETR = 0.5 × 0.84×PPFD×[(Fm’ − Fs)/Fm’] (Maxwell and Johnson Citation2000). Values of 0.5 and 0.84 were used as the fraction of excitation energy distributed to PSII and incoming light absorbed by the leaves, respectively. PPFD, Fm’ and Fs’ represent the photosynthetic photon flux density, maximum and steady state chlorophyll a (Chla) fluorescence, respectively.
2.3. Reactive oxygen cultivar metabolism
After the gas exchange and Chla fluorescence measurement, the third fully expanded young leaf of the main stem from the terminal end were sampled and preserved at −80°C for the determination of reactive oxygen cultivar metabolism. Frozen leaf segments (0.5 g) were homogenized to fine powder and the powders were suspended in extraction buffer containing 50 mM potassium phosphate buffer (pH 7.0) and 1% (volume/volume) Triton X-100. After centrifugation at 12,000 g for 15 min, the resulting supernatant was used for assays of the activities of superoxide dismutase (SOD), peroxidases (POD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR), according to the methods of Parida et al. (Citation2004). The malondialdehyde (MDA) content was determined with thiobarbituric acid reaction method (Heath and Packer Citation1968). Superoxide anion (O2−) production was measured according to the method of Elstner and Heupel (Citation1976). The accumulation of hydrogen peroxide (H2O2) was measured spectrophotometrically (EU-2600, Onlab, China), following the methods in Alexieva et al. (Citation2001).
2.4. Measurement of Na+ and K+
The seedlings were harvested after 9 d of 150 mM salinity treatment, and their roots were immersed in deionised water for 10 min. Then, seedlings were separated into roots and shoots and oven-dried at 80°C for 72 h. After weighing, dried tissue samples were ground to a fine powder and soaked in 1.0 mol l−1 hydrochloric acid (HCl) solution for 24 h at 30°C, and then shaken for 60 min. Na+ and K+ content in the extracts were analyzed by atomic absorption spectrophotometer (SpectAA-50/55, Varian, Australia).
2.5. Measurement of Na+ and K+ flux
For ion flux experiments, cotton seedlings were germinated over 9 d in a sand medium and were measured by Xuyue Science & Technology Co. (Beijing, China) using the non-invasive micro-test (NMT) system (NMT-YG-100, Younger, USA). The ion-selective electrodes were calibrated using the following solutions: Na+, 0.5 and 5.0 mmol l−1 NaCl; K+, 0.05 and 0.5 mmol l−1 potassium chloride (KCl) (Sun et al. Citation2009). Taproot segments were immobilized on the bottom of a measuring dish and then incubated in the measuring solution (0.2 mM Na+, 0.1 mM K+, 0.1 mM calcium (Ca2+), pH 6.0 adjusted with Tris or HCl) to equilibrate for 20 min. Steady fluxes of Na+ and K+ in the meristematic zone (about 300 µm from the root tip) of intact roots were measured in a fresh measuring solution. Ion fluxes of the NaCl shock treatments were recorded for 10 min to ensure steady initial values, then NaCl treatment was applied to reach a final 100 mM NaCl concentration and the transient ion flux kinetics was measured at specified times. The steady-state ion fluxes of the salt treatment measurements were continuously recorded for 10–15 min. The flux data were recorded with MageFlux developed by Xuyue Science & Technology Company.
2.6. Statistical analysis
Data (n ≥ 3) were examined by one-way analysis of variance using SPSS10.0 (SPSS Inc. Chicago, USA) software and significant differences among means (P < 0.05) were assessed using Duncan’s multiple range test.
3. Results
3.1. Effects of NaCl treatment on plant growth
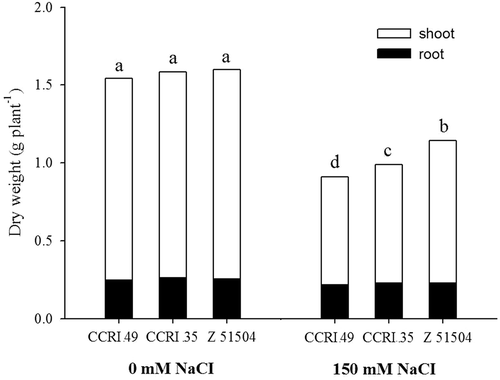
In the present study, young cotton plants were exposed to salt stress (150 mM NaCl) for 9 d. Salinity promoted a significant reduction in the dry matter of the shoot but no change occurred in the root dry weight, compared with the respective controls (). Under NaCl treatment, more pronounced effects were observed in CCRI 49 and CCRI 35, such that the dry weight decreased by 41.6% and 35.3%, respectively, while the dry weight only decreased by 28.1% in Z 51504 compared to the control.
3.2. Effects of NaCl treatment on ion content
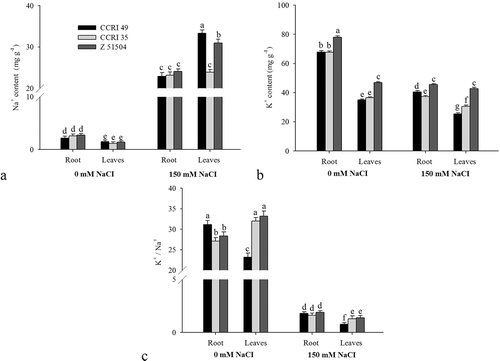
The concentration of Na+ and K+ in the roots and leaves were significantly affected by the NaCl treatment (). The sodium content of roots and leaves of all cultivars increased dramatically, by 8.3- and 20.6-fold, after NaCl treatment. Interestingly, the extents of the Na+ rise in seedling leaves were CCRI 49 > Z 51504 > CCRI 35, and those in roots showed similar rising extents (). In addition, the K+ content of the different cultivars decreased in the salinity treatment but the Z 51504 exhibited a higher K+ concentration in both roots and leaves compared with the other two cultivars. The K+ concentration in seedling roots was CCRI 49 > CCRI 35, whereas the K+ concentration in the leaves showed opposite results (). As expected, the K+/Na+ ratio in roots and leaves decreased with salt stress (). More pronounced effects were observed in CCRI 49, such as exhibiting a lower K+/Na+ ratio in leaves compared with the other cultivars, and the ratio in roots was also greatly inhibited (decreased by 94.5%).
Figure 2. The (a) Na+ and (b) K+ concentrations, and (c) K+/Na+ ratios in the leaves of the cotton cultivars CCRI 49, CCRI 35 and Z 51504 after treatment with sodium chloride (NaCl) for 9 d. The different letters present on the columns indicate significant differences at P < 0.05 between the control and the treatment.
3.3. Effects of NaCl treatment on net Na+ and K+ flux
In order to understand how salt treatment influences the uptake of ions in cotton seedlings, salt shock treatment experiments were conducted, and steady flux profiles of Na+ and K+ were measured using NMT. Immediately after the onset of salt shock, we observed a sustained increase of Na+ influx and a more pronounced effect was observed in CCRI 49 with a steady-state value of −4201 pmol cm−2 s−1, compared with CCRI 35 and Z 51504 (−3062 and −2582 pmol cm−2 s−1, respectively; , ). Under acute salt stress, the K+ efflux showed a biphasic pattern with a rapid increase to peaking levels after the onset of salt exposure, then followed by a gradual decrease, finally reaching a stable level, and K+ efflux was significantly increased by the NaCl shock (). However, the extent of the K+ loss in seedling roots was greatest in CCRI 49 and lowest in Z 51504 at the stable level ().
Figure 3. The effects of salt shock [100 mM sodium chloride (NaCl)] on (a) Na+ and (b) K+ flux kinetics of cotton seedlings of three different cultivars (CCRI 49, CCRI 35 and Z 51504) that were grown for 9 d in a sand medium (n = 8). The mean (c) Na+ and (d) K+ fluxes of steady state of cotton seedlings within the measuring periods are shown. The different letters present on the columns indicate significant differences at P < 0.05 between the control and the treatment.
![Figure 3. The effects of salt shock [100 mM sodium chloride (NaCl)] on (a) Na+ and (b) K+ flux kinetics of cotton seedlings of three different cultivars (CCRI 49, CCRI 35 and Z 51504) that were grown for 9 d in a sand medium (n = 8). The mean (c) Na+ and (d) K+ fluxes of steady state of cotton seedlings within the measuring periods are shown. The different letters present on the columns indicate significant differences at P < 0.05 between the control and the treatment.](/cms/asset/0bb9b861-16f0-42c8-a7ee-5e74b9c46c3d/tssp_a_1172022_f0003_b.gif)
3.4. Effects of NaCl treatment on photochemical efficiency
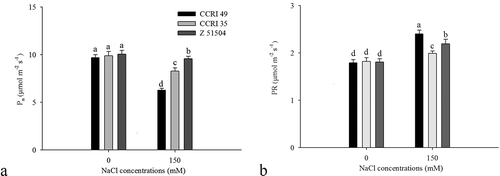
Salinity treatment promoted a significant reduction in Pn, which decreased by 35.3%, 16.3% and 6.1% in CCRI 49, CCRI 35 and Z 51504, respectively (). Salt stress also caused a significant increase in PR. However, more pronounced effects were observed in CCRI 49 and Z 51504, as the PR increased by 34.1% and 21.1%, respectively, while the PR increased by only 9.3% in CCRI 35 ().
Figure 4. Changes in (a) photosynthesis (Pn) and (b) photorespiration (PR) in the leaves of the cotton cultivars CCRI 49, CCRI 35 and Z 51504 after treatment with sodium chloride (NaCl) for 9 d. The different letters present on the columns indicate significant differences at P < 0.05 between the treatment and the control.
No obvious changes were observed in the maximum efficiency of photosystem II (PSII) photochemistry (Fv/Fm) under salt stress, but the photochemical quenching (qP), photochemical quantum yield of photosystem II (ΦPSII) and ETR significantly decreased (). The decrease of those parameters was greater in CCRI 49 compared to those observed in the CCRI 35 and Z 51504. Salt stress caused a significant increase in non-photochemical quenching (qN), which strongly increased in the leaves of CCRI 49 and Z 51504, while only slightly increasing in the leaves of CCRI 35 (). The ETR/Pn of CCRI 49 and CCRI 35 increased under the salt stress condition by 41.1% and 16.9%, respectively, while no differential change was observed in Z 51504 ().
Table 1. Changes in the maximum photochemical efficiency of photosystem II (PSII; Fv/Fm), actual photochemical efficiency of PSII (ΦPSII), photochemical quenching (qP), non-photochemical quenching (qN), electron transport rate (ETR) and ETR/Pn in the leaves of the cotton cultivars CCRI 49, CCRI 35 and Z 51504 after treatment with NaCl for 9 d.
3.5. Effects of NaCl treatment on reactive oxygen cultivar metabolism
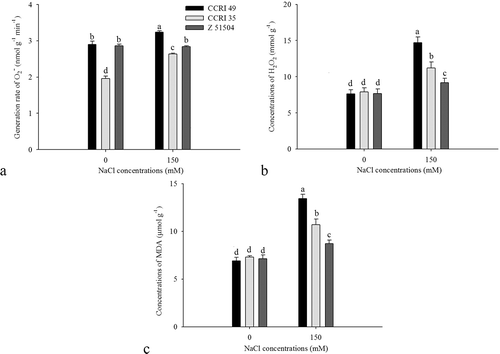
As shown in Fig. 5a, salt stress increased the O2− generation rate in CCRI 49 and CCRI 35 leaves; however, no differential change was observed in the leaves of Z 51504. Salinity treatment also resulted in the accumulation of H2O2 and MDA in leaves, and the increase of H2O2 and MDA was greatest in CCRI 49 and lowest in Z 51504 (). Several antioxidative enzymes were also significantly affected by the NaCl treatment (). The activity of SOD and POD decreased in leaves after treatment with NaCl, but Z51504 was less affected than other cultivars. The SOD activity of CCRI 49 was much lower than that of CCRI 35 after treatment with salt stress, whereas the POD activity was the opposite. Under salt stress, the CAT activity increased by 88.7% in CCRI 35 and 89.5% in Z 51504, but decreased by 50.3% in CCRI 49. The activity of APX and GR in different cultivars also varied significantly in response to salt stress, and the activity of APX and GR was highest in Z 51504 and lowest in CCRI 49.
Table 2. Changes in the activity of superoxide dismutase (SOD), peroxidases (POD), catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR) in the leaves of the cotton cultivars CCRI 49, CCRI 35 and Z 51504 after treatment with sodium chloride (NaCl) for 9 d.
4. Discussion
Salt treatment significantly reduced cotton cultivar seeding growth in both our study and previous studies (Kong et al. Citation2012; Li et al. Citation2013; Dai et al. Citation2014; ). In this study, we also indicated that Z 51504 showed a higher salt tolerance than other cotton cultivars did. It has been described that avoiding the accumulation of Na+ and maintaining K+ retention is a pivotal strategy for plants to grow in high-salt conditions (Xu and Fujiyama Citation2013; Bose et al. Citation2014). These traits may contribute to differences in the salinity tolerance among cotton cultivars. Compared with CCRI 49 and CCRI 35, the leaves and roots of Z 51504 had lower levels of Na+ but higher levels of K+ under salt stress (). Those results indicated that Z 51504 exhibited fine regulation of ion movements, which was supported by less Na+ influx and better K+ retention under salt stress (). Similarly, Dai et al. (Citation2014) indicated that there was a clear interaction between unequal salt distribution and unequal nutrient distribution on plant growth and salt tolerance in cotton. Zhu (Citation2003) also suggested that the high-salinity condition disturbed the intracellular K+/Na+ balance and maintained the optimal K+/Na+ ratio, which he suggested was an important salt-tolerance mechanism in many plant species.
PSII has been shown to be sensitive to salt stress in some studies (Chen et al. Citation2013; Zhang et al. Citation2014). Therefore, the highly efficient utilization of photochemical energy was an important factor for photoprotection in plants under high salinity (Takahashi and Badger Citation2011). In the current study, salt stress decreased the PSII activity (e.g., Pn, qP, ΦPSII and ETR; ) but those photochemical parameters were different among cotton cultivars under salt treatment. The superior salt tolerance of Z 51504 exhibited more effective photochemical energy utility during salt stress, such as displaying higher photochemical efficiency and excess-energy dissipation ( and ). These results suggest that fewer electrons were driven to other sinks (lower ETR/Pn; ), and it could reduce the excess generation of ROS and alleviate damage of the PSII core under salt stress (Takahashi and Badger Citation2011). Hence, the higher salt tolerance of Z 51504 under saline conditions could be attributed to an effective adjustment in the stoichiometry of the photosystem, which may be an important mechanism to resist external salinity condition. Similar results were also observed in previous studies (Zhang et al. Citation2009; Wituszynska et al. Citation2013; Lima-Neto et al. Citation2014).
Salt stress can cause physiological disorders of plants and lead to excess generation of ROS (Chen et al. Citation2013; Zhang et al. Citation2014). The excess ROS induced by salt can be scavenged by the antioxidant system, and antioxidant capacity is usually regarded as the defining criterion of a salt-tolerant cultivar (Abogadallah Citation2010). Several studies have also suggested salt-tolerant species generally have greater antioxidant enzyme activity under salt stress than salt-sensitive species do (Gosset et al. Citation1994; Chen et al. Citation2013; Zhang et al. Citation2014). With respect to those parameters, lower activities of antioxidant enzymes were detected in CCRI 49 and CCRI 35 leaves compared with those of Z 51504 (), which indicates a less efficient antioxidant capability against oxidative stress and lipid peroxidation. Accompanied by the tendency to produce more excessive excitation energy in CCRI 49 and CCRI 35 (see above), this resulted in greater accumulation of ROS (e.g., O2− and H2O2 production) under NaCl treatment (). Therefore, CCRI 49 and CCRI 35 suffered more severe oxidation degradation of Chl and membranes, as indicated by their higher photoinhibition () and MDA content (Fig. 5c), respectively. Those results suggest that the differences in antioxidant capability, at least in part, explained the higher salt tolerance exhibited by Z 51504 compared to that exhibited by CCRI 49 and CCRI 35.
In conclusion, our results indicate that the different physiological responses of CCRI 49, CCRI 35 and Z 51504 to salt stress were highly related to the retention of ions, photochemical energy utility and ROS-removing capability. The cotton cultivar Z 51504 had a high salt tolerance due to (1) avoiding accumulation of Na+ and maintaining K+ retention in leaves to protect the photosynthetic apparatus from salt damage; (2) maintaining photosynthetic function to utilize more light energy and decrease ROS generation; and (3) possessing a more efficient antioxidant defense system against oxidative stress. Moreover, although CCRI 49 was derived from the cross CCRI 35 × Z 51504, and although it was a close relative of CCRI 35 and Z 51504, it did not show enhanced salt tolerance. This bad agronomic trait for salt tolerance in CCRI 49 needs to be highly emphasized and further investigated in future work.
Acknowledgments
This research was supported by the China Agricultural Research System (No. CARS-18-05), the Modern Agricultural Industry Technology System in Henan (No. S2013-07-1), and the Central Research Institutes of Basic Research and the Public Service Special Foundation (No. 1610162014002).
References
- Abogadallah GM 2010: Antioxidative defense under salt stress. Plant Signal. Behav., 5, 369–374. doi:10.4161/psb.5.4.10873
- Alexieva V, Sergiev I, Mapelli S, Karanov E 2001: The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ., 24, 1337–1344. doi:10.1046/j.1365-3040.2001.00778.x
- Aliyev JA 2012: Photosynthesis, photorespiration and productivity of wheat and soybean genotypes. Physiol. Plant., 145, 369–383. doi:10.1111/j.1399-3054.2012.01613.x
- Ashraf M, Ahmad S 2000: Influence of sodium chloride on ion accumulation, yield components and fiber characteristics in salt-tolerant and salt-sensitive lines of cotton (Gossypium hirsutum L.). Field Crop Res., 66, 115–127. doi:10.1016/S0378-4290(00)00064-2
- Bose J, Shabala L, Pottosin I, Zeng F, Velarde-Buendía AM, Massart A, Poschenrieder C, Hariadi Y, Shabala S 2014: Kinetics of xylem loading, membrane potential maintenance, and sensitivity of K+-permeable channels to reactive oxygen species: physiological traits that differentiate salinity tolerance between pea and barley. Plant Cell Environ., 37, 589–600. doi:10.1111/pce.12180
- Chen P, Yan K, Shao HB, Zhao SJ 2013: Physiological mechanisms for high salt tolerance in wild soybean (Glycine soja) from yellow river delta, China: photosynthesis, osmotic regulation, ion flux and antioxidant capacity. Plos One, 8, 1–12.
- Dai JL, Duan LS, Dong HZ 2014: Improved nutrient uptake enhances cotton growth and salinity tolerance in saline media. J. Plant Nutr., 37, 1269–1286. doi:10.1080/01904167.2014.881869
- Ding MQ, Hou PC, Shen X et al. 2010: Salt-induced expression of genes related to Na+/K+ and ROS homeostasis in leaves of salt-resistant and salt-sensitive poplar species. Plant Mol. Biol., 73, 251–269. doi:10.1007/s11103-010-9612-9
- Duarte B, Santos D, Marques JC, Cacador I 2013: Ecophysiological adaptations of two halophytes to salt stress: photosynthesis, PSII photochemistry and anti-oxidant feedback-implications for resilience in climate change. Plant Physiol. Bioch., 67, 178–188. doi:10.1016/j.plaphy.2013.03.004
- Elstner EF, Heupel A 1976: Formation of hydrogen peroxide by isolated cell walls from horseradish (Armoracia lapathifolia Gilib). Planta, 130, 175–180. doi:10.1007/BF00384416
- Flowers T, Yeo A 1995: Breeding for salinity resistance in crop plants: where next? Funct. Plant Biol., 22, 875–884.
- Gosset DR, Millhollon EP, Lucas MC 1994: Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci., 34, 706–714. doi:10.2135/cropsci1994.0011183X003400030020x
- Greenway H, Munns R 1980: Mechanisms of salt tolerance in nonhalophytes. Ann. Rev. Plant Physiol., 31, 149–190. doi:10.1146/annurev.pp.31.060180.001053
- Heath RL, Packer L 1968: Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125, 189–198. doi:10.1016/0003-9861(68)90654-1
- Kong XQ, Luo Z, Dong HZ, Eneji AE, Li WJ 2012: Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J. Exp. Bot., 63, 2105–2116. doi:10.1093/jxb/err420
- Leidi EO, Saiz JF 1997: Is salinity tolerance related to Na+ accumulation in upland cotton (Gossypium hirsutum) seedlings? Plant Soil, 190, 67–75. doi:10.1023/A:1004214825946
- Li MY, Li FJ, Yue YS, Tian XL, Li ZH, Duan LS 2013: NaCl-induced changes of ion fluxes in roots of transgenic Bacillus thuringiensis (Bt) cotton (Gossypium hirsutum L.). J. Integr. Agr., 12, 436–444. doi:10.1016/S2095-3119(13)60244-0
- Lima-Neto MC, Lobo AK, Martins MO, Fontenele AV, Silveira JA 2014: Dissipation of excess photosynthetic energy contributes to salinity tolerance: a comparative study of salt-tolerant Ricinus communis and salt-sensitive Jatropha curcas. J. Plant Physiol., 171, 23–30. doi:10.1016/j.jplph.2013.09.002
- Luo QY, Yu BJ, Liu YL 2005: Differential sensitivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. J. Plant Physiol., 162, 1003–1012. doi:10.1016/j.jplph.2004.11.008
- Maxwell K, Johnson GN 2000: Chlorophyll fluorescence-a practical guide. J. Exp. Bot., 51, 659–668. doi:10.1093/jexbot/51.345.659
- Miller G, Suzuki N, Ciftci-Yilmaz SRM 2010: Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Environ. Expe. Bot., 33, 453–467.
- Mittova V, Guy M, Tal M, Volokita M 2004: Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J. Exp. Bot., 55, 1105–1113. doi:10.1093/jxb/erh113
- Munns R, Tester M 2008: Mechanisms of salinity tolerance. Ann. Rev. Plant Biol., 59, 651–681. doi:10.1146/annurev.arplant.59.032607.092911
- Neto ADD, Prisco JT, Eneas J, de-Abreu CEB, Gomes E 2006: Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Expe. Bot., 56, 87–94. doi:10.1016/j.envexpbot.2005.01.008
- Parida AK, Das AB, Mohanty P 2004: Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some antioxidative enzymes. J. Plant Physiol., 161, 531–542. doi:10.1078/0176-1617-01084
- Rivas R, Oliveira MT, Santos MG 2013: Three cycles of water deficit from seed to young plants of Moringa oleifera woody species improves stress tolerance. Plant Physiol. Biochem., 63, 200–208. doi:10.1016/j.plaphy.2012.11.026
- Shabala S, Cuin TA 2008: Potassium transport and plant salt tolerance. Physiol. Plant., 133, 651–669. doi:10.1111/j.1399-3054.2007.01008.x
- Sperdouli I, Moustakas M 2014: A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol., 171, 587–593. doi:10.1016/j.jplph.2013.11.014
- Sun J, Chen S, Dai S, Wang R, Li N, Shen X 2009: NaC-linduced alternations of cellular and tissue ion fluxes in roots of salt-resistant and salt-sensitive poplar species. Plant Physiol., 149, 41–53.
- Takahashi R, Nishio T, Ichizen N, Takano T 2007: Cloning and functional analysis of the K+ transporter, PhaHAK2, from salt-sensitive and salt-tolerant reed plants. Biotechnol. Lett., 29, 501–506. doi:10.1007/s10529-006-9246-9
- Takahashi S, Badger MR 2011: Photoprotection in plants: a new light on photosystem II damage. Trends Plant Sci., 16, 53–60. doi:10.1016/j.tplants.2010.10.001
- Teaklea NL, Bazihizinaa N, Shabalac S, Colmerb TD, Barrett-Lennarda EG, Rodrigo-Morenoc A, Shabala S, Mackay A 2011: Ion transport in halophytes. Adv. Bot. Res., 57, 151–199.
- Wang N, Hua HB, Eneji AE, Li ZH, Duan LS, Tian XL 2012: Genotypic variations in photosynthetic and physiological adjustment to potassium deficiency in cotton (Gossypium hirsutum). J. Photoch. Photobio. B., 110, 1–8. doi:10.1016/j.jphotobiol.2012.02.002
- Wituszynska W, Gałazka K, Rusaczonek A, Vanderauwera S, Van Breusegem F, Karpinski S 2013: Multivariable environmental conditions promote photosynthetic adaptation potential in Arabidopsis thaliana. J. Plant Physiol., 170, 548–559. doi:10.1016/j.jplph.2012.11.016
- Xu R, Fujiyama H 2013: Comparison of ionic concentration, organic solute accumulation and osmotic adaptation in Kentucky bluegrass and Tall fescue under NaCl stress. Soil Sci. Plant Nutr., 59, 168–179. doi:10.1080/00380768.2012.763215
- Zhang HJ, Dong HZ, Li WJ, Sun Y, Chen SY, Kong XQ 2009: Increased glycine betaine synthesis and salinity tolerance in AhCMO transgenic cotton lines. Mol. Breed., 23, 289–298. doi:10.1007/s11032-008-9233-z
- Zhang L, Ma HJ, Chen TT, Pen J, Yu SX, Zhao XH 2014: Morphological and physiological responses of cotton (Gossypium hirsutum L.) plants to salinity. Plos One, 9, 1–14.
- Zhu JK 2003: Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol., 6, 1–5. doi:10.1016/S1369-5266(03)00085-2