ABSTRACT
Nitrogen is an essential element for plant growth. Development of the root system architecture is highly correlated with nitrogen availability from the environment. Recent studies show that auxin response modules are involved in nitrate-dependent lateral root growth. However, the role of auxin in nitrogen metabolism remains to be elucidated. Few researchers have addressed the effect of auxin signaling modules on the use of ammonium for nutrition. The purpose of this study was to describe and examine the relation between auxin signaling modules and ammonium for nutrition. The growth of T-DNA insertion lines for auxin signaling modules was compared with that of a wild type under different nitrogen regimes. Nitrogen use efficiency consists of two components: metabolism and uptake. The nitrogen usage index was calculated following elemental analysis to evaluate nitrogen metabolism. Isotope-labeled ammonium uptake was measured under nitrogen-deficient and -sufficient conditions. Transcriptional levels and accumulation of enzymes necessary for primary ammonium assimilation, glutamine synthetase and glutamate synthase were evaluated. One of the T-DNA insertion lines for the auxin signaling module, IAA17, showed severe growth reduction in hydroponic solution containing ammonium as a major nitrogen source. The accumulation of cytosolic glutamine synthetase was reduced in the roots of iaa17. The expression of cytosolic glutamine synthetase 1;2 in iaa17 did not respond to ammonium supply. Here we show that the auxin signaling module has an effect on ammonium use by regulating the transcriptional level of cytosolic glutamine synthetase 1;2 in the root, the gene essential for ammonium assimilation.
KEY WORDS:
1. Introduction
Nitrogen is an essential element for plant growth (Marschner Citation1995). Nitrogen is supplied as a fertilizer to the plant. Nitrogen fertilizer is a limited resource because its synthesis requires a vast amount of fossil fuels. Therefore, it is important to understand the underlying mechanism of nitrogen transport and metabolism in a plant (Lea and Azevedo Citation2006). There are two primary components of nitrogen use efficiency, which are referred to as ‘uptake efficiency’ and ‘usage efficiency’ (Good et al. Citation2004). In this work, nitrogen use efficiency was evaluated by considering uptake of isotope-labeled nitrogen compounds and by calculating the usage index (Good et al. Citation2004). Root system architecture is a crucial trait for resource acquisition (Forde Citation2014). Root development is influenced by several environmental factors, including nutrient availability (Zhang and Forde Citation2000; Kellermeier et al. Citation2014) and the presence of phytohormones (Petricka et al. Citation2012; Ma et al. Citation2014). The phytohormone auxin acts as a current integrator of endogenous and environmental signals, regulating the root system architecture in a plant (Lavenus et al. Citation2013; Ma et al. Citation2014). The auxin response module consists of several components, including Aux/IAA proteins and auxin response factor (ARF) transcription factors. Aux/IAA proteins act as transcriptional repressors by heterodimerizing with ARF. Aux/IAA proteins are degraded at high auxin levels, and ARFs can modulate the transcription of their target genes (Lavenus et al. Citation2013). Kiba et al. (Citation2011) recently suggested the hormonal control of nitrogen acquisition. It is known that nitrate availability regulates Arabidopsis root development (Zhang and Forde Citation2000). Owing to the progress of systems biology, a link has been suggested between auxin signaling and nitrate-dependent changes in the root morphology. The importance of ARF8 and IAA14 on nitrate-dependent lateral root development was pointed out in previous studies (Gifford et al. Citation2008; Vidal et al. Citation2013). ARF8 is a nitrogen-inducible ARF and is one of the targets of microRNA167 (miR167) (Gifford et al. Citation2008). ARF8 and miR167 regulate lateral root growth in response to organic nitrogen. IAA14/SLR is considered a master regulator of lateral root initiation, controlling the initial pericycle cell divisions that give birth to new roots (Fukaki et al. Citation2002). Recently, a crucial role of auxin biosynthetic gene TAR2 in root architecture under nitrogen-limited conditions was suggested (Ma et al. Citation2014). However, the role of auxin in nitrogen metabolism remains to be fully elucidated. The nitrogen status and auxin content are closely correlated (Kiba et al. Citation2011). For example, when nitrate is supplied to a plant, the auxin content is decreased in the roots (Caba et al. Citation2000; Tian et al. Citation2008). Ammonium supply is also found to reduce the auxin content in roots, while it increases the IAAsp content (Tamura et al. Citation2010). The conjugation of IAA to aspartic acid forms IAAsp (Kramer and Ackelsberg Citation2015). Because IAAsp is not hydrolyzed by plants, it appears to be a second pathway for auxin catabolism. Previous studies have mainly focused on the effect of the auxin signaling module on root development. However, few researchers have addressed the relation between the auxin signaling module and nitrogen use in the plant. Here we suggest that the auxin signaling module impacts the ammonium use by controlling transcriptional regulation of the ammonium assimilatory gene. The research describes the comparison of a set of (1) dry weights, (2) carbon and nitrogen contents, (3) ammonium uptake, and (4) protein and mRNA contents of ammonium assimilatory genes in T-DNA insertion lines for Aux/IAA and ARF with a wild type using hydroponic culture.
2. Materials and methods
2.1. Plant material
Insertion lines were purchased from the Arabidopsis thaliana T-DNA insertion mutant collection at the Arabidopsis Biological Resource Centre (ABRC), including arf3, arf7, arf8, arf11, arf13, arf15, arf16, arf21 and arf22 (Okushima et al. Citation2005) and iaa4, iaa6, iaa8, iaa9, iaa11, iaa12, iaa14, iaa17-5, iaa17-6, iaa19 and iaa31 (Overvoorde et al. Citation2005). The ammonium transporter quadruple knockout line has been previously reported (Yuan et al. Citation2007). Arabidopsis thaliana Columbia was used as the wild type.
2.2. Hydroponics
Arabidopsis (Arabidopsis thaliana Col-0) seeds were sown on moistened rock wool in half-cut 1.5-mL tubes. Tubes were set in a Styrofoam plate and floated on water in a plastic container. Plants were grown for 3 d in the dark. The seedlings were thinned and transferred to a nutrient solution and cultured as previously reported (Konishi et al. Citation2017).
2.3. Quantitative real-time PCR
Total RNA was extracted from plant samples frozen in liquid nitrogen using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Reverse transcription and DNase treatment were performed using a PrimeScript RT reagent kit with a gDNA Eraser (Takara Bio Inc., Otsu, Shiga, Japan) with 300 ng of total RNA in a 20-µL final volume. Quantitative real-time polymerase chain reaction (PCR) analysis was conducted using gene-specific primers for NADH-GOGAT, Fd-GOGAT1 and Fd-GOGAT2 (Konishi et al. Citation2014) and GLN1;1, GLN1;2, GLN1;3 and GLN2 (Ishiyama et al. Citation2004). Gene-specific primers for IAA17 were as follows: IAA17 cds realtimeF01 (5′-GCTTTCTAATGCTTTGTCCAAC-3′) and IAA17 cds realtimeR03 (5′-AACGCTTGCATGTATCGACG-3′). The PCR products were quantified on optical 96-well plates, using a LightCycler 480 (Roche Diagnostics KK, Tokyo, Japan). Ubiquitin (UBQ2) was used as a reference gene to standardize the signal intensity, according to the method used by Ishiyama et al. (Citation2004).
2.4. Element analysis and nitrogen usage index (UI)
The amounts of carbon and nitrogen in samples were determined with a Flash 2000 Elemental Analyzer (Thermo Fisher Scientific KK, Yokohama, Japan), similar to that used in a previous study (Konishi et al. Citation2017). After milling, 0.95–1.05 mg of powdered samples was used. Nitrogen usage index (UI) was calculated based on a method used by Siddiqi and Glass (Citation1981).
2.5. 15N-labeled ammonium uptake analysis
Ammonium uptake in roots was measured during incubation for 6 min in the nutrient solution, containing 0.2 or 2 mM 15N-labeled NH4Cl (ammonium chloride) (99.2 atom% 15N), following the method used by Loqué et al. (Citation2006). After milling, 0.95–1.05 mg of powdered samples was used for 15N determination, using isotope ratio mass spectrometry (Thermo Fisher Scientific KK, Yokohama, Japan).
2.6. Protein gel blot analysis
The protein extraction for GS (glutamine synthetase) was performed as per the method followed by Hayakawa et al. (Citation1990). Western blot analysis for GS, with anti-GS antibody, was performed according to the method followed Ishiyama et al. (Citation2004). The protein extraction for GOGAT was conducted according to the method followed by Ishiyama et al. (Citation1998). Western blot analysis for GOGAT was performed following the method of Hayakawa et al. (Citation1993).
2.7. Statistics
Microsoft Excel, with the add-in software Ekuseru-Toukei (Social Survey Research Information Co., Ltd. Tokyo, Japan) was used for the statistical analysis.
3. Results
3.1. T-DNA insertion line for IAA17 showed a reduction in biomass in ammonium nutrient
The plant nitrogen status influences the auxin content. However, the relationship between the auxin signaling module and nitrogen use remains to be elucidated. Here, the effect of the auxin signaling module on ammonium use was investigated using a reverse genetic approach. T-DNA insertion lines for Aux/IAA and ARF were grown in hydroponic culture. The dry weight of T-DNA insertion lines was compared with that of a wild type. Quadruple knockout for ammonium transporters (amt1;1, amt1;2, amt1;3 and amt2;1) was grown as a control. Some T-DNA insertion lines for ARF or Aux/IAA showed a reduction in dry weight. The T-DNA insertion line for IAA17 showed a severe growth reduction. illustrates the dry weights of all tested insertion lines grown in a nutrient solution containing 1 mM ammonium. The dry weights of T-DNA insertion lines for ARF7, ARF13, ARF15 and ARF22 were lower than that of Col-0, their genetic background. These lines showed reduced dry weight of their shoots and roots (). The decrease in dry weights ranged between 25 and 33% in the root and between 12 and 25% in the shoot. Dry weights of T-DNA insertion lines for IAA6, IAA14 and IAA17 were lower than that of Col-0 (). The dry weight of the insertion line for IAA17 was the lowest in the insertion lines for IAA. The dry weight decreased by 72% in the root and by 39% in the shoot. There were no significant differences in the dry weights between wild type and other T-DNA insertion lines for ARF, ARF3, ARF8, ARF11, ARF16 and ARF21 () or Aux/IAA, IAA4, IAA8, IAA9, IAA11, IAA12, IAA19 and IAA31 (). The dry weight of qko was lower than that of the wild type (). Dry weight data in indicate a specific growth reduction of insertion lines for IAA17 under ammonium conditions. Two independent T-DNA insertion lines for IAA17, iaa17-5 and iaa17-6 (Overvoorde et al. Citation2005), were grown in a nutrient solution containing 1 mM nitrate or 1 mM ammonium as a primary nitrogen source in hydroponic culture. First, the expression of IAA17 was quantified by qPCR (). The expression of IAA17 in iaa17-5 was decreased by 60% in the shoot and by 80% in the root, whereas that in iaa17-6 was decreased by 99% in the shoot and by 97% in the root (). Second, the dry weight of both insertion lines for IAA17 was lower than that of the wild type under ammonium-supplied conditions (); it was decreased by 29–38% in the shoot and by 36–42% in the root. However, under the nitrate-supplied conditions, the dry weight of iaa17-5 was not significantly different from that of the wild type, whereas that of iaa17-6 was decreased by 49% in the shoot and by 58% in the root (). Because previous studies have indicated the importance of IAA14 (Vidal et al. Citation2013) and ARF8 (Gifford et al. Citation2008) in nitrate stimulation-induced lateral root development, T-DNA insertion lines for IAA17, IAA14 and ARF8 were grown in 0.1 mM or 1 mM ammonium supply (). shows that growth reduction in the insertion line for IAA17 is alleviated by a lower ammonium supply. Neither iaa14 nor arf8 showed a significant difference in the dry weight from the wild type under both higher and lower ammonium supply (). Although there was no significant difference in the dry weight of the shoot between the wild type and iaa17-6 under 0.1 mM ammonium supply, the dry weight of iaa17-6 was decreased by 33% in the root (). The dry weight of iaa17-6 was reduced by 41% in the shoot and by 49% in root under 1 mM ammonium conditions (). The dry weight of qko was decreased by 40% in the root and shoot under 0.1 mM ammonium conditions, and it was reduced by 35% only in the shoot under 1 mM ammonium conditions (). Because insertion lines for IAA17 showed the most severe growth reduction among the mutants related to the auxin signaling module, we focused on the insertion line for IAA17.
Figure 1. Growth of T-DNA insertion lines for ARF and Aux/IAA in hydroponic culture solution containing ammonium as a major nitrogen source.
(A) Dry weight of the shoot and root of the T-DNA insertion lines for ARF (dark gray column), wild type (closed column), and quadruple knockout for the ammonium transporter (open column) grown in hydroponic solutions containing 1 mM NH4Cl as the major nitrogen source supplemented with 10 μM nitrate for 6 weeks. (B) Dry weight of the shoot and root of the T-DNA insertion lines for Aux/IAA (light gray column), wild type (closed column), and quadruple knockout for ammonium transporter (open column) grown in hydroponic solutions containing 1 mM NH4Cl as the major nitrogen source supplemented with 10 μM nitrate for 6 weeks. Bars indicate means ± standard deviation (SD; n = 6). Significant differences between wild type and mutant, identified by the Student’s t-test, are marked with asterisks: *P < 0.05.
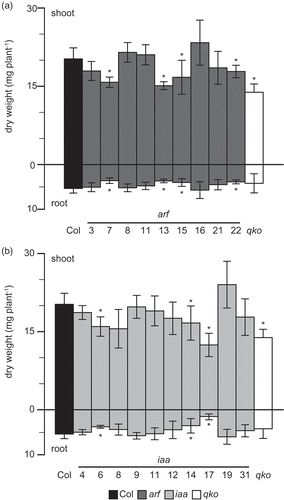
Figure 2. Growth of T-DNA insertion lines for IAA17 in hydroponic solution containing nitrate or ammonium.
(A) Quantitative real-time PCR (polymerase chain reaction) analysis of root RNA from wild type (filled columns) and iaa17-5 (dark gray columns) and iaa17-6 (light gray columns) using gene-specific primer sets for IAA17. Bars indicate the mean signal intensity of the IAA17 in shoot or in root ± standard deviation (SD; n = 6). (B) Growth of the wild type in hydroponic solutions containing either 1 mM NH4+ or 1 mM NO3− as the major nitrogen source supplemented with 10 μM nitrate for 6 weeks. Bars indicate the mean dry weight of the shoot or root ± standard deviation (SD; n = 6). Significant differences between wild type and mutant, identified by the Student’s t-test, are marked with asterisks: **P < 0.01, ***P < 0.005.
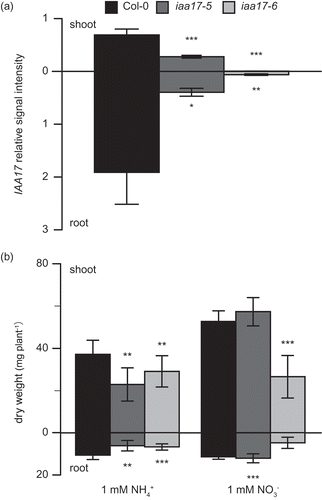
Figure 3. Growth of the wild type and T-DNA insertion lines for IAA14, IAA17 and ARF8 under ammonium supply conditions.
(A) Dry weight of the shoot and root of the T-DNA insertion lines for IAA14 or IAA17 (light gray column), ARF8 (dark gray column), wild type (closed column), and quadruple knockout for ammonium transporter (open column) grown in hydroponic solutions containing either 0.1 or 1 mM NH4Cl as the major nitrogen source supplemented with 10 μM nitrate for 6 weeks. Bars indicate means ± standard deviation (SD; n = 6). One-way analysis of variance (ANOVA) followed by Bonferroni tests was used, and significant differences at P < 0.05 within each group are indicated by different letters. (B) Images of the wild type and insertion line for IAA17.
3.2. Ammonium assimilation was decreased in the insertion line for IAA17
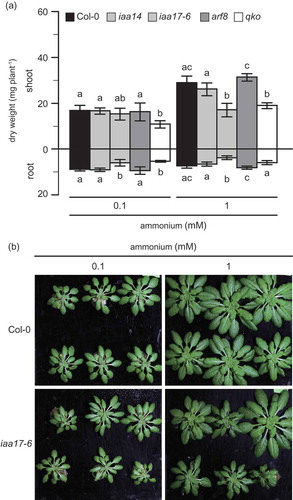
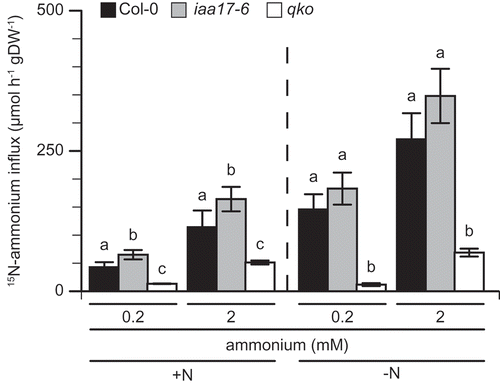
There are two primary components of nitrogen use efficiency, which are referred to as ‘uptake efficiency’ (the efficiency of absorption or uptake of supplied nitrogen) and ‘usage efficiency’ (the efficiency with which the total plant nitrogen is used to produce biomass) (Good et al. Citation2004). The two components of the insertion line for IAA17 were compared with those of the wild type to investigate the relation between the loss of IAA17 function and growth inhibition under ammonium conditions. Nitrogen usage efficiency was estimated as the biomass per unit of tissue nutrient concentration (Siddiqi and Glass Citation1981). Ammonium uptake was measured by short-term labeling using 15N-labeled ammonium (von Wirén et al. Citation2000). Because of the decrease in nitrogen metabolism, IAA17 insertion showed a reduction in dry weight under ammonium conditions. shows nitrogen and carbon content in the plant and illustrates the nitrogen UI. The nitrogen content in the wild type plant ranged from 2 to 3% under 0.1 mM ammonium supply and increased by 5% under 1 mM ammonium (). Under 0.1 mM ammonium supply, there was no significant difference in nitrogen content between the wild type and mutants. The nitrogen content in qko was significantly lower than that in the wild type, under 1 mM ammonium supply (). The carbon content in the wild type was approximately 60% in shoots under 1 mM ammonium supply and a higher ammonium supply decreased it to 50% (). The carbon content in wild type roots was not changed by ammonium supply (). The UI for nitrogen was calculated by nitrogen content and dry weight (Siddiqi and Glass Citation1981). The UI of the wild type under 0.1 mM ammonium supply was higher than that under 1 mM ammonium supply (). The UI of iaa17-6 was slightly lower than that of the wild type in the roots under 0.1 mM ammonium supply, and it was obviously lower than that of the wild type under 1 mM ammonium supply (). The UI of qko was lower under 0.1 mM ammonium supply. Conversely, there was no significant difference with the wild type under 1 mM ammonium supply (Fig. 4C). summarizes the 15N-labeled ammonium influx under nitrogen-sufficient and -deficient conditions. Ammonium influx at 0.2 mM ammonium supply in the wild type under nitrogen-deficient conditions was 3-fold higher than that under nitrogen-sufficient conditions (). Neither 0.2 nor 2 mM ammonium supply showed a significant difference in ammonium uptake between the wild type and iaa17-6 under nitrogen-deficient conditions (); however, ammonium uptake in iaa17-6 was significantly higher than that in the wild type under nitrogen-sufficient conditions (). Ammonium uptake of qko was always lower than that of the wild type ().
Figure 4. Nitrogen usage index (UI) in wild type and insertion line for IAA17.
Nitrogen (A) and carbon (B) amounts in roots and shoots of Col-0 (filled column), insertion line for IAA17 (gray column), and qko (open column). Plants were grown in a hydroponic culture solution containing either 0.1 or 1 mM NH4+ for 6 weeks. (c) UI was calculated based on the following formula:UI = Dw × (Dw ÷ N), where Dw is dry weight and N is nitrogen in organs. Bars indicate means ± standard deviation (SD; n = 6). One-way analysis of variance (ANOVA) followed by Bonferroni tests was used, and significant differences at P < 0.05 within each group are indicated by different letters.
Figure 5. 15N-labeled ammonium uptake in wild type, iaa17-6 and qko.
Uptake of 15N-labeled ammonium into the roots of Col-0 (filled column), iaa17-6 (gray column), and qko (open column). Plants were grown in a hydroponic solution containing 2 mM NH4NO3 for 6 weeks, after which they were transferred to a solution containing either 2 mM NH4NO3 (A) or no nitrogen (B) for 3 days. 15N-labeled ammonium was supplied at either 0.2 or 2 mM. Bars indicate means ± standard deviation (SD). Individual root samples were taken from six plants.
3.3. Ammonium-responsive expression of GLN1;2 was dependent on IAA17
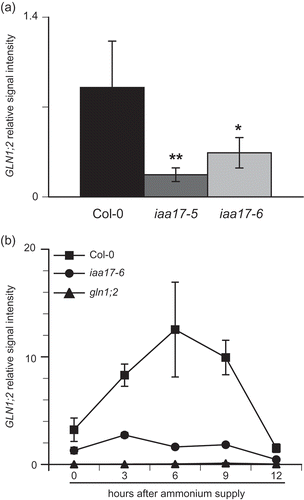
Because a decrease in nitrogen metabolism, and the other effect of T-DNA insertion in IAA17, resulted in growth inhibition of iaa17-6, the expression of genes was compared with the accumulation of proteins related to ammonium assimilation. GLN1;2 (Konishi et al. Citation2017) and NADH-GOGAT (Konishi et al. Citation2014) were the key enzymes for ammonium assimilation in a root. The expression of ammonium assimilatory genes was quantified by qPCR. Protein gel blot analysis quantified the accumulation of ammonium assimilatory proteins. A loss of IAA17 leads to a disruption of ammonium-responsive expression of GLN1;2. shows the gene expression and accumulation of GS and GOGAT under 2 mM NH4NO3 (ammonium nitrate) conditions. GLN2 was expressed at high levels in the shoot, but at low levels in the root (). The expression of GLN1;2 and GLN2 in the roots of iaa17-6 was significantly lower than that of the wild type (), whereas there was no difference in expression of GLN1;2 and GLN2 in the shoot between the wild type and iaa17-6. The expression of GLN1;2 was decreased by 25% in iaa17-6 (). There was no significant difference in the expression of GLN1;1 and GLN1;3 between the wild type and iaa17-6 (). Protein gel blot analysis showed that GS2 protein was highly accumulated only in the shoot (, ), whereas GS1 protein was accumulated in the shoot and root (, ). There was no difference in the accumulation of GS in the shoot between the wild type and iaa17-6 (); however, in the root, the accumulation of GS1 showed a marked reduction in iaa17-6 compared with the wild type (Fig. 6C). The expression of GLU1 was high in the shoot, whereas it was almost not expressed in the root (Fig. 6D). GLT1 was expressed in both the shoot and root (Fig. 6D). The expression of GLU2 was low in the shoot and root (Fig. 6D). The expression of GLU1 in iaa17-6 was lower than that in the wild type (Fig. 6D). However, there was no difference in the accumulation of Fd-GOGAT in the shoot between the wild type and iaa17-6 (). summarizes the expression of GLN1;2 in the root. GLN1;2 was reduced by approximately 80% in iaa17-5 and 60% in iaa17-6, respectively, in root under nitrogen-sufficient conditions (). Ammonium chloride was provided to plants grown in hydroponic culture at 1 mM. Before ammonium supply, plants were transferred to a hydroponic solution containing no nitrogen and kept for 3 days. The expression of GLN1;2 in the wild type sharply increased after ammonium supply; it was highest at 6 h after ammonium supply () and then it decreased. The expression of GLN1;2 in iaa17-6 did not respond to the ammonium supply, and it maintained similar transcriptional levels during ammonium supply (). The T-DNA insertion line for GLN1;2 did not express GLN1;2 (Fig. 7B).
Figure 6. mRNA expression and accumulation of the genes related to ammonium use.
Plants were grown in a hydroponic solution containing 2 mM NH4NO3 for 6 weeks. Transcriptional levels of glutamine synthetase (A, GLN1;1, GLN1;2, GLN1;3 and GLN2) and glutamate synthase [D, GLU1, (Fd-GOGAT1), GLU2 (Fd-GOGAT2), and GLT1 [NADH-glutamine oxoglutarate aminotransferase (NADH-GOGAT)] were determined with qPCR. Bars indicate the relative signal intensity of the genes in either shoot or root ± standard deviation (SD; n = 6). Significant differences between wild type and mutant, identified by the Student’s t-test, are marked with asterisks: *P < 0.05. The accumulation of GS (B and C) and GOGAT (E) were determined using protein gel blot analysis. Antibodies raised against recombinant rice GS1 (B for shoot sample, and C for root sample) and antibodies raised against rice Fd-GOGAT (E for shoot sample), were used for staining.
![Figure 6. mRNA expression and accumulation of the genes related to ammonium use.Plants were grown in a hydroponic solution containing 2 mM NH4NO3 for 6 weeks. Transcriptional levels of glutamine synthetase (A, GLN1;1, GLN1;2, GLN1;3 and GLN2) and glutamate synthase [D, GLU1, (Fd-GOGAT1), GLU2 (Fd-GOGAT2), and GLT1 [NADH-glutamine oxoglutarate aminotransferase (NADH-GOGAT)] were determined with qPCR. Bars indicate the relative signal intensity of the genes in either shoot or root ± standard deviation (SD; n = 6). Significant differences between wild type and mutant, identified by the Student’s t-test, are marked with asterisks: *P < 0.05. The accumulation of GS (B and C) and GOGAT (E) were determined using protein gel blot analysis. Antibodies raised against recombinant rice GS1 (B for shoot sample, and C for root sample) and antibodies raised against rice Fd-GOGAT (E for shoot sample), were used for staining.](/cms/asset/d71d97b8-252f-4d8d-9156-1dae8d8aab86/tssp_a_1314178_f0006_b.gif)
Figure 7. Ammonium-responsive expression of root GLN1;2 in wild type and T-DNA insertion line for IAA17 and GLN1;2.
(A) Plants were grown in hydroponic solution containing 2 mM NH4NO3 for 6 weeks. The transcriptional levels of GLN1;2 in wild-type (opened column), iaa17-5 (dark gray column) and iaa17-6 (light gray column) were determined by qPCR. Bars indicate means ± standard deviation (SD; n = 4 plants).(B) Plants were grown in hydroponic solution containing 2 mM NH4NO3 for 6 weeks, following which they were transferred to a solution containing no nitrogen for 3 d, and were then transferred to a solution containing 1 mM NH4Cl. After transfer to the ammonium solution, the roots were sequentially harvested at 0, 3, 6, 9 and 12 h. The transcriptional level of GLN1;2 was determined by qPCR. Spots indicate means ± standard deviation (SD; n = 6 plants).
4. Discussion
Nitrate supply suppresses primary root elongation and stimulates lateral root development (Vidal et al. Citation2013). An approach using systems biology reveals that nitrate-responsive lateral root development is regulated by auxin signal transduction modules (Gifford et al. Citation2008). The plant uses not only nitrate but also ammonium. Few researchers have addressed the effect of auxin signaling on ammonium. This study proposed a relation between ammonium use and auxin signaling because previous studies have shown that ammonium supply to a root alters the levels of auxin in the root. For example, supplying ammonium to rice (Oryza sativa L) roots decreases activated auxin but increases inactivated auxin (Tamura et al. Citation2010). This study compares the growth of T-DNA insertion lines for auxins with that of the wild type in a hydroponic culture solution containing ammonium as a primary nitrogen source. Supplying higher levels of ammonium reduced the dry weight of the T-DNA insertion line for IAA17 (–). The insertion line for IAA17 inhibited ammonium metabolism () but did not reduce the ammonium uptake in the root (Fig. 5). IAA17 insertion reduced the ammonium-responsive expression of GLN1;2 and reduced the accumulation of GS1 in the root (–). These results indicate that Aux/IAA contributed not only to nitrate-induced lateral root formation but also to ammonium-responsive gene expression. The IAA17 insertion-dependent decrease in GLN1;2 could reduce the assimilation of ammonium to glutamine, which in turn may reduce the ammonium-responsive gene expression which is necessary for ammonium use. Ammonium is assimilated to glutamine, and either glutamine itself or a glutamine metabolite regulates the gene expression (Hirose et al. Citation1997; Ishiyama et al. Citation1998; Hirose and Yamaya Citation1999). GLN1;2 is an essential gene for ammonium assimilation in the root (Lothier et al. Citation2011; Konishi et al. Citation2014) because T-DNA insertion for GLN1;2 leads to the reduction of glutamine under higher ammonium conditions (Konishi et al. Citation2017). This study expands the understanding of the physiological function of the auxin signaling module. The increase in auxin concentration leads to Aux/IAA protein degradation, and the auxin signal transduction is derepressed (Worley et al. Citation2000). However, T-DNA insertion for IAA17 does not change the auxin signaling (Overvoorde et al. Citation2005). This is because the Arabidopsis genome encodes several Aux/IAA genes, and it substitutes other Aux/IAA genes for IAA17 (Overvoorde et al. Citation2005). Our study provides two possibilities: (1) GLN1;2-dependent ammonium use might be regulated by IAA17-dependent auxin signaling, or (2) IAA17-dependent transcriptional control is not limited in auxin signaling. Auxin signaling module-dependent regulation of ammonium-responsive gene expression may relate to the lateral root development stimulated by ammonium supply. Previous studies have suggested that lateral root development in barley (Hordeum vulgare L.) root is dependent not only on nitrate but also on ammonium (Drew Citation1975); ammonium transporter 1;3 is one of the essential components for ammonium to trigger lateral root branching (Lima et al. Citation2010). However, the effects of IAA17 on ammonium-dependent lateral root branching remain to be determined. Future studies should focus on lateral root branching in insertion lines for IAA17 and GLN1;2 to investigate the mechanisms of ammonium-dependent root development.
Acknowledgments
We are grateful to Mrs. Ikumi Sakurada-Enomoto for technical assistance and figure preparation. The Japan Advanced Plant Research Network supported by JSPS is also acknowledged for the use of the Elemental Analyzer.
Additional information
Funding
References
- Caba JM, Centeno ML, Fernandez B, Gresshoff PM, Ligero F 2000: Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type. Planta, 211, 98–104. doi:10.1007/s004250000265
- Drew MC 1975: Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist, 75, 479–490. doi:10.1111/nph.1975.75.issue-3
- Forde BG 2014: Nitrogen signalling pathways shaping root system architecture: an update. Curr. Opin. Plant Biol., 21, 30–36. doi:10.1016/j.pbi.2014.06.004
- Fukaki H, Tameda S, Masuda H, Tasaka M 2002: Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of Arabidopsis. Plant J., 29, 153–168. doi:10.1046/j.0960-7412.2001.01201.x
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD 2008: Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. U.S.A., 105, 803–808. doi:10.1073/pnas.0709559105
- Good AG, Shrawat AK, Muench DG 2004: Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci., 9, 597–605. doi:10.1016/j.tplants.2004.10.008
- Hayakawa T, Kamachi K, Oikawa M, Ojima K, Yamaya T 1990: Response of glutamine-synthetase and glutamate synthase isoforms to nitrogen-sources in rice cell-cultures. Plant . Physiol., 31, 1071–1077.
- Hayakawa T, Yamaya T, Mae T, Ojima K 1993: Changes in the content of 2 glutamate synthase proteins in spikelets of rice (Oryza sativa) plants during ripening. Plant Physiol., 101, 1257–1262. doi:10.1104/pp.101.4.1257
- Hirose N, Hayakawa T, Yamaya T 1997: Inducible accumulation of mRNA for NADH-dependent glutamate synthase in rice roots in response to ammonium ions. Plant . Physiol., 38, 1295–1297. doi:10.1093/oxfordjournals.pcp.a029120
- Hirose N, Yamaya T 1999: Okadaic acid mimics nitrogen-stimulated transcription of the NADH-glutamate synthase gene in rice cell cultures. Plant Physiol., 121, 805–812. doi:10.1104/pp.121.3.805
- Ishiyama K, Hayakawa T, Yamaya T 1998: Expression of NADH-dependent glutamate synthase protein in the epidermis and exodermis of rice roots in response to the supply of ammonium ions. Planta, 204, 288–294. doi:10.1007/s004250050258
- Ishiyama K, Inoue E, Watanabe-Takahashi A, Obara M, Yamaya T, Takahashi H 2004: Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. J. Biol. Chem., 279, 16598–16605. doi:10.1074/jbc.M313710200
- Kellermeier F, Armengaud P, Seditas TJ, Danku J, Salt DE, Amtmann A 2014: Analysis of the root system architecture of arabidopsis provides a quantitative readout of crosstalk between nutritional signals. Plant Cell, 26, 1480–1496. doi:10.1105/tpc.113.122101
- Kiba T, Kudo T, Kojima M, Sakakibara H 2011: Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot., 62, 1399–1409. doi:10.1093/jxb/erq410
- Konishi N, Ishiyama K, Beier MP et al. 2017: Contribution of two glutamine synthetase isozymes to ammonium assimilation in Arabidopsis roots. J. Exp. Bot. 68(3), 613–625.
- Konishi N, Ishiyama K, Matsuoka K et al. 2014: NADH-dependent glutamate synthase plays a crucial role in assimilating ammonium in the Arabidopsis root. Physiol. Plant, 152, 138–151. doi:10.1111/ppl.12177
- Kramer EM, Ackelsberg EM 2015: Auxin metabolism rates and implications for plant development. Front Plant Sci, 6. doi:10.3389/fpls.2015.00150
- Lavenus J, Goh T, Roberts I et al. 2013: Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci., 18, 455–463. doi:10.1016/j.tplants.2013.04.006
- Lea PJ, Azevedo RA 2006: Nitrogen use efficiency. 1. Uptake of nitrogen from the soil. Ann. Appl. Biol., 149, 243–247. doi:10.1111/aab.2006.149.issue-3
- Lima JE, Kojima S, Takahashi H, von Wirén N 2010: Ammonium triggers lateral root branching in arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell, 22, 3621–3633. doi:10.1105/tpc.110.076216
- Loqué D, Yuan L, Kojima S et al. 2006: Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J., 48, 522–534. doi:10.1111/j.1365-313X.2006.02887.x
- Lothier J, Gaufichon L, Sormani R et al. 2011: The cytosolic glutamine synthetase GLN1;2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. J. Exp. Bot., 62, 1375–1390. doi:10.1093/jxb/erq299
- Ma W, Li J, Qu B et al. 2014: Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J., 78, 70–79. doi:10.1111/tpj.12448
- Marschner H 1995: Mineral Nutrition of Higher Plants, Academic Press, London.
- Okushima Y, Overvoorde PJ, Arima K et al. 2005: Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell, 17, 444–463. doi:10.1105/tpc.104.028316
- Overvoorde PJ, Okushima Y, Alonso JM et al. 2005: Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell, 17, 3282–3300. doi:10.1105/tpc.105.036723
- Petricka JJ, Winter CM, Benfey PN 2012: Control of Arabidopsis root development. Annu. Rev. Plant Biol, 63, 563–590. doi:10.1146/annurev-arplant-042811-105501
- Siddiqi MY, Glass ADM 1981: Utilization index - a modified approach to the estimation and comparison of nutrient utilization efficiency in plants. J Plant Nutr, 4, 289–302. doi:10.1080/01904168109362919
- Tamura W, Hidaka Y, Tabuchi M et al. 2010: Reverse genetics approach to characterize a function of NADH-glutamate synthase1 in rice plants. Amino Acids, 39, 1003–1012. doi:10.1007/s00726-010-0531-5
- Tian QY, Chen FJ, Liu JX, Zhang FS, Mi GH 2008: Inhibition of maize root growth by high nitrate supply is correlated with reduced IAA levels in roots. J. Plant Physiol., 165, 942–951. doi:10.1016/j.jplph.2007.02.011
- Vidal EA, Moyano TC, Riveras E, Contreras-Lopez O, Gutierrez RA 2013: Systems approaches map regulatory networks downstream of the auxin receptor AFB3 in the nitrate response of Arabidopsis thaliana roots. Proc. Natl. Acad. Sci. U.S.A., 110, 12840–12845. doi:10.1073/pnas.1310937110
- Von Wirén N, Gazzarrini S, Gojon A, Frommer WB 2000: The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol., 3, 254–261. doi:10.1016/S1369-5266(00)00073-X
- Worley CK, Zenser N, Ramos J et al. 2000: Degradation of Aux/IAA proteins is essential for normal auxin signalling. Plant J., 21, 553–562. doi:10.1046/j.1365-313x.2000.00703.x
- Yuan L, Loque D, Kojima S et al. 2007: The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell, 19, 2636–2652. doi:10.1105/tpc.107.052134
- Zhang HM, Forde BG 2000: Regulation of Arabidopsis root development by nitrate availability. J. Exp. Bot., 51, 51–59. doi:10.1093/jxb/51.342.51
