ABSTRACT
Uncertainties exist about the importance of rhizobia inoculant and starter nitrogen (N) application in dry pea (Pisum sativum L.) production. Three field experiments were conducted to evaluate how rhizobia inoculant and starter N fertilizer affect pea seed yield and protein concentration in a semi-arid environment in central Montana. Commercial rhizobia inoculant was mixed with seed prior to planting at the manufacturer’s recommended rate. Starter N fertilizers were applied into the same furrow as seed at 0, 22, 44 and 88 kg ha−1 as urea, slow-release polymer-coated N fertilizer (ESN), and a combination of both. The application of rhizobia inoculant had no or a very small beneficial effect on pea yield in lands with a previous history of peas. In a land without pea history, application of rhizobia increased pea seed yield by 16%. The positive effect of starter N was only pronounced when initial soil N was low (≤ 10 kg ha−1 nitrate-nitrogen), which increased net return by up to US$ 42 ha−1. In this condition, application of slow-release N outperformed urea. However, application of starter N (especially with urea) had a negative effect on pea establishment, vigor and seed yield when soil initial N was high (≥ 44 kg ha−1 NO3-N). The results indicate that the rate, placement and form of the starter N must be optimized to benefit pea yield and protein without detrimental effects on germination and nodulation. Moreover, application of starter N must be guided by the soil nitrate content.
1. Introduction
Dry peas (Pisum sativum Linn.) have long been recognized as an excellent source of protein in the diet of humans and livestock, as well as an important component of cropping systems worldwide (Ito et al. Citation2016; Tao et al. Citation2017). Today, dry peas are grown in more than 100 countries, and the Food and Agriculture Organization of the United Nations declared the year 2016 as the International Year of Pulses. Dry pea production in the USA has been increasing in the past few decades, especially in Montana, where pea production exceeded 243,000 ha in 2014, and this state has become the number one dry pea producer in the USA (Montana Agricultural Statistics Citation2014).
Numerous studies have been conducted evaluating nitrogen (N) fertilization and N2 fixation in grain legumes in various countries. The majority of these studies, however, have focused on soybean (Glycine Max (L.) merr.) (see review by Salvagiotti et al. Citation2008), and other important legumes, including dry peas, have not received much attention. Growers are still facing uncertainties in the cold semi-arid environments in terms of optimal N fertilization strategies for peas. Responses of grain legumes to N fertilization are reportedly highly variable as a function of overall environmental conditions (Van Kessel and Hartley Citation2000); the plant cultivar used (Kutcher et al. Citation2002); the availability of compatible bacteria strains or rhizobia inoculation (Chemining’wa and Vessey Citation2006); and the form, amount, time and method of N application (McKenzie et al. Citation2001). Some producers rely solely on rhizobia inoculation with no additional N fertilizer while others use starter N fertilizer in addition to rhizobia inoculants. Therefore, additional studies on starter N and rhizobia inoculant for peas are needed to enhance yield and quality of the produce, improve N use efficiency, reduce N losses, and maximize economic returns.
The necessity of using rhizobia inoculants every year is still in question. In some studies, the beneficial effect of rhizobia inoculation on agriculturally important legumes has been reported (Kutcher et al. Citation2002; Chemining’wa and Vessey Citation2006), whereas in many others rhizobia inoculants were found to be only partially effective or completely ineffective. McKenzie et al. (Citation2001) evaluated pea yield response to rhizobia inoculant in 22 field trials and found significant yield increases due to inoculation in only 41% of the trials, with an average increase of 14%. Vessey (Citation2004) reviewed field studies in the Canadian prairies and reported that pea yields responded to rhizobia inoculation in only 21 of 58 site-years. They declared that fields with no response to inoculation were those with a recent history of the crop in which an appreciable population of effective rhizobia is usually already present. In favorable environments, and in the absence of conditions affecting soil rhizobia populations such as extreme soil pH or temperature, osmotic stress, and high concentrations of heavy metals (Catroux et al. Citation2001), refraining from using commercial rhizobia inoculants can be a cost-saving measure, especially for large-scale farms. However, in the wheat-based cropping systems in Montana, peas are only grown once every 3 years in the crop rotations. It is not clear whether inoculation is necessary for such a crop rotation scheme.
The next important question is about the efficiency of starter N in addition to rhizobia inoculants. The percentage of plant N derived from N2 fixation for major legumes including pea has been estimated at 63 and 65% in experiments and farmers’ fields, respectively (Herridge et al. Citation2008). The remaining N demand must be provided from the soil pool or from inorganic fertilizer applications. It has been reported that in soils with low levels of mineral N, a period of ‘N hunger’ before the establishment of an effective plant–bacteria symbiosis can negatively affect yield in grain legumes (van Kessel and Hartley Citation2000). This is because the crop root system and root nodulation are not fully developed at this stage to supply the crop’s N demand. The application of starter N eliminates this N deficiency period during early plant growth and development (Starling et al. Citation1998). McKenzie et al. (Citation2001) found that when spring soil nitrate-nitrogen (NO3-N) to 30 cm depth was less than 20 kg N ha–1, application of starter N fertilizer increased pea yield in 33% of the trials by an average of 11%. They reported a modest benefit of rhizobia inoculation or starter N fertilizer in most cases, which they attributed to adequate nodulation of the crop by soil indigenous rhizobia.
In addition to yield, pea protein concentration is also an important criterion. Lack of N during seed filling can cause a reduction in seed protein concentration in peas (Holl and Vose Citation1980) and mineral N supplementation is expected to improve seed pea.
In the polymer coated urea fertilizers, direct contact of urea to urease is minimized (Huang et al. Citation2016). Therefore, the N of these fertilizers releases more slowly than that of regular fertilizers, suppling a higher amount of N at later growth stages. Therefore, a positive impact of these fertilizers on protein concentration is expected. There is little documentation to support this idea in peas.
The aim of this study was to determine the efficiency of commercial rhizobia inoculant and of starter N application at different rates and in different forms on the performance of pea in a temperate semi-arid rainfed environment. We hypothesized that application of commercial rhizobia inoculant and/or starter N is not necessarily useful, and soil available N and rotation history should play an important role in determining the necessity of using commercial rhizobia and/or starter N. We also hypothesized that the application of slow-release N has a positive impact on pea seed protein concentration.
2. Materials and methods
2.1. Site description
In 2012, field trials were conducted at two sites: the Central Agricultural Research Center (CARC) and a cooperating farmer’s field (SG Farm), both located in central Montana (47°03ʹN, 109°57ʹW; 1311 m elevation). The CARC site has been planted with peas in the past, while the SG site had no history of pea. The experiment was repeated at the CARC in 2015. Selected soil characteristics are presented in . The soil series in this region is classified as Judith-Danvers or as leptic Cambisol according to the World Reference Base, with gravelly clay loam (0–66 cm) and extremely gravelly sandy clay loam (66–152 cm) textures. Soils are well drained with water table depths > 200 cm, 60% calcium carbonate in profile, and non-saline to slightly saline conditions (0.0 to 4.0 mmhos cm−1).
Table 1. Soil characteristics at the experimental sites (on the station [CARC] and on the farmer’s field [SG Farm]).
Weather parameters, including cumulative precipitation and average temperature during pea growing seasons in 2012 and 2015, are shown in . The mean annual precipitation in this region is 355–457 mm, and the mean annual air temperature is 4–7°C with frost-free periods of 100–120 d.
Table 2. Monthly average air temperature and monthly cumulative precipitation during the experiment in 2012 and 2015, and their long-term average (LTA) in central Montana.
2.2. Experimental details
Montech 4152, a mid-maturing yellow pea cultivar with large seed size and tall plants, was used in the study. The trials were conducted on a randomized complete block design with four replications. Treatments comprised four levels of starter N (0, 22, 44 and 88 kg ha−1) applied as either urea (22 U with 22 kg N ha−1), or as the polymer-coated slow-release N product ESN® with 44% N obtained from Agrium Inc., Calgary, Canada (22 ESN with 22 kg N ha−1 in the form of ESN), or as a combination of these (22 U + 22 ESN). In 2015, an additional level of starter N at 88 kg ha−1 was used (44 U + 44 ESN). All starter N treatments were applied with and without rhizobia inoculation. The commercial inoculant (Nodulator, BASF Corporation, Triangle Park, NC) was mixed with seeds prior to planting at the manufacturer-recommended rate. Starter N fertilizers were applied into the seed furrow at seeding time using a locally made no-till plot drill pulled by a 65-hp tractor.
Pea seeds were sown in mid-April (according to the local production guide) of 2012 and 2015 at a seeding rate of 128 seeds m−2 at a 0.3-m row spacing. Plots were 6 m long and 1.5 m wide. No phosphorus (P), potassium (K) or molybdenum (Mo) fertilizers were used, based on soil test results. Prior to seeding, seeds were pre-treated with fludioxonil and mefenoxam fungicide (Apron MAXX® RTU, Syngenta, Greensboro, NC) and thiamethoxam insecticide (Cruiser MAXX®, Syngenta, Greensboro, NC) to control soil-borne diseases and pea leaf weevil infestation. The compatibility of these pesticides with rhizobia inoculants had previously been tested. Crops were grown under strict rainfed conditions with no supplemental irrigation.
In 2012, only seed yield was evaluated. In 2015, however, seedling establishment and vigor, root nodulation, final biomass, seed protein, and post-harvest soil residual NO3-N were additionally determined. Eighteen days after seeding, plants in each plot were counted to determine seedling establishment. Ten random plants were carefully uprooted from each plot to measure seedling root and shoot lengths and weights. The seedling vigor index (SVI) was calculated as follows (Sparg et al. Citation2006):
At the seven-leaf stage and full-bloom stage, two additional samples (10 plants) were taken from each plot, and the number of active nodules per plant was counted following the method described by (Xiang and Hongyu Citation2007). Plant total biomass was measured after drying the samples at 65°C for 96 h.
At seed maturity in August, plants were hand harvested from three center rows 1 m long in each plot. After measuring total biomass the seeds were threshed; then, seed weight and moisture content were determined. A sample of seed from each plot was taken for N analysis and converted to protein content by multiplying by a factor of 6.25. Nitrogen concentration in seed was determined by the standard Kjeldahl method. Seed yield and protein reported are adjusted to 13% (weight/weight) moisture content.
Following the harvest in 2015, soil core samples (0–15 and 15–30 cm depths) were taken from all experimental plots, and residual NO3-N (mg kg−1) was determined. Five samples (3 cm in diameter each) were randomly taken from 0–15 and 15–30 cm depths in each plot, then mixed to make a composite sample. Two subsoil samples were weighed (10 g each). One subsoil sample was placed in a specimen bottle; 40 mL 2 M potassium chloride (KCl) was added and the sample was shaken for 1 h at 200 rpm. After extraction, the supernatant was gravity-filtered through filter paper. The filtered solution was then used to determine soil NO3-N following the QuickChem method 12-107-04-1-B through cadmium (Cd) reduction with a flow injection auto-analyzer (Sechtig Citation1992). The other soil subsample was dried at 105°C for 48 h to determine gravimetric soil moisture content. Using the soil moisture content, the NO3-N concentration measured in the KCl extract was converted to soil NO3-N concentration on a dry weight basis (mg kg−1).
2.3. Data analysis
Data was subjected to analysis of variance (ANOVA) using Proc General Linear Model in SAS. Data of each year was analyzed individually. Means were separated using Fisher’s Least Significant Difference (LSD) test at P < 0.05.
3. Results
3.1. Seed yield
In 2012, average seed yields at CARC and at the SG Farm were 1401 and 1469 kg ha−1, respectively (). The response of pea seed yield to N treatments varied between sites. At CARC, pea seed yield responded positively to the application of starter N, irrespective of application rate and rhizobium inoculation. At this location, the lowest yield (1303 kg ha−1) was obtained in the control treatment without N application (0 N). Starter N increased yield by 9% and 12.5% in 22 U and 22 U + 22 ESN, respectively, which were statistically similar. Application of rhizobium inoculant increased pea yield slightly, by 4% (P ≤ 0.05), at CARC, but the impact was much stronger at the farmer’s field site (SG; ). In the 2015 on-station (CARC) experiment, pea final biomass and seed yield also responded significantly to starter N, but not to the application of rhizobia inoculant (). The interaction of starter N and rhizobia inoculation was also non-significant. Application of starter N negatively impacted final biomass and seed yield. As a higher rate of N was used, the negative effect was larger. It should be noted that the negative impact of starter N was less pronounced when ESN was applied.
Table 3. ANOVA and mean comparisons for the effect of rhizobia inoculation and starter N fertilizer on pea yield at two experimental sites (on-station [CARC] and on farmer’s field [SG Farm]) in central Montana during 2012.
Table 4. Effects of rhizobia inoculation and starter N fertilizer application on growth, selected performance attributes, and seed yield of pea (on-station experiment, CARC- 2015).
3.2. Seedling establishment and nodule formation
In the 2015 experiment, the application of starter N had a significant effect on the seedling establishment and the SVI, but the effect of rhizobia inoculant on these traits was not significant (). Application of starter N had a disadvantageous effect on pea seedling establishment and SVI. As a higher rate of starter N was used (urea and/or ESN), lower seedling establishment and vigor were obtained. The slow-release fertilizer (ESN) had less destructive effects on pea establishment compared to urea. Starter N negatively impacted seed germination and establishment, irrespective of rhizobia inoculation.
Averaged over all treatments, individual pea plants had about 19 active nodules at the seven-leaf stage (). As plants aged, the number of nodules declined and only five active nodules (on average) were found on plants’ root at the full-bloom stage. Seed inoculation with rhizobium bacteria did not affect root nodulation. On the other hand, starter N had a negative effect on root nodulation. In the 0 N treatment, each plant had 35.4 active nodules at the seven-leaf stage (averaged over rhizobia inoculation treatments). The number of active nodules on plant’s roots declined as the starter N was applied, and decreased to only 3.7 nodules plant−1 when 44 U + 44 ESN was used.
Application of starter N not only affected root nodulation but also reduced plant biomass at early growth stages (). This could be a consequence of detrimental effects of excessive N on SVI, which were later reflected in smaller and less vigorous plants at the seven-leaf stage. Lower biomass in plants receiving starter N was recovered later (full-bloom stage), and no significant difference was observed between N treatments in terms of single-plant biomass.
3.3. Seed protein
Among the pea characteristics evaluated in this study, seed protein was the only trait that responded positively to the application of starter N in 2015 (). Application of higher rates of starter N (22 U + 22 ESN and 44 U + 44 ESN) significantly increased seed protein concentration. It is worth noting that the high protein concentration in the 44 U + 44 ESN treatment was associated with very low seed yield.
3.4. Soil residual N
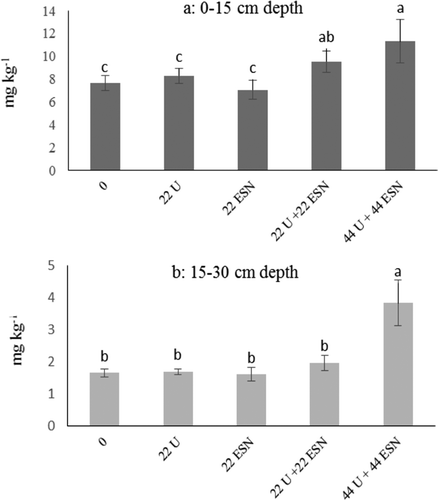
Following pea harvest, the soil of each plot was sampled in 0–0.15 m and 0.15–0.30 m depths to monitor post-harvest residual NO3-N. As shown in , in plots that received high rates of starter N, a high concentration of NO3-N remained in the soil after harvest. Post-harvest nitrate is highly prone to leaching, especially in shallow soils of central Montana, which can contribute to the contamination of below-ground water resources.
Figure 1. Post-harvest soil residual nitrate-nitrogen (NO3-N) in (a) 0–15 cm and (b) 15–30 cm depths for the on-station experiment at CARC in 2015. Vertical bars represent standard error of the means (n = 8). 22 N U: 22 kg N ha−1 in the form of urea; 22 N ESN: 22 kg N ha−1 in the form of ESN; 44 U: 44 kg N ha−1 in the form of urea; 44 ESN: 44 kg N ha−1 in the form of ESN.
4. Discussion
4.1. Effect of seed inoculation on pea growth and yield
Pea production area has been rapidly increasing in the last decade, from < 15,000 ha in 2004 to > 243,700 ha in 2015 in Montana (), and the production area is expanding from NE Montana to other regions across the state. While NE Montana has a longer pea production history (> 8 yr), this crop is new to the other regions. Questions have been raised by both new and experienced producers regarding whether rhizobia inoculant should be used every time they grow peas. Reports in the literature show that grain legumes usually respond to rhizobia inoculation when they are introduced into new fields where soils lack a sufficient population of compatible indigenous bacteria. In soils with a history of a targeted legume, however, a reasonable population of the effective rhizobia is usually present; sufficient to establish an effective plant–bacteria symbiosis. In the current study, the soil at CARC had a history of pea rotation and the population of the indigenous bacteria seemed to be capable of supporting adequate levels of N fixation. As no pea was previously grown on the SG Farm, application of commercial rhizobia inoculant provided a benefit in this field. Our results are in line with the reports of others (van Kessel and Hartley Citation2000; McKenzie et al. Citation2001). In 22 field trials, McKenzie et al. (Citation2001) found significant yield increases due to inoculation in 41% of the trials, with an average increase of 14%, and Vessey (Citation2004) reported that pea yields responded to rhizobia inoculation in only 21 of 58 site-years. However, it is not clear how many of these studies were conducted in a field with pea production history. To prevent a buildup of root diseases, Montana farmers normally rotate pea with wheat (Triticum aestivum L.) or barley (Hordeum vulgare L.) and only grow pea once every 3 years. More investigations are needed to reveal whether this once in a 3-year rotation scheme creates an adequate pool of indigenous rhizobia in the soil to remove the need for exogenous application. Our study at the CARC site indicated that the benefit of application of exogenous bacteria is small in this situation. However, to prevent a crop failure due to a low indigenous rhizobia population in this semi-arid rainfed environment, application of commercial rhizobia inoculant with pea seed is still warranted.
4.2. Effect of starter N fertilizer on plant–rhizobium symbiosis and pea yield
Establishment of an effective plant–bacteria symbiosis in legumes’ root system takes time. This ‘N hunger’ period can negatively affect yield in grain legumes (van Kessel and Hartley Citation2000). Starter N, in fact, is used to eliminate this N-deficiency period, especially when soil initial N is low. Our study at CARC showed that when pre-planting soil NO3-N was low (10 kg ha−1), application of starter N showed a positive effect (up to 19% yield response). When the initial soil NO3-N was higher (44 kg ha−1), however, application of starter N reduced pea yield. Furthermore, we found detrimental effects of excessive N on seedling establishment, vigor and plant nodulation. Similarly, McKenzie et al. (Citation2001) found that when spring soil NO3-N to 30 cm depth was less than 20 kg N ha–1, application of starter N increased pea yield in 33% of their trials, by an average of 11%. The soil in our 2015 experiment contained high levels of mineral N; therefore, the application of starter N did not provide a positive response. It should be noted that yield potential in rainfed farming systems of central Montana is mainly limited by moisture availability, not nutrient availability.
In the current experiment, the starter fertilizer was placed in the same furrow with seeds, which probably is the reason for the negative impact of N fertilizer on seed germination. It is expected that the influence on germination would be smaller if the fertilizer were placed 5 cm deeper than the seed furrow. Therefore, farmers may use planters with openers that were designed to place starter fertilizer 5 cm or more away from the seed. If this is not possible, the application rate of N that goes into the same furrow with seeds should be lowered to prevent its negative impact on seed germination and establishment. Above all, soil testing before seeding is strongly recommended to determine whether starter N is needed.
Overapplication of starter N not only negatively affects N fixation, but may also increase the post-harvest residual N in the soil. In a shallow soil, such as that in central Montana, higher nitrate residual will increase the likelihood of nitrate leaching and environmental pollution.
Application of starter N must be economically profitable. We ran a simple economic analysis on the cost/benefit of starter N based on our 2012 results (we did not consider the 2015 results since starter N had a negative effect on yield in that year). In 2012, Montana dry pea was traded at a price of US$ 0.321 kg−1. Application of the best-performing fertilizer (22 ESN + rhizobia inoculant) at CARC and the SG Farm increased yield by 241 and 177 kg ha−1 compared to the control, which equals a gross return of $77 and $57 ha−1, respectively. The urea price in spring (March) 2012 was about $410 metric ton−1 commodity = urea&months = 60). Therefore, the cost of 22 ESN would be around $23 ha−1 (note the ESN price is 15% higher than that of regular urea). When $12 ha−1 for the cost of rhizobia inoculant is considered, application of 22 ESN+ rhizobia inoculant treatment would cost about $35 ha−1. Therefore, the net benefit obtained from this treatment was about $22 ha−1 (at SG Farm) to $42 ha−1 (at CARC). Therefore, starter N seems warranted, especially when soil initial N is limited. This conclusion is in agreement with Salvagiotti et al. (Citation2008) who suggested in their review that deep placement of slow-release fertilizers below the nodulation zone, or late applications during reproductive stages, may be promising alternatives for achieving a yield response to N fertilization in grain legumes. Furthermore, application of slow-release N increased pea protein concentration. Although peas are not currently traded based on protein, pea protein has received increasing attention and its market is growing. Lack of N during seed filling can cause a reduction in seed protein concentration in pea (Holl and Vose Citation1980). Pea nodules start decaying after flowering; additional mineral N in the soil during the late growing stage could improve pea seed protein. Further studies are needed to optimize starter N rate and placement to benefit yield and protein without a negative impact on germination and nodulation.
5. Conclusion
Application of commercial rhizobia inoculants in lands with a history of pea had no considerable influence on yield, but in lands without pea history improved seed yield by 16%. Application of starter N had an appreciable benefit (up to $42 ha−1) only when soil initial N was low (≤ 10 kg ha−1). Excessive application of starter N had a negative impact on seed germination and nodule formation. The rate, placement and form of the starter N must be optimized to benefit pea yield and protein without a detrimental effect on germination and nodulation.
Acknowledgments
The authors want to thank Johnna Heser, Sally Dahlhausen and Yesuf Mohammed for their assistance in field and laboratory. Funding support from the Montana Agricultural Experiment Station and from the Chinese Ministry of Agriculture for Project 948, # 2014-Z36 is acknowledged.
Additional information
Funding
References
- Catroux G, Hartmann A, Revellin C 2001: Trends in rhizobial inoculant production and use. Plant Soil, 230, 21–30. doi:10.1023/A:1004777115628
- Chemining’wa GN, Vessey KJ 2006: The abundance and efficacy of Rhizobium leguminosarum bv. viciae in cultivated soils of the eastern Canadian prairie. Soil Biol. Biochem., 38, 294–302. doi:10.1016/j.soilbio.2005.05.007
- Herridge DF, Peoples MB, Boddey RM 2008: Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil., 311, 1–18. doi:10.1007/s11104-008-9668-3
- Holl FB, Vose JR 1980: Carbohydrate and protein accumulation in the developing field pea seed. Can. J. Plant Sci., 60, 1109–1114. doi:10.4141/cjps80-161
- Huang J, Keshavarz Afshar R, Chen C 2016: Lentil response to nitrogen application and rhizobia inoculation. Comm. Soil Sci. Plant Anal., 47, 2458–2464. doi:10.1080/00103624.2016.1254786
- Ito D, Keshavarz Afshar R, Chen C, Miller P, Kephart K, McVay K, Lamb P, Miller J, Bohannon B, Knox M 2016: Multi-environmental evaluation of dry pea and lentil cultivars in Montana using AMMI model. Crop Sci, 56, 520–529. doi:10.2135/cropsci2015.01.0032
- Kutcher HR, Lafond G, Johnston AM, Miller PR, Gill KS, May WE, Hogg T, Johnson E, Biederbeck VO, Nybo B 2002: Rhizobium inoculant and seed-applied fungicide effects on field pea production. Can. J. Plant Sci., 82, 645–651. doi:10.4141/P01-180
- McKenzie RH, Middleton AB, Solberg ED, DeMulder J, Flore N, Clayton GW, Bremer E 2001: Response of pea to rhizobia inoculation and starter nitrogen in Alberta. Can. J. Plant Sci., 81, 637–643. doi:10.4141/P01-006
- Montana Agricultural Statistics 2014: [Online]. http://www.nass.usda.gov/Statistics_by_State/Montana/Publications/Annual_Statistical_Bulletin/2015/Montana_Annual_Bulletin_2014.pdf
- Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A 2008: Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crop. Res., 108, 1–13. doi:10.1016/j.fcr.2008.03.001
- Sechtig A 1992: Nitrate in 2 M Kcl Soil Extracts. Quikchem Method 12-107-04-1-B. Lachat Instruments, Milwaukee, WI, USA.
- Sparg SG, Kulkarni MG, Van Staden J 2006: Aerosol smoke and smoke-water stimulation of seedling vigor of a commercial maize cultivar. Crop Sci, 46, 1336–1340. doi:10.2135/cropsci2005.07-0324
- Starling ME, Wood C, Weaver DB 1998: Starter nitrogen and growth habit effects on late-planted soybean. Agron. J., 90, 658–662. doi:10.2134/agronj1998.00021962009000050015x
- Tao, A, Afshar, RK, Huang, J, Mohammed, YA, Espe, M, Chen, C 2017: Variation in yield, starch, and protein of dry pea grown across Montana. Agron. J., 109: 1–11. doi:10.2134/agronj2016.07.0401
- Van Kessel C, Hartley C 2000: Agricultural management of grain legumes: has it led to an increase in nitrogen fixation? Field Crop. Res., 65, 165–181. doi:10.1016/S0378-4290(99)00085-4
- Vessey JK 2004: Benefits of inoculating legume crops with rhizobia in the northern Great Plains. Crop Manage. doi:10.1094/CM-2004-0301-04-RV
- Xiang L, Hongyu Y 2007: Preliminary study on the root nodule formation of the legume plant. Southwest. China J. Agric. Sci., 20, 95–98.