?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Co-inoculation of nitrogen-fixing bacteria with plant growth-promoting bacteria has become more popular than single inoculation of rhizobia or plant-growth-promoting bacteria because of the synergy of these bacteria in increasing soybean yield and nitrogen fixation. This study was conducted to investigate the effects of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 co-inoculation on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of the ‘Yezin-6’ soybean cultivar. Nitrogen fixation was measured using the acetylene reduction assay and ureide methods. Uptake of major nutrients [nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg)] was also measured. This study showed that single inoculation of SAY3-7 significantly increased shoot biomass; nodulation; Relative Ureide Index (RUI %), percent nitrogen derived from N fixation (% Ndfa); N, P, K, Ca, and Mg uptakes; during the later growth stages (R3.5 and R5.5), compared with control. These observations indicate that SAY3-7 is an effective N-fixing bacterium for the plant growth, nodulation, and nitrogen fixation with an ability to compete with native bradyrhizobia. Co-inoculation of SAY3-7 and P4 significantly improved nodule number; nodule dry weight; shoot and root biomass; N fixation; N, P, K, Ca, and Mg uptake; at various growth stages and seed yield in ‘Yezin-6’ soybean cultivar compared with the control, but not the single inoculation treatments. Significant differences in plant growth, nodulation, N fixation, nutrient uptake, and yield between co-inoculation and control, not between single inoculation and control, suggest that there is a synergetic effect due to co-inoculation of SAY3-7 and P4. Therefore, we conclude that Myanmar Bradyrhizobium strain SAY3-7 and P4 will be useful as effective inoculants in biofertilizer production in the future.
1. Introduction
Legumes are the second largest crops in Myanmar after rice according to the cultivated areas. Seventeen kinds of pulse, including soybean, are produced widely in Myanmar. Among them, black gram, green gram, soybean, butter bean, pigeon pea, and cowpea are mainly exported. Due to the relatively low cost of cultivation and increasing demand for domestic consumption and export, cultivation areas for legumes have increased substantially from 0.73 million hectares in 1988–1989 to 5.91 million hectares in 2014–2015 (MOAI Citation2015). Among these legumes, soybean is a major grain legume crop for domestic consumption. In addition to being a rich source of protein and oil, soybeans are very efficient in fixing nitrogen (N) among the leguminous crops (Unkivich and Pate Citation2000; Van Kessel and Hartley Citation2000).
The application of effective inoculants is an alternative strategy for agricultural sustainability and reduction of the environmental impact of chemical fertilizer application due to decreased use (Adesemoye et al. Citation2009; Hungria et al. Citation2013). Rhizobia are very important for crop production because biological N fixation, the process by which atmospheric elemental N2 is converted to ammonia (NH3) (Franche et al. Citation2009), provides the required N to leguminous plants and accounts for 65% of the N currently used in agriculture (Matiru and Dakora Citation2004). Moreover, legume–rhizobia symbiosis is an easy and inexpensive way to maintain soil fertility and improve crop production. Therefore, rhizobium inoculation has become a popular agronomic practice to provide adequate amounts of N to leguminous plants, instead of the use of nitrogenous fertilizer.
Successful rhizobium–legume symbiosis depends largely on whether the host and inoculum are compatible to form N fixation nodules. In the case of soybean symbiosis, this compatibility is related to the nodulation type of bradyrhizobia and nodulation regulatory genes (Rj genes) of the soybean cultivar. Nodulation types and Rj genes have been reported to play roles in the control of nodule formation (Ishizuka et al. Citation1991a, Citation1991b). Type A strains induce nodulation on all Rj genotype cultivars but prefer non-Rj soybean cultivars. Type B and C strains have restricted nodule formation on Rj 2 Rj 3 and Rj 4 genotype cultivars, respectively, preferring the Rj 4 and Rj 2 Rj 3 genotype cultivars, respectively, for nodulation. Therefore, type A, B, and C strains must be inoculated with their specific soybean cultivars to successfully nodulate and fix N.
Co-inoculation of N-fixing bacteria with plant-growth-promoting bacteria has become more popular than single inoculation of rhizobia or plant-growth-promoting bacteria because co-inoculation increases soybean yield and improves the sustainability of agriculture (Hungria et al. Citation2015). Vessey and Buss (Citation2002) reported that co-inoculation of rhizobia with plant-growth-promoting bacteria improved nodulation and N fixation. Streptomyces griseoflavus P4, which is an endophytic bacterium, is known to enhance symbiotic nitrogen fixation of some bradyrhizobia under controlled greenhouse and open field conditions (Soe et al. Citation2012; Soe and Yamakawa Citation2013a; Soe and Yamakawa Citation2013b). Our previous research demonstrated that co-inoculation of Bradyrhizobium japonicum SAY3-7 with the endophytic bacterium Streptomyces griseoflavus P4 increases N fixation rates and plant growth compared with single inoculation of B. japonicum SAY3-7 under environmentally controlled conditions (Htwe and Yamakawa Citation2016). However, field investigation is needed to determine the performance of single and co-inoculation of SAY3-7 and/or P4 under open field conditions. Therefore, this study was conducted to investigate the effects of co-inoculation of SAY3-7 and P4 on plant growth, nodulation, N fixation, nutrient uptake, and seed yield of the ‘Yezin-6’ soybean cultivar.
2. Materials and methods
2.1. Experimental site
The experiment was conducted at Kyushu University Farm, Fukuoka Prefecture, Japan (33°37ʹN, 130°25ʹE) from July to November 2016.
2.2. Experimental design and treatments
In this study, a randomized complete block design was used with three replications. The four treatments were an uninoculated control, single inoculation of P4, single inoculation of SAY3-7, and co-inoculation of SAY3-7 and P4.
2.3. Soil analysis
Before conducting the field experiment, soil samples were collected from five locations in the field at 10 cm depth using a 5-cm-diameter soil sampling tube. The soil samples were spread and air dried at room temperature for 24 h. Then, they were crushed by hand and sieved through a 2-mm-mesh sieve. They were stored at 4°C before the soil analysis. Soil (1:2.5 soil:H2O) was measured using a pH meter. The soils were digested using the salicylic acid–H2SO4–H2O2 digestion method (Ohyama et al. Citation1991); total N was determined using the indophenol method (Cataldo et al. Citation1974); and total phosphorus (P) was analyzed using the ascorbic acid method (Murphy and Riley Citation1962). Mineralisable N was assessed using the soil incubation method (Sahrawat Citation1983) followed by Cataldo et al. (Citation1974). Available P was measured using Truog’s method (Truog Citation1930). Cation exchange capacity and exchangeable cations were determined using the ammonium acetate shaking extraction method (Muramoto et al. Citation1992). Exchangeable cations were measured by atomic absorption spectrophotometry (Z-5300; Hitachi, Tokyo, Japan). The physicochemical properties of the experimental soils are described in .
Table 1. Physicochemical properties of soil.
The rhizobial populations of the collected soil samples were evaluated by the most probable number (MPN) method (Vincent Citation1970) using ‘Yezin-6’ (non-Rj) as the host plant.
2.4. Bacterial inoculant preparation
B. japonicum SAY3-7 (type A) strain isolated from soybean at Aungban City, Myanmar (Htwe et al. Citation2015). The type A described in parentheses expresses the nodulation type of Bradyrhizobium strain and it was identified by Htwe et al. (Citation2015). B. japonicum SAY3-7 was incubated in A1E broth culture medium (Kuykendall Citation1987) on a rotary shaker at 30°C for 7 days. S. griseoflavus P4 isolated from sweet pea root at Kurima, Tsu City, Japan (Thapanapongworakul Citation2003), was incubated in IMA-2 broth culture medium (Shimizu et al. Citation2000) on a rotary shaker at 30°C for 7 days. A peat-based inoculum was used in this experiment. Peat soil was collected from Heho, Myanmar. A total of 100 soybean seeds were mixed thoroughly with 10 g peat soil, 7 mL 20% liquid solution of gum arabic, and 0.1 mL 1 × 108 cell mL−1 SAY3-7 and/or 0.1 mL 1 × 108 cell mL−1 P4 to obtain the required inoculation density (1 × 105 cells seed−1) for both bacteria during preparation of the peat-based inoculum. Our previous experiment highlighted that the proper inoculation density for SAY3-7 and P4 was 1 × 105 cells mL−1 for co-inoculation.
2.5. Soybean variety
The soybean ‘Yezin-6’ (non-Rj) was used as the host plant. Rj gene described in parentheses indicates the nodulation regulatory gene of soybean and it was identified by Htwe et al. (Citation2015). Yezin-6 is one of the most widely grown soybean cultivars in Myanmar. The term ‘non-Rj’ in parentheses expresses the nodulation regulatory gene. Although non-Rj is compatible with all types of bacterium (Vest and Caldwell Citation1972), it prefers type A strain bacteria for nodulation. Therefore, the non-Rj soybean cultivar is suitable for use as a host plant when inoculating SAY3-7 (type A strain).
2.6. Cultivation and crop management
The experimental field was prepared for conventional practice in Japan. Compound fertilizer (Kumiai Mame-kasei 300, Ryoto Fertilizer Co., Ltd., Ooita, Japan) which contains 3% N, 10% P2O5, and 10% K2O was applied at a rate of 800 kg ha−1 1 week before the final land preparation. After the basal application of fertilizer, the experimental field was leveled and divided into individual plots. The experimental site was 15 m long and 20 m wide. Each plot was 3 m long and 6 m wide. The row and plant spacing were 60 and 40 cm, respectively. Four inoculated seeds were sowed in one hill and covered with soil just after seed sowing. To eliminate contamination, seeds were first sown in control plots, followed by the single inoculation of P4, single inoculation of SAY3-7, and co-inoculation of SAY3-7 and P4. After sowing, granular forms of herbicide and pesticide were applied to prevent competition with weeds and to prevent insect and pest infestation. Sixteen days after sowing (DAS), the first, inter-cultivation was done using an inter-cultivator to control weeds and reduce competition with the soybean plants. At 28 DAS, the second inter-cultivation was done for earthing-up. Then, plants were thinned as necessary to maintain two plants per hill. Pesticides were sprayed fortnightly from the flowering stage (R2) to the beginning of maturation (R7).
2.7. Plant sampling
Plant samples were collected from five growing stages: V6 (six unfolded trifoliate leaves), R2 (Full flowering stage), R3.5 (early pod-fill stage), R5.5 (early seed-fill stage), and R8 (maturity stage). The growth stages refer to Fehr et al. (Citation1971).
2.8. Acetylene reduction assay
Two plants at the V6 and R2 stages from one hill in each plot were uprooted and carefully washed with water so as not to detach the nodules. The acetylene reduction assay (ARA) was performed according to Haider et al. (Citation1991) to measure nitrogenase activity. The soybean plants were cut at the cotyledonary nodes. Then, the soybean root with intact nodules was placed in a 200-mL conical flask and sealed with a serum stopper. A 25-mL aliquot of acetylene (C2H2) gas was injected into the flask to replace the air with acetylene. The flasks containing roots with intact nodules were incubated at room temperature and 1.0-mL subsamples were analyzed at 5 and 65 min, respectively. The ARA value, in terms of ethylene (C2H4) production per plant, was measured using a flame ionization gas chromatograph (GC-14A, Shimadzu, Kyoto, Japan) equipped with a stainless steel column (3 mm diameter, 0.5 m length). The column was filled with Porapak R 60–80 mesh (Nacalai Tesque, Inc., Kyoto Japan). Colum, injection, and detection temperatures were 35, 45, and 170°C, respectively. N gas was used as the carrier gas at a flow rate of 45 mL min−1. The number of nodules was counted after the assay. Shoots, roots, and nodules were collected separately and oven dried at 70°C for 72 h to record their dry weights.
2.9. Xylem sap collection
Two plants at the R3.5 and R5.5 stages from one hill in each plot were cut just under the cotyledonary nodes and inserted into a silicon tube. The xylem sap was collected for 1 and 2 h after cutting at the R3.5 and R5.5 stages, respectively. The sap samples were stored at −30°C for long-term use. Amino acid (Moore and Stein Citation1954), nitrate (Cataldo et al. Citation1975), and ureide (Young and Conway Citation1942) were analyzed from the root-bled sap. The relative ureide index (RUI) of the root-bled sap was calculated using the following formula (Peoples et al. Citation1989):
The percentage of N derived from N fixation was calculated using the following formula (Herridge and Peoples Citation1990):
where y is RUI (%) and x is the percentage of N derived from N fixation (%Ndfa), respectively. After xylem sap collection, the roots were uprooted and washed. Then, the nodules were counted. The shoots, roots, and nodules were oven dried at 70°C for 72 h to record their dry weights.
2.10. Measurements of total N, P, K, Ca, and Mg uptake
The shoots were divided into leaves, stems and petioles, shells, unfilled seeds, and filled seeds. Then, each plant part was dried and separately ground into a powder using a mill (100–120 mesh, Tecator AB, Hoedanaes, Sweden). After digestion of the nutrients using the H2SO4–H2O2 digestion method (Ohyama et al. Citation1991), total N accumulation in the shoot was measured by the indophenol method (Cataldo et al. Citation1974); total P was analyzed using the ascorbic acid method (Murphy and Riley Citation1962); and total K, Ca, and Mg were measured using atomic absorption spectrophotometry (Z-5300, Hitachi, Tokyo, Japan).
2.11. Yield and yield components
Four hills from each plot at R8 were selected randomly and plants were cut at the cotyledon nodes to determine seed yield and yield component parameters, such as the number of pods per plant, number of seeds per pod, 100-seed weight.
2.12. Data analysis
Data were statistically analyzed using STATISTIX 8 (Analytical Software, Tallahassee, FL, USA) and the means were compared by Tukey’s HSD test at P < 0.05.
3. Results
3.1. Indigenous rhizobia
Before cultivation, the population of indigenous rhizobia in the soil from the experimental field was estimated by the MPN method using the Yezin-6 cultivar as the host plant. The population density of indigenous rhizobia was 0.85 × 107 rhizobia in 1 g dried soil.
3.2. Effects of single and co-inoculation on biomass production at different growth stages
The results of shoot and root biomass production are shown in . No difference in shoot and root dry biomass was observed at the V6 and R2 stage. Significantly different shoot and root dry biomasses were detected at the R3.5, R5.5, and R8 stages. A synergistic effect was formed by combined inoculation of both bacteria as significant increase in shoot dry weight and root dry weight was observed between co-inoculation and control, but not between single inoculation and control at R5.5 and R8 stages.
Table 2. Effect of co-inoculation of B. japonicum SAY3-7 and S. griseoflavus on nodule number, nodule, shoot, and root dry weights of Yezin-6 soybean cultivar at different growth stages.
3.3. Effects of single and co-inoculation on nodulation at different growth stages
The nodule count and nodule dry weight results are shown in . Nodulation, in terms of nodule number and nodule dry weight, differed significantly among treatments at the V6 stage. Co-inoculation of SAY3-7 and P4 resulted in significantly more nodules and a higher nodule dry weight compared with the control treatment, but not the single inoculation treatments. At R2 and R3.5 stages, the numbers of nodules, but not nodule dry weight, differed significantly among treatments. The number of nodules and nodule dry weight differed significantly among treatments at the R5.5 stage. Co-inoculation of SAY3-7 and P4 produced the greatest nodule dry weight compared with other treatments. Moreover, co-inoculation resulted in significantly higher nodules than control treatment. The abundance of nodulation was occurred due to co-inoculation compared with control, but not the single inoculation at each growth stages. These results indicate that a synergetic effect was formed by co-inoculation.
3.4. Effects of single and co-inoculation on nitrogen fixation at different growth stages
The results of N fixation in terms of C2H4 production are shown in . N fixation at the V6 and R2 stages in terms of C2H4 production was measured. C2H4 production at the V6 stage did not differ significantly among treatments. N fixation at the R2 stage in terms of C2H4 production differed significantly among treatments. Co-inoculation significantly increased N fixation by approximately 47%, compared with the control.
Figure 1. Effect of co-inoculation of B. japonicum SAY3-7 and S. griseoflavus on (A) acetylene reduction activity (ARA), (B) Relative Ureide Index (%), (C) N derived from N fixation (%Ndfa) of Yezin-6 soybean cultivar at different growth stages. The histograms with the same letter at each growth stage are not significantly different at P < 0.05 (Tukey’s test).
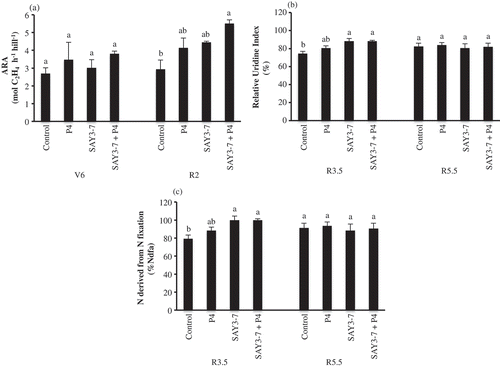
The results of N fixation in terms of the RUI (%) are shown in . The RUI (%) at the R3.5 stage differed significantly among treatments. Single inoculation of SAY3-7 and co-inoculation of SAY3-7 and P4 resulted in significantly higher RUIs (%) of 88.21% and 88.30%, respectively, compared with the control (74.43%). The RUI (%) at the V5.5 stage did not differ among treatments.
The N fixation results in terms of %Ndfa are shown in . %Ndfa at the R3.5 stage differed significantly among the treatments. Single inoculation of SAY3-7 and co-inoculation of SAY3-7 and P4 resulted in significantly higher %Ndfa values of 99.87% and 99.99%, respectively, compared with those of the control (79.30%). %Ndfa did not differ among treatments at the R5.5 stage.
3.5. Effects of single and co-inoculation on N, P, K, Ca, and Mg uptake at different growth stages
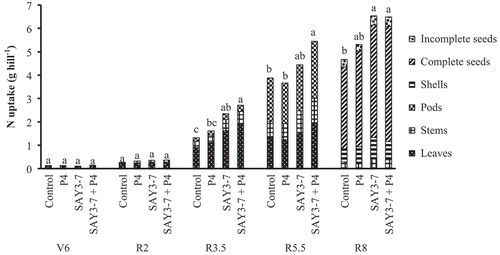
The results of N uptake are shown in . The uptake results for other nutrients are shown in . Total N, P, K, Ca, and Mg uptakes were not significant difference from other treatments at V6 and R2 stages. However, significantly different total N, P, K, Ca, and Mg uptakes occurred at the R3.5 and R5.5 stages. Total N, P, and K uptakes at the R8 stage differed significantly among treatments. However, Ca and Mg uptakes did not differ among treatments. Moreover, K, Ca, and Mg uptakes decreased at the R8 stage compared with those at the R5.5 stage. N and P uptakes did not decline until maturation (R8 stage).
Table 3. Effect of co-inoculation of B. japonicum SAY3-7 and S. griseoflavus on P, K, Ca, and Mg uptakes of Yezin-6 soybean cultivar at different growth stages.
3.6. Effects of single and co-inoculation on yield at maturity stage
The yield and yield component results are shown in . Yield component parameters, such as the number of pods per plant, number of seeds per pod, and 100-seed weight, did not differ significantly among treatments. However, co-inoculation of SAY3-7 with P4 resulted in significantly higher yield (3.11 t ha−1) than the control (2.28 t ha−1). In this study, significant difference in yield was observed between co-inoculation and control, not between co-inoculation and single inoculations. Significant difference in yield between co-inoculation and control, not between single inoculation and control, suggests that there is a synergetic effect in increasing soybean productivity due to co-inoculation of SAY3-7 and P4.
Table 4. Effect of co-inoculation of B. japonicum SAY3-7 and S. griseoflavus on yield and yield components of Yezin-6 soybean cultivar at maturity stage.
4. Discussion
The combination of P4 with SAY3-7 resulted in significant increases in shoot and root dry biomasses at R3.5, R5.5, and R8 (). These findings are similar to those of our previous studies, in which the combination of P4 and SAY3-7 had a synergistic effect on shoot and root dry weights (Htwe and Yamakawa Citation2015, Citation2016). This plant-growth-promoting effect of P4 might be due to secretion of growth-promoting hormones. The P4 used in this experiment can induce production of the plant growth hormone indole acetic acid (Soe Citation2013).
The number of nodules and nodule dry weight was significantly increased due to after co-inoculation compared with the other treatments at some growth stages (). Similar findings were documented by Soe et al. (Citation2012), in which co-inoculation of P4 with USDA110 significantly increased the nodule dry weight of a Myanmar soybean cultivar. Aung et al. (Citation2013) reported a significant increase in nodulation of a Myanmar soybean cultivar due to co-inoculation of Azospirillum sp. with B. japonicum USDA110, compared with the uninoculated control and single inoculations of each bacterium in rhizobia-established soils collected from Myanmar and Thailand. In this study, soybean plants in the un-inoculated control and the P4 treatment group formed root nodules via indigenous rhizobia because the plants inoculated with P4 and control did not form nodules in sterilized vermiculite media (Htwe and Yamakawa Citation2016). One of the major problems in inoculation technology with soybean is the establishment of an inoculated strain from populations of indigenous bradyrhizobia existing in the soils (Tang Citation1979). Therefore, the occupancy of inoculated strains in nodules is relatively low compared with indigenous rhizobia because of their competition to occupy in root nodules (Weaver and Frederick Citation1974; Kvien et al. Citation1981). However, in this study, nodulation in control treatment was significantly lower than co-inoculation. The abundance of nodulation in co-inoculation treatment shows that the inoculated strain had the competitive ability with indigenous rhizobia for nodulation.
N fixation has been measured using various methods, such as N-difference and ureide methods, as well as ARA and 15N isotope methods. In this study, ARA and ureide method were used for assessment of nitrogen fixation. The ethylene production will be governed by the proportion of active nodules that remained intact on the plants in uprooting process (Danso Citation1995). At the early growth stages, most of the nodules were remained intact by carefully uprooting whereas nodules were easily detached from the plant at the later growth stages because a large number of nodules were formed on lateral roots. Danso (Citation1995) stated that it is very difficult to recover 100% of intact nodules even in normal friable soil, especially where a large number of nodules are formed on lateral roots. In contract, root bled saps were difficult to collect at early growth stages because of lower flow rate, but not at later growth stages. Therefore, ARA and ureide method are suitable for early growth stages (V6 and R2) and the later growth stages (R3.5 and R5.5), respectively. For ARA measurement, uprooted plants with intact nodules were used to detect the acetylene reduction to ethylene. In this study, co-inoculation of Bradyrhizobium with P4 has been reported to have a synergistic effect on symbiotic N fixation by increasing nitrogenase activity (Soe and Yamakawa Citation2013a). These findings are in agreement with our results, in which co-inoculation of B. japonicum SAY3-7 with the plant-growth-promoting bacterium S. griseoflavus P4 significantly increased nitrogenase activity at the R2 stage (). Our previous study showed that low-density co-inoculation of B. japonicum SAY3-7 and P4 improved nitrogenase activity by 15–75% in various soybean cultivars compared with single inoculation of SAY3-7 under environmentally controlled conditions (Htwe and Yamakawa Citation2016). Yamakawa et al. (Citation2000) revealed that ureide is the main nitrogenous compound transported in xylem sap of the soybean. Streeter (Citation1979) also stated that ureide-N, allantoin, and allantoic acid are the major forms of N transported from nodulated roots to shoots of soybean. Therefore, the RUI has become one of the most widely used methods to measure N fixation, particularly in soybean. In this study, RUI (%) was determined at the R3.5 and R5.5 stages. Our results show that the RUI (%) increased significantly in soybean plants inoculated with SAY3-7 and SAY3-7 + P4 at the R3.5 stage, although no significant increase occurred at the R5.5 stage (). Consequently, N derived from N fixation was significantly increased at the R3.5 stage, but not at the R5.5 stage, compared with the control (). These results support the findings of Soe et al. (Citation2012) and Soe and Yamakawa (Citation2013b), in which single and co-inoculation of bradyrhizobia with P4 promoted higher RUI (%) and %Ndf at the R3.5 stage.
Symbiotic N fixation of soybean can provide 40–70% of the total N requirement (Klubeck et al. Citation1988). In this study, single and co-inoculation of SAY3-7 improved total N uptakes in the shoot at R3.5, R5.5, and R8 stages (). Similarly, Soe and Yamakawa (Citation2013b) reported that total N accumulation was significantly affected by single and co-inoculation at the V6 and R8 stages. Moreover, Soe et al. (Citation2010) found that co-inoculation with Streptomyces spp. P4 and indigenous Myamar Bradyrhizobium was more effective for N uptake than single inoculation of Bradyrhizobium. In the present study, co-inoculation of SAY3-7 with P4 significantly improved P, K, Ca, and Mg uptakes at R3.5 and R5.5 stages (). This is the first report that P, K, Ca, and Mg uptakes were affected by co-inoculation of Myanmar Bradyrhizobium strain and P4.
Our group has reported significant increases in soybean yield after inoculation of bradyrhizobia (Soe et al. Citation2010; Yamakawa and Fukushima Citation2014) and co-inoculation of bradyrhizobia with P4 (Soe et al. Citation2012; Soe and Yamakawa Citation2013a, Citation2013b). Significant increase in yield was observed due to inoculation (). This results support the findings of our group (Soe et al. Citation2012; Soe and Yamakawa Citation2013a; Soe and Yamakawa Citation2013b) in which there is synergetic effect in increasing soybean productivity due to co-inoculation of P4 with Myanmar Bradyrhizobium.
In our previous study, we observed the effects of co-inoculation of the same combination of SAY3-7 and P4 bacteria on plant growth, nodulation, and nitrogen fixation in Yezin-6 in the controlled pot experiment where these bacteria were inoculated on the sterilized seeds and vermiculite (Htwe and Yamakawa Citation2016). Compared to the previous studies, the new findings in this study were the effects of co-inoculation SAY3-7 and P4 bacteria on plant growth, nodulation, nitrogen fixation, nutrient uptake, and yield in a field condition where indigenous bacteria were already established in the soil.
5. Conclusion
The results show that co-inoculation of SAY3-7 and P4 significantly improved plant growth; nodulation; nitrogen fixation; N, P, K, Ca, and Mg uptake; and yield of Yezin-6 compared with those in the control group. Therefore, we conclude that the combined use of SAY3-7 and P4 will be helpful for soybean production by enhancing plant growth, nodulation, N fixation, and major nutrient uptake.
Acknowledgment
This work was supported by Ministry of Education, Culture, Sports, Sciences and Technology (MEXT) of Japan.
Additional information
Funding
References
- Adesemoye AO , Torbert HA , Kloepper JW 2009: Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb Ecol. , 58(4), 921–929. doi:10.1007/s00248-009-9531-y
- Aung TT , Tittaburt P , Boonkerd N , Herridge D 2013: Co-inoculation effects of Bradrhizobium japonicum and Azospirillum sp. on competitive nodulation and rhizosphere eubacterial community structures of soybean under rhizobia-established soil conditions. African J. Biotech. , 12(20), 2850–2862, doi:10.5897/AJB12.2557
- Cataldo DA , Haroon M , Schrader LE , Youngs VL 1975: Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. , 6, 71–80. doi:10.1080/00103627509366547
- Cataldo DA , Schrader LE , Youngs VL 1974: Analysis by digestion and Colorimetric assay of total nitrogen in plant tissues high in nitrate. Crop Sci. , 14, 854–856. doi:10.2135/cropsci1974.0011183X001400060024x
- Danso SKA 1995: Assessment of biological nitrogen fixation. Fertilizer Res. , 42, 33–41. doi:10.1007/BF00750498
- Fehr WR , Caviness CE , Burmood DT , Pennington JS 1971: Stage of development descriptions for soybeans, Glycine max (L) Merrill. Crop Sci. , 11, 929–931. doi:10.2135/cropsci1971.0011183X001100060051x
- Franche C , Lindström K , Elmerich C 2009: Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil , 321, 35–59. doi:10.1007/s11104-008-9833-8
- Haider J , Hussam AKMA , Ikeda M , Yamakawa T , Ishizuka J 1991: Effects of nitrate application on growth, nodulation and nitrogen fixation of nitrate-tolerant mutant soybean. Soil Sci Plant Nutr. , 37, 521–529. doi:10.1080/00380768.1991.10415065
- Herridge DF , Peoples MB 1990: Ureide assay for measuring nitrogen fixation by nodulated soybean calibrated by 15N methods. Plant Physiol. , 93, 495–503. doi:10.1104/pp.93.2.495
- Htwe AZ , Yamakawa T 2015: Enhanced plant growth and/or nitrogen fixation by leguminous and non-leguminous crops after single or dual inoculation of Streptomyces griseoflavus P4 with Bradyhizobium Strains. African J. Of Microbiol. Res. , 9, 2337–2344. doi:10.5897/AJMR2015.7796
- Htwe AZ , Yamakawa T 2016: Low-density co-inoculation with Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 promotes plant growth and nitrogen fixation in soybean cultivars. American J. Plant Sci. , 7, 1652–1661. doi:10.4236/ajps.2016.712156
- Htwe AZ , Yamakawa T , Sarr PS , Sakata T 2015: Diversity and distribution of soybean-nodulation bradyrhizobia isolated from major soybean-growing regions in Myanmar. African J. Microbiol. Res. , 9(43), 2183–2196, doi:10.5897/AJMR2015.7702
- Hungria M , Nogueira MA , Araujo RS 2013: Co-inoculation of soybeans and common beans with rhizobia and azospirilla: strategies to improve sustainability. Biol. Fertil. Soils , 49, 791–801. doi:10.1007/s00374-012-0771-5
- Hungria M , Nogueira MA , Araujo RS 2015: Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. American J. Plant Sci. , 6, 811–817. doi:10.4236/ajps.2015.66087
- Ishizuka J , Suemasu Y , Mizogami K 1991a: Preference of Rj-soybean cultivars for Bradyrhizobium japonicum for nodulation. Soil Sci Plant Nutr. , 37, 15–21. doi:10.1080/00380768.1991.10415005
- Ishizuka J , Yokoyama A , Suemasu Y 1991b: Relationship between serotypes of Bradyrhizobium japonicum and their compatibility with Rj-cultivars for nodulation. Soil Sci Plant Nutr. , 37, 23–30. doi:10.1080/00380768.1991.10415006
- Klubeck BP , Hendrickson LL , Zablotowicz RM et al. 1988: Competitiveness of selected Bradyrhizobium japonicum strains in Midwestern USA soils. Soil Sci. Soc. American J. , 52, 662–666. doi:10.2136/sssaj1988.03615995005200030012x
- Kuykendall LD 1987: Isolation and identification of genetically marked strains of nitrogen-fixing microsymbionts of soybeans. In Practical Symbiotic Nitrogen Fixation Methodology, ed Elkan GH , pp. 205–220. Marcel Dekker, New York
- Kvien CS , Ham GE , Lambert JW 1981: Recovery of introduced Rhizobium japonicum strains by soybean genotypes. Agronomy J. , 73, 900–905. doi:10.2134/agronj1981.00021962007300050034x
- Matiru VN , Dakora FD 2004: Potential use of rhizobial bacteria as promoters of plant growth for increased yield in landraces of African cereal crops. African J. Biotechnol. , 3, 1–7. doi:10.5897/AJB2004.000-2002
- MOAI 2015: Myanmar Agriculture in Brief. pp. 16–23. Department of planning, Ministry of Agriculture and Irrigation, Naypyitaw, Union of Myanmar
- Moore S , Stein WH 1954: A Modified ninhydrin reagent for the photometric determination of amino acids and realted compounds. J. Biol. Chem. , 211, 907–913.
- Muramoto J , Goto I , Ninaki M 1992: Rapid analysis of exchangeable cation and cation exchange capacity (CEC) of soils by shaking extraction method. Soil Sci Plant Nutr. , 63, 210–215.
- Murphy J , Riley JP 1962: A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta. , 27, 31–36. doi:10.1016/S0003-2670(00)88444-5
- Ohyama T , Ito M , Kobayashi K et al. 1991: Analytical procedures of N, P, K contents in plant and manure materials using H2SO4–H2O2 Kjeldahl digestion method. Jpn. Bull. Facul. Agric. Niigata Univ. , 43, 110–120. (in Japanese with English summary)
- Peoples MB , Faizah AW , Rerkasem B , Herridge DF 1989: Methods for evaluating nitrogen fixation by nodulated legumes in the field. ACIAR Monograph. , 11(7), 39–40
- Sahrawat KL 1983: Nitrogen availability indexes for submerged rice soils. Adv. Agron. , 36, 415–451.
- Shimizu M , Nakagawa Y , Sato Y , Furumai T , Igarashi Y , Onaka H , Yoshida R , Kunoh H 2000: Studies on endophytic actinomycetes (I) Streptomyces sp. isolate from rhododendron and its antifungal activity. J. Gen. Plant Patho. , 66, 360–366. doi:10.1007/PL00012978
- Soe KM 2013: Effect of co-inoculation of Myanmar bradyrhizobia with Streptomyces griseoflavus P4 on nodulation, nitrogen fixation and seed yield of Myanmar soybean cultivars. PhD Thesis. Laboratory of Plant Nutrition, Kyushu University, Japan.
- Soe KM , Bhromsiri A , Karladee D 2010: Effect of selected endophytic actinomycetes (Steptomyces sp.) and bradyrhizobia from Myanmar on growth, nodulation, nitrogen fixation and yield of different soybean varieties. CMU J. Nat. Sci. , 9(1), 95–109
- Soe KM , Bhromsiri A , Karladee D , Yamakawa T 2012: Effects of endophytic actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of different soybean varieties. Soil Sci. Plant Nutr. , 58, 319–325. doi:10.1080/00380768.2012.682044
- Soe KM , Yamakawa T 2013a: Evaluation of effective Myanmar Bradyrhizobium strains isolated from Myanmar soybean and effects of coinoculation with Streptomyces griseoflavus P4 for nitrogen fixation. Soil Sci Plant Nutr. , 59, 361–370. doi:10.1080/00380768.2013.794437
- Soe KM , Yamakawa T 2013b: Low-density co-inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to enhance symbiosis and seed yield in soybean varieties. American J. Plant Sci. , 4, 1879–1892. doi:10.4236/ajps.2013.49231
- Streeter JG 1979: Allantoin and allantoic acid in tussues and stem exudate from field-grown soybean plants. Plant Physiol. , 63, 478–480. doi:10.1104/pp.63.3.478
- Tang S 1979: Study on the nodulation and nitrogen fixation of soybean in lessive soils. Acta Pediologica Sinica. , 16, 9–16.
- Thapanapongworakul P 2003: Characterization of endophytic actinomycetes capable of controlling sweet pea root rot diseases and effects on root nodule bacteria. Master Thesis, Chiang Mai University, Thailand.
- Truog E 1930: The determination of the readily available phosphorus in soils. J. American Soc. Agron. , 22, 874–882. doi:10.2134/agronj1930.00021962002200100008x
- Unkivich MJ , Pate JS 2000: An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crops Res. , 65, 211–228. doi:10.1016/S0378-4290(99)00088-X
- Van Kessel C , Hartley C 2000: Agricultural management of grain legumes: has it led to an increased in nitrogen fixation? Field Crops Res. , 65, 165–181. doi:10.1016/S0378-4290(99)00085-4
- Vessey JK , Buss TJ 2002: Bacillus cereus UW85 inoculation effects on growth, nodulation, and N accumulation in grain legumes: Controlled-environment studies. Canadian J. Plant Sci. , 82, 282 –290. doi:10.4141/P01-047
- Vest G , Caldwell BE 1972: Rj 4-A gene conditioning ineffective nodulation in soybean. Crop Sci. , 12, 692–693. doi:10.2135/cropsci1972.0011183X001200050042x
- Vincent JM 1970: A Manual for the Practical Study of Root Nodule Bacteria. IBP Handbbok No.15 Blackwell Scientific Publications, Oxford, UK
- Weaver RW , Frederick LR 1974: Effect of inoculum rate on competitive nodulation of Glycine max L. Merrill. II. Field studies. Agronomy J. , 66, 233–236. doi:10.2134/agronj1974.00021962006600020015x
- Yamakawa T , Fukushima Y 2014: Low inoculum densities of Bradyrhizobium japonicum USDA110 is effective on production of soybean (Glycine max L. Merr.) Cultivar Fukuyutaka. J. Fac. Agr., Kyushu Univ. , 59(1), 45–53
- Yamakawa T , Shirai T , Ishizuka J 2000: Effects of symbiosis with Rhizobium fredii on transport of fixed nitrogen in the xylem of soybean plants. Soil Sci Plant Nutr. , 46, 885–892. doi:10.1080/00380768.2000.10409154
- Young EG , Conway CF 1942: On the estimation of Allantoin by the Rimini-Schryver reaction. J. Biol. Chem. , 142, 839–853.