?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The need for salinity resistance in turfgrass is increasing because of the enhanced use of effluent and other low-quality water for turfgrass irrigation. Although most turfgrasses form an arbuscular mycorrhizal fungus (AMF) symbiosis, there is little information on the mycorrhization of turfgrass species. Therefore, the aim of this study was to determine the effects of three AMF species, Glomus intraradices Schenck & Smith, Glomus etunicatum Becker & Gerdemann, and Glomus deserticola Trappe & John, and a mixture thereof on the growth, productivity, and nutrient uptake of two species of cool-season turfgrasses, Challenger Kentucky bluegrass (Poa pratensis L.) and Arid tall fescue (Festuca arundinacea Schreb.), and to relate the effects to colonization of the roots by mycorrhiza to assess the dependency of the plants (mycorrhizal dependency [MD]). Following the experimental period (4 months) and measurements, the mycorrhizal inoculated plants had significantly greater biomass production compared to that of non-inoculated plants. MD and shoot mineral contents (particularly P) differed among turfgrass hosting AMF, and the highest value (13%) occurred for P. pratensis and F. arundinacea seedlings colonized with G. intraradices and G. deserticola, respectively. The P content was highest for the F. arundinacea/mixed AMF combination compared to other treatments. We confirmed that mycorrhizal inoculation (P. pratensis/G. intraradices and F. arundinacea/mixed AMF combinations) enhanced plant productivity and nutrient uptake (especially P) even under non-optimum conditions.
1. Introduction
Arbuscular mycorrhizal fungi (AMFs) are obligate symbionts that form symbiotic relationships with more than 80% of terrestrial plants. Mycorrhizae are of extreme importance in the agricultural ecosystems because they aid plants in nutrient uptake, enhance soil aggregation and fertility, and may even help plants resist biotic and abiotic stresses (Smith and Read Citation2008). Extraradical hypha of AMF covers greater sections of soil than do plant roots, thereby enhancing nutrient uptake, especially those that are immobile, such as P, Cu, and Zn. Consequently, mycorrhizal symbiosis is an invaluable relationship for plants under abiotic stress conditions, such as salinity stress (Dodd and Ruiz-Lozano Citation2012; Mbadi et al. Citation2015; Ruíz-Lozano et al. Citation2011). The growth enhancement role of mycorrhiza was reported by Johnson (Citation1998) in his study on turfgrass (Panicum virgatum) and by Smith and Read (Citation2008), who stated that mycorrhizal-associated prairie plants had a greater biomass than non-mycorrhizal ones, in their study on roadside prairie plants. The positive mycorrhizal effects are not always the same, and the level of improvement varies depending on the host plant and mycorrhizal strain, and under some ecological conditions, it may even be a negative relationship (Asrar and Elhindi Citation2011; Dhar et al. Citation2015; Maherali Citation2014). In the turfgrass community, the level of positive effects related to mycorrhizal symbiosis (mycorrhizal dependency [MD]) is widely variable (Al-Qarawi et al. Citation2012). The root architecture of turfgrass strongly affects its response toward the mycorrhizal symbiosis. C4 grasses have coarse roots with a low percentage of root hairs. Thus, these plants need to form a relationship with an organism that has fine-root hair abilities that would be beneficial to the host plant, and C4 grasses greatly benefit from the mycorrhizal symbiosis. Conversely, the C3 grasses have fibrous roots with a high percentage of root hairs. Thus, they are not in great need of the mycorrhizal symbiosis (Anjum, Gill, et al., Citation2012). However, this hypothesis neglects that the mycorrhizal fungi need a living root with which to form a symbiotic relationship. Consequently, the higher the abundance of living roots on the plant, the higher the colonization rate. This may better explain the positive correlation between root density and MD, as reported by Al-Qarawi et al. (Citation2014).
Two species of cool-season turfgrass, Challenger Kentucky bluegrass (Poa pratensis L.) and Arid tall fescue (Festuca arundinacea Schreb), are widely distributed in Saudi Arabia. Ahmad (Citation2010) demonstrated that they could be easily cultivated and established from seed, stem cuttings, root stumps, or rhizomes, particularly in salt stressed and waterlogged areas where other species may not grow successfully. These grasses supply high-quality forage that is palatable for sheep and goats. However, there is little information about the domestication of these grasses with AM fungi and management practices for their growth and adaptation must be developed and tested. To understand the ecosystem functions of grasses, the determination of the value of symbiosis to plant growth responses under controlled conditions is a beneficial first step. Therefore, the present study was designed to study the effects of the AMFs, Glomus intraradices, Glomus etunicatum, and Glomus deserticola, on the growth and nutrition of P. pratensis and F. arundinacea seedlings under greenhouse conditions.
2. Materials and methods
The treatments were set up using a factorial set (two turf grass levels × five mycorrhizal treatment levels). The experimental units were randomized according to the complete block experimental design. Every treatment combination was replicated 10 times.
The experiment was conducted for a period of 4 months under greenhouse conditions at the Botany and Microbiology Department, College of Science, King Saud University (225 μmol m− 2 s− 1 light intensity, 27/20°C day/night temperatures, 70–80% relative humidity, and 16-h light photoperiod). P. pratensis and F. arundinacea seeds were brought from a local nursery in Riyadh City, Saudi Arabia. Healthy grass seeds were surface sterilized with 1% NaCIO solution for 10 min, then vigorously rinsed with sterilized double distilled water (DDW) before sowing. The seeds were sown in plastic bags (25-cm diameter and 24-cm height) filled with sterilized soil brought from sandy soil of Dharama, west of Riyadh. Pots were irrigated every week with DDW (200 mL) and supplied with Hogland and Arnon’s nutrient solution. The soil was crushed, passed through a 2-mm sieve, and autoclaved for 1 h at 120°C to eliminate native microorganisms. Some chemical and physical characteristics of the soil included pH (water), 7.61; electrical conductivity, 0.35 dS m−1; clay, 3.6%; fine silt, 0.0%; coarse silt, 0.8%; fine sand, 55.5%; coarse sand, 39.4%; total carbon, 0.17%; available nitrogen, 25.0 mg kg−1; available phosphorus, 7.02 mg kg−1; potassium, 52 mg kg−1; magnesium, 85 mg kg−1; and C/N, 8.5.
The mycorrhizal inocula were obtained from naturally saline sites in the Algassab region (northwest of Riyadh City), and the spores were extracted from the saline soils of this site via the wet-sieving method according to Gerdemann and Nicolson (Citation1963). The mycorrhizal strains were identified according to the methods Gerdemann and Trappe (Citation1974), Fisher and Vovides (Citation2004), and Walker et al. (Citation2007). The spores of G. intraradices Schenck and Smith, G. etunicatum Becker and Gerdemann, and G. deserticola Trappe and John were increased via trapping on the host plant Sorghum vulgare var. sudanese and the inocula were extracted from the roots after trapping. This inoculum was then used in the present experiment. The roots at the end of the trapping were cut into 1-cm pieces (the roots were examined to assess the colonization rate, which was approximately 85%), mixed with the surrounding soil containing the hypha and spores, and 20 g inoculum was used for every plant. The inoculum contained 45 propagules in every gram as measured according to Guissou et al. Citation1998). The inoculum was placed in the hole during transplanting. The mixed AMF contained approximately the same infective propagules of each fungal species. Non-inoculated controls also received 20 g of autoclaved sand–root mixture. The fungal association with the two turfgrass was tested using Trypan blue, according to the method of Phillips and Hayman (Citation1970).
Plants were watered every day with tap water until soil saturation. After 4 months of growth, parameters of grasses (height of the culm and number of stems) were determined. The plants were sampled to assess their growth characteristics (root fresh weight plant−1 [RFW], root dry weight plant−1 [RDW], shoot fresh weight plant−1, shoot dry weight plant−1 [SDW]) for each of the 10 plant replicates per treatment. After harvest, the shoots and roots were separated and dried at 65°C for 48 h. The MD of each plant species was calculated using the formula
where TDWM and TDWNM are total dry weight of mycorrhizal and non-mycorrhizal plants, respectively (Plenchette et al. Citation1983). Dried shoots were chemically analyzed. Total P content was determined by automatic calorimetry according to the method of Dabin et al. (Citation1968). The total C and N contents were quantified using the combustion system CHN Thermo Finnigan EA 1112 Series Flash Elemental Analyzer. Plant content of some mineral elements, such as K+ and Mg2 +, were determined according to the Association of Official Analytical Chemistry methods (AOAC Citation1990) using the Atomic Absorption Spectrophotometer AA-675 Series.
The data were analyzed with SPSS-12 statistical software (SPSS Inc., Chicago, IL, USA). Means were compared using the least significant different method at the p ≤ 0.05 significance level.
3. Results
3.1. Effect of AM fungi on root inoculation and growth rate of grasses
The data shown in indicate colonization success in all mycorrhizal treatments, with variation in the colonization extent among the treatments depending on the AMF and grass species. AMF colonization of P. pratensis by G. intraradices and G. deserticola was more marked than those colonized by G. etunicatum and mixed AMF. For F. arundinacea seedlings, AMF colonization was significantly higher with G. deserticola than the other AMF treatments. Examination of non-inoculated plants showed no trace of mycorrhizal colonization (). Mycorrhizal root inoculation of P. pratensis and F. arundinacea was not correlated with any growth characteristics (data not shown). The data in (ANOVA of plant growth characteristics) indicate differences among plants treated with the mycorrhizal inoculation depending on the AMF and turfgrass species. There was no significant correlation between the mycorrhizal inoculation and plant heights for both P. pratensis and F. arundinacea. The number of stems was significantly enhanced in P. pratensis plants when inoculated with G. intraradices and G. deserticola. Conversely, there was no significant effect of the other mycorrhizal treatments in relation to stem number (for the two turfgrass species). The RWD of F. arundinacea seedlings was significantly enhanced by colonization with G. intraradices and the mixed AMF compared to non-inoculated seedlings. However, the RWD of P. pratensis seedlings colonized with AMF did not differ from the control treatment. The SDW of the two grasses was significantly enhanced by colonization with G. deserticola. The total dry weight of P. pratensis and F. arundinacea inoculated by G. intraradices and G. deserticola, respectively, was higher than that of the controls. Colonization with G. intraradices and mixed AMF significantly enhanced the root/shoot ratios of F. arundinacea seedlings compared to that of the control but had no significant influence in P. pratensis.
Table 1. Effect of inoculation with arbuscular mycorrhizal fungi (AMF) and mixed AMF on growth responses and AMF inoculation of Poa pratensis and Festuca arundinacea seedlings.
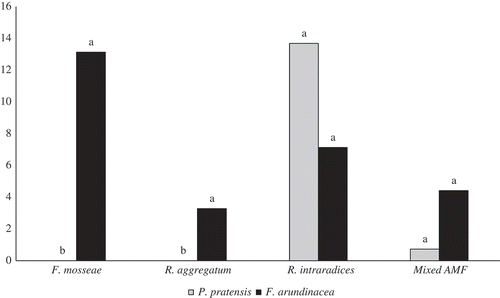
Data showed that the two grass species varied in their MD following AMF colonization (). P. pratensis and F. arundinacea had similar MD values when colonized with G. intraradices and G. deserticola, respectively (). P. pratensis in symbiosis with G. intraradices, G. etunicatum, and mixed AMF had the lowest MD. F. arundinacea in symbiosis with G. deserticola, G. etunicatum, and mixed AMF also had the lowest MD.
3.2. Changes in nutrient contents of grass species
The data related to the N, K, Mg, and C content are presented in . There was no significant difference among the inoculated grasses regardless of the inoculation type. The nutrient content of inoculated plants in the two grass varieties was higher than that in the non-inoculated ones. There was a positive relationship between the mycorrhizal inoculation and the shoot content of N for P. pratensis (r = 0.982, p < 0.05). Similarly, this was true for F. arundinacea shoots with mycorrhizal inoculation and C and K content and dry weight (r = 0.993, p < 0.01; r = 0.981, p < 0.05, respectively). In P. pratensis shoots, P concentration did not differ significantly. In contrast, concentration of P in shoots was significantly increased in the F. arundinacea/mixed AMF combination compared to that of non-inoculated grasses and other plant-AMF combinations. P concentration may have contributed to the total biomass production of F. arundinacea more than the other nutrients.
Table 2. Concentrations (%) of N, P, K, Mg, and C in seedlings of arbuscular mycorrhizal fungi (AMF) and (mixed AMF) Poa pratensis and Festuca arundinacea.
4. Discussion
The data from our study clearly indicated that AMFs have beneficial effects on the growth and productivity of P. pratensis and F. arundinacea. This was in agreement with the findings of previous studies on other plant species (Khakpour and Khara Citation2012; Ramakrishnan and Bhuvaneswari Citation2014). The root/shoot ratio of F. arundinacea was significantly affected by the inoculation with G. intraradices and mixed AMF colonization. This might explain the positive effect of mycorrhizal inoculation on the RDW, whereas there was a large reduction in SDW production in F. arundinacea.
The ability of mycorrhiza to associate with the roots is highly affected by the host plant (Klironomos Citation2003). This occurred in our experiment, as the percentage of mycorrhizal colonization of the roots differed between P. pratensis and F. arundinacea. This might have resulted from differing compatibility between the introduced mycorrhizal strains and the turfgrass species. Declerck et al. (Citation2002) reported that the competition between mycorrhizal strains might lower the significance of their effects; this was the case in the present study, as the lowest positive effect was in the mixed AMF treatment.
In our experiment, there was a notable reduction in AMF root inoculation in response to colonization with multiple AMF. Tagu et al. (Citation2004) demonstrated that forming a mycorrhizal symbiotic relationship is a genetically controlled process and the colonization level significantly differs based on the genetics of the plant species and the mycorrhizal strains. Furthermore, other studies showed that plants perform roles that affect the soil microbial communities, and this role is dependent on the plant genotype communities (Korkama et al. Citation2007; Mari et al. Citation2003). Genetic effects of fallen plant parts (leaves and branches) on the soil biota have been reported by several authors (Lojewski et al. Citation2009); less differences in bacterial communities and arbuscular fungi have been observed in a previous study (Madritch and Hunter Citation2002).
MD is the degree to which plants depend on the mycorrhizal fungi to maximize growth (at a known fertility level) (Plenchette et al. Citation1983). MD measurements may be highly variable or similar, depending on the plant species response to the non-inoculated treatment. MD classification was made by Cruz et al. (Citation1999) who reported that the MD is divided into three main groups: high dependency (MD ˃ 40%), moderate dependency (10 ˂ MD ˂ 40%), and not dependent (MD ≤ 10%). Zangaro et al. (Citation2007) reported that MD depends on the plant species, and Nogueira and Cardoso (Citation2007) reported that MD depends on the mycorrhizal species. The plants used in the present study (P. pratensis and F. arundinacea) fall in the moderate MD category in relation to the arbuscular mycorrhiza (G. intraradices and G. deserticola).
AM fungi inoculation, when succeeding in forming a symbiotic relationship with plants, can enhance nutrient uptake, thus enhancing tissues mineral content (Smith and Read Citation2008). Our results demonstrated that the P content in plants inoculated with mixed AMF was significantly higher than that in the other treatments. This result supports the findings of other authors (Smith and Read Citation2008) who reported that inoculating plants with AMF (primarily G. intraradices) resulted in enhanced plant shoot content of P. This may have occurred because plants were able to enhance their P uptake abilities by the utilization of many AMF (Cheng et al. Citation2011). The most important and inevitable strategy is forming a symbiotic relationship with AMF (Sheng et al. Citation2013), because AMF has an extensive hyphal network that can reach and cover a larger section of soil than can the roots alone (Ruiz-Lozano and Azcón Citation2000). In fact, mycorrhizal hyphae extend beyond the depletion zones around roots and acquire nutrients that are several centimeters away from the root surface (Augé et al. Citation2007). Although the role of AM in relation to P content is of extreme importance to highly AMF-dependent plants, it is important to state that mycorrhizae also help in supplying the host plants with other nutrients, including Mg, K, and N (Vaingankar and Rodrigues Citation2014). This was the case in the inoculated P. pratensis grasses with AMF in relation to the N content in shoots. Conversely, there was no correlation between the F. arundinacea grasses and AMF inoculation, this difference may have resulted from the different percentages of AMF colonization among the grasses. This was not the case in our experiment because all AMF tested did not significantly increase contents of Mg, K, and N in shoots of P. pratensis and F. arundinacea. However, such increases in nutrient content in response to the mycorrhizal effects were highly associated with the level of mycorrhizal infection (Fattah Citation2013). Furthermore, lower positive responses to AMF treatment in shoot nutrient concentration were related to grass species that have an extensive enough root system and consequently could meet their own nutrient demands without the arbuscular mycorrhizal symbiosis.
The symbiotic relationship between the AMF and the turfgrass plants in the present study was highly efficient in enhancing the growth ratio and rate of seedling growth. The RDW was not significantly affected by the AMF inoculation, and this might be explained by the fact that the AMF hypha partially assumes some of the root’s work. These results agree with those of Hetrick et al. (Citation1992). Moreover, Diagne and Ingleby (Citation2003) reported that in semiarid conditions, AMF inoculation was not able to enhance the plants root and shoot growth in all cases. The K content in plants was enhanced in a positive relationship to the enhanced content of P in AMF inoculated P. pratensis. There was a positive relationship between the shoot K content and the RDW in F. arundinacea grasses, but this relationship was not reported in P. pratensis grasses. The results of our experiment indicated a positive relationship between plant inoculation with AMF and K uptake, thus improving the plant growth. Furthermore, the shoot C ratio was positively affected by AMF inoculation, especially in F. arundinacea grasses that had higher MD. This might explain the higher dry weights of those plants compared to others. Plants optimize C expenditure to avoid limiting any one resource (N, C, or P). In addition, species of low MD would limit C expenditure for AMF inoculation more than species highly dependent on the symbiosis. Asrar and Elhindi (Citation2011) reported that mycorrhizal symbiosis is of extreme importance in plants as it enhances their nutrient uptake, especially in non-fertile soils.
5. Conclusions
Taken together, the results of this study indicate that improved growth performance of two turfgrass species was accompanied by increased nutrient accumulation in plants inoculated with mycorrhiza under greenhouse conditions. Thus, the present study corroborates that AMF symbioses can play a role in growth enhancement in both P. pratensis and F. arundinacea compared to non-mycorrhizal grasses. Further research is required to clarify the domestication of P. pratensis and F. arundinacea with selected AMF, and management practices for their propagation and adaptation must be developed and tested in saline areas.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research, King Saud University, Saudi Arabia for funding of this research through the Research Group: [Grant Number RG-1436-020].
Additional information
Funding
References
- Ahmad F 2010: Leptochloa fusca cultivation for utilization of salt - affected soil and water resources in Cholistan Desert. Sociedade & Natureza , 22, 141–149. doi:10.1590/S1982-45132010000100010
- Al-Qarawi AA , Mirdha MAU , Alghamdi OM 2012: Diversity of structural colonization and spore population of arbuscular mycorrhizal fungi in some plants from Riyadh, Saudi Arabia. J. Pure Appl. Microbiol. , 6, 1119–1125.
- Al-Qarawi AA , Mirdha MAU , Dhar PP , Alghamdi OM 2014: Management of arbuscular mycorrhizal fungi by growing Petunia hybrid (L.) Mill. as ornamental plant in Saudi Arabia-a case study. Pak. J. Bot , 46, 749–752.
- Anjum NA , Gill SS , Umar S , Ahmad I , Duarte AC , Pereira E 2012: Improving growth and productivity of oleiferous brassicas under changing environment: significance of nitrogen and sulphur nutrition, and underlying mechanisms. Sci. World J. , 2012, 12. doi:10.1100/2012/657808
- AOAC 1990: Official methods of analysis of AOAC international. Association of official analytical chemists, Washington, DC.
- Asrar A-WA , Elhindi KM 2011: Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. , 18, 93–98. doi:10.1016/j.sjbs.2010.06.007
- Augé RM , Toler HD , Moore JL , Cho K , Saxton AM 2007: Comparing contributions of soil versus root colonization to variations in stomatal behavior and soil drying in mycorrhizal Sorghum bicolor and Cucurbita pepo. J. Plant Physiol. , 164, 1289–1299. doi:10.1016/j.jplph.2006.08.005
- Cheng L , Bucciarelli B , Liu J , Zinn K , Miller S , Patton-Vogt J , Allan D , Shen J , Vance CP 2011: White Lupin Cluster root acclimation to phosphorus deficiency and root hair development involve unique glycerophosphodiester phosphodiesterases. Plant Physiol , 156, 1131–1148. doi:10.1104/pp.111.173724
- Cruz R , De La Zarade J , Agganzae N , Lorilla E 1999: Differential mycorrhizal development of some agricultural, horticultural and forestry crops to inoculation of mycorrhizal fungi. In Proceedings of the International Symposium on Management of Mycorrhizas in Agriculture, Horticulture and Forestry, Ed. Jasper D , pp. 54. Australian Institute of Agricultural Sciences, Australia.
- Dabin B , Brion J-C , Pelloux P , Rivoalen J , Robin F 1968: Application des dosages automatiques à l’analyse des sols: 4ème partie: dosage de la silice dans des extraits de sol. Symposium Technicon , 6, 225–233.
- Declerck S , Risede J-M , Delvaux B 2002: Greenhouse response of micropropagated bananas inoculated with in vitro monoxenically produced arbuscular mycorrhizal fungi. Sci. Hortic. , 93, 301–309. doi:10.1016/S0304-4238(01)00347-8
- Dhar PP , Al-Qarawi AA , Mridha MAU 2015: Arbuscular mycorrhizal fungal association in asteraceae plants growing in the arid lands of Saudi Arabia. J. Arid Land , 7, 676–686. doi:10.1007/s40333-015-0081-5
- Diagne O , Ingleby K 2003: Ecologie des champignons mycorhiziens arbusculaires infectant Acacia raddiana. In Un Arbre Au Désert: acacia Raddiana, Eds. Grouzis M , Le Floc’h E , pp. 205–228. IRD, Paris.
- Dodd IC , Ruiz-Lozano JM 2012: Microbial enhancement of crop resource use efficiency. Curr. Opin. Biotechnol. , 23, 236–242. doi:10.1016/j.copbio.2011.09.005
- Fattah OA 2013: Effect of mycorrhiza and phosphorus on micronutrients uptake by soybean plant grown in acid soil. Int. J. Agro. Plant Prod. , 4, 429–437.
- Fisher JB , Vovides AP 2004: Mycorrhizae are present in cycad roots. The Botanical Review , 70, 16–23. doi:10.1663/0006-8101(2004)070[0016:MAPICR]2.0.CO;2
- Gerdemann JW , Nicolson TH 1963: Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society , 46, 235–244. doi:10.1016/S0007-1536(63)80079-0
- Gerdemann JW , Trappe JM 1974: The Endogonaceae in the Pacific Northwest. Mycologia Memoirs, 5, 1-76.
- Guissou T , Moustapha A , Guinko BS , Duponnois R , Plenchette C 1998: Influence des phosphates naturels et des mycorhizes à vésicules et à arbuscules sur la croissance et la nutrition minérale de Zizyphus mauritiana Lam. dans un sol à pH alcalin. Annales des Sciences Forestières , 55, 925–931. doi:10.1051/forest:19980805
- Hetrick BAD , Wilson GWT , Cox TS 1992: Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can. J. Bot. , 70, 2032–2040. doi:10.1139/b92-253
- Johnson NC 1998: Responses of Salsola kali and Panicum virgatum to mycorrhizal fungi, phosphorus and soil organic matter: implications for reclamation. J. Appl. Ecol. , 35, 86–94. doi:10.1046/j.1365-2664.1998.00277.x
- Khakpour O , Khara J 2012: Spore density and root colonization by arbuscular mycorrhizal fungi in some species in the northwest of Iran. Int. Res. J. Appl. Basic Sci. , 3, 977–982.
- Klironomos JN 2003: Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology , 84, 2292–2301. doi:10.1890/02-0413
- Korkama T , Fritze H , Kiikkilä O , Pennanen T 2007: Do same-aged but different height Norway spruce (Picea abies) clones affect soil microbial community? Soil Biol Biochem , 39, 2420–2423. doi:10.1016/j.soilbio.2007.03.033
- Lojewski NR , Fischer DG , Bailey JK , Schweitzer JA , Whitham TG , Hart SC 2009: Genetic basis of aboveground productivity in two native populus species and their hybrids. Tree Physiol , 29, 1133–1142. doi:10.1093/treephys/tpp046
- Madritch MD , Hunter MD 2002: Phenotypic diversity influences ecosystem functioning in an Oak sandhills community. Ecology , 83, 2084–2090. doi:10.1890/0012-9658(2002)083[2084:PDIEFI]2.0.CO;2
- Maherali H 2014: Is there an association between root architecture and mycorrhizal growth response? New Phytol , 204, 192–200. doi:10.1111/nph.12927
- Mari M , Bertolini P , Pratella GC 2003: Non-conventional methods for the control of post-harvest pear diseases. J. Appl. Microbiol. , 94, 761–766. doi:10.1046/j.1365-2672.2003.01920.x
- Mbadi SH , Alipour ZT , Asghari H , Kashefi B 2015: Effect of the salinity stress and arbuscular mycorhizal fungi (AMF) on the growth and nutrition of the Marigold (Calendula officinalis). J. Biodiv. Environ. Sci. , 6, 215–219.
- Nogueira MA , Cardoso EJBN 2007: Phosphorus availability changes the internal and external endomycorrhizal colonization and affects symbiotic effectivenes. Sci. Agric. , 64, 295–300.
- Phillips JM , Hayman DS 1970: Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society , 55, 158–IN118. doi:10.1016/S0007-1536(70)80110-3
- Plenchette C , Fortin JA , Furlan V 1983: Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility. Plant Soil , 70, 211–217. doi:10.1007/BF02374781
- Ramakrishnan K , Bhuvaneswari G 2014: Effect of inoculation of AM fungi and beneficial microorganisms on growth and nutrient uptake of Eleusine coracana (L.) Gaertn. (Finger Millet). Int. Let. Nat. Sci. , 13, 59–69.
- Ruiz-Lozano JM , Azcón R 2000: Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza , 10, 137–143. doi:10.1007/s005720000075
- Ruíz-Lozano JM , Del Carmen Perálvarez M , Aroca R , Azcón R 2011: The application of a treated sugar beet waste residue to soil modifies the responses of mycorrhizal and non mycorrhizal lettuce plants to drought stress. Plant Soil , 346, 153–166. doi:10.1007/s11104-011-0805-z
- Sheng M , Lalande R , Hamel C , Ziadi N 2013: Effect of long-term tillage and mineral phosphorus fertilization on arbuscular mycorrhizal fungi in a humid continental zone of eastern Canada. Plant Soil , 369, 599–613. doi:10.1007/s11104-013-1585-4
- Smith SE , Read D 2008: Growth and carbon economy of arbuscular mycorrhizal symbionts, pp. 117–144. Elsevier, London.
- Tagu D , Bastien C , Faivre-Rampant P , Garbaye J , Vion P , Villar M , Martin F 2004: Genetic analysis of phenotypic variation for ectomycorrhiza formation in an interspecific F1 poplar full-sib family. Mycorrhiza , 15, 87–91. doi:10.1007/s00572-004-0302-9
- Vaingankar JD , Rodrigues BF 2014: Effect of arbuscular mycorrhizal (AM) inoculation on growth and flowering inCrossandra infundibuliformis(L.). Nees. J. Plant Nutr. , 38, 1478–1488. doi:10.1080/01904167.2014.957398
- Walker C , Vestberg M , Demircik F , Stockinger H , Saito M , Sawaki H , Nishmura I , Schüßler A 2007: Molecular phylogeny and new taxa in the archaeosporales (Glomeromycota): ambispora fennica gen. sp. nov., Ambisporaceae fam. Nov., and emendation of archaeospora and archaeosporaceae. Mycol. Res , 111, 137–153. doi:10.1016/j.mycres.2006.11.008
- Zangaro W , Nishidate FR , Vandresen J , Andrade G , Nogueira MA 2007: Root mycorrhizal colonization and plant responsiveness are related to root plasticity, soil fertility and successional status of native woody species in southern Brazil. J. Trop. Ecol. , 23, 53–62. doi:10.1017/S0266467406003713