ABSTRACT
Growth of the weed Monochoria vaginalis (Burm. f.) Kunth under the conditions of organic rice production is a serious problem. The reason for the growth of M. vaginalis being dominant, especially in organic rice production, is not fully understood. In this study, laboratory experiments were conducted to analyze soil and seed factors in relation to the promotion of germination. (1) After incubation of flooded soil with or without the addition of rice bran (0.3%, 0.6%, and 0.9% in an air-dried soil basis), soil solutions were recovered and seeds of M. vaginalis were incubated in the soil solutions. Germination in the soils solutions without and with 0.3% rice bran was greater than that in distilled water. However, germination was suppressed in the presence of 0.6% and 0.9% of rice bran. These findings indicate that the solution from the soils with rice bran has different effects that may either increase or decrease germination. (2) A mixture of air-dried soil and distilled water was filtered to obtain a soil solution. Seeds were incubated in the soil solution (same as above). Environmental and physiological factors affected germination: exposure of seeds to light was an environmental factor and high germination activity and shallow dormancy of seed were physiological. The recovered soil solution promoted germination when these factors were not optimized. (3) There was a negative and significant correlation between dissolved oxygen (DO) in the soil solution and germination, indicating that a low content of DO was a promotive factor for germination. (4) Based on an experiment using pH buffers, germination increased with decrease in pH of soil solution, as long as the pH ranged from 4.0 to 7.0. This finding indicates that pH is also a factor that promotes germination.
1. Introduction
Recently, rice (Oryza sativa. L.) cultivation by organic farming has received much attention because consumers are concerned about food safety. Although herbicides, such as butachlor, easily kill Monochoria vaginalis (Burm. f.) Kunth (De Datta and Bernasor Citation1973), its growth under the conditions of organic rice production is a very serious problem (). Control via application of rice bran to paddy fields has been adopted by farmers in many areas in Japan. The application of rice bran increases the electrical conductivity (EC) of soil solutions (Nozoe et al. Citation2012) as well as the settled soil volume in water (the reciprocal of mud density in soil surfaces) (Nozoe et al. Citation2016). These chemical and physical changes are responsible for the suppression of germination of M. vaginalis. Although this method is expected to be useful, its use has been limited because its effectiveness varies for unknown reasons.
The reason why the growth of M. vaginalis is dominant, especially in organic rice production, is not fully elucidated. One hypothesis is the promotion of germination by rice seeds. Takeuchi et al. (Citation2001) reported that this phenomenon was observed only in M. vaginalis and Monochoria korsakowii Regel et Maack. Allelochemicals extracted from rice (Kawaguchi et al. Citation1997) were suggested to be associated with the promotion. However, the study does not fully explain the primary reason why germination was promoted. As another reason, M. vaginalis exhibit strong tolerance to iron (Fe) toxicity (Nozoe et al. Citation2009). The amount of ferrous iron (Fe2+) increases in soil solution under reducing conditions and sometimes even suppresses the growth of rice (Nozoe et al. Citation2008). However, the Fe-tolerance of M. vaginalis is not the main factor for the growth dominance because other weeds such as Echinochloa oryzicola Vasing (early watergrass) (Nozoe et al. Citation2010) and Schoenoplectus juncoides (= Scirpus juncoides var. ohwianus) (Nozoe et al. Citation2009) also exhibit strong tolerance to Fe-toxicity.
In this report, laboratory experiments were conducted to analyze firstly the relationship between the promotion of germination in soil solution and the suppression in the presence of rice bran. Secondly, we investigated seed factors that were associated with the promotion of germination in soil solution; physiological and environmental factors are also discussed. The former are germination activity and seed dormancy and the latter is exposure to light. Thirdly, it is well known that M. vaginalis requires very little oxygen (O2) for germination (Takeuchi et al. Citation2001). Reduction of O2 is the first step for the decrease in oxidative–reductive status (Eh) after flooding of soil (Tanji et al. Citation2003). In addition, soil contains reducing agents such as fulvic acid (Scott et al. Citation1998). Thus, the effects of dissolved oxygen (DO) and the decrease in Eh on germination were analyzed. Finally, we analyzed the effect of pH on germination.
2. Materials and methods
2.1. Seed
Seeds of M. vaginalis were collected from plants grown at the National Agricultural and Food Research Organization (NARO), Central Region Agricultural Research Center (CARC) at Yawara experimental paddy field, Tsukubamirai City, Japan (36°00´27´´N, 140°01´19´´E) after the harvest of rice in 2012, 2014, and 2015. In 2015, a portion of the collected seeds were multiplied in 1/2000 a Wagnel pots (1 plant per pot) at CARC, Tsukuba City, Japan (36°01´41´´N, 140°05´58´´E). The seeds were air-dried and stored in a refrigerator at 5°C for more than 6 months and a portion of them was stored in tap water for more than 2 months in a refrigerator at 5°C. In this study, collection year of seed, collection place, and stored condition were expressed as [year (field or pot), air-dried, or in water].
2.2. Soil and rice bran
Five soils were sampled from the plow layer (upper 10 cm) of paddy fields at three sites: NARO, Hokkaido Agricultural Research Center, Sapporo City, Japan (43°00´31´´N, 141°24´41´´E) (soil A); NARO, CARC at Yawara experimental paddy field (soils B and C); and a farmer’s paddy field, Ryugasaki City, Japan (35°55´18´´N, 140°14´29´´E) (soils D and E). The soil samples were air-dried and passed through a 2-mm mesh sieve. Soil properties are shown in . Commercial rice bran was used for the experiment. Granulated rice bran was powdered before the experiments. Total N and total C were 20.8 (g kg−1) and 410.6 (g kg−1), respectively. The C:N ratio was 19.7:1.
Table 1. Description of topsoil.
2.3. Statistical analysis
Statistical data analyses were performed with SPSS Statistics version 20 (IBM Japan, Tokyo, Japan).
2.4. Experiment: factors relating to the promotion of germination in soil solution
2.4.1. Rice bran
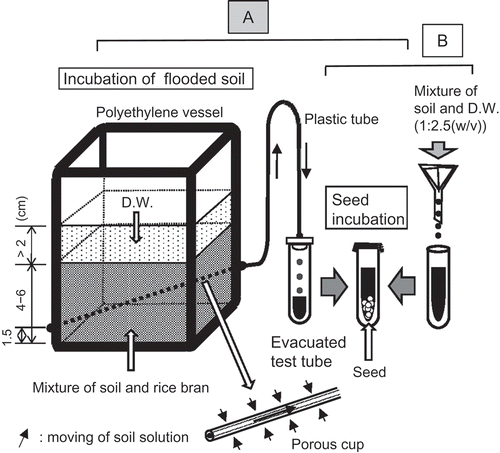
We conducted two consecutive procedures: incubation of flooded soil and seed incubation in soil solutions. The apparatus used for the incubation is shown in and in a photograph (Watanabe et al. Citation2017).
Incubation of flooded soil: 100 g of soil, with a given amount of rice bran, was put into a polyethylene vessel (60 mm × 60 mm × 85 mm in height). Distilled water (D.W.) was added to the vessel, followed by thorough stirring to remove air in the soil. The depth of flooded water was maintained at more than 2 cm throughout the incubation. The vessels were incubated at 30°C. A small hole was made in the side of each vessel 1.5 cm from the bottom. A porous cup (3 mm in diameter and 9 cm in length, Daiki Rika Kogyo, Tokyo, Japan) was inserted into the hole and horizontally set relative to the soil surface. The space between the vessel and the porous cup was sealed with epoxy adhesive. The porous cup was connected to a flexible plastic tube (TYGON®), and the soil solution obtained was introduced into a 10 mL evacuated test tube (VP-P100K, TERUMO®). After a given period of incubation, the soil solution was collected. The amount of collected solution was 6 mL.
Seed incubation in the soil solution: 30 seeds of M. vaginalis were put into the collected soil solution in the tube. The tube was sealed with a flexible film (PARAFILM®) followed by incubation at 30°C. After a given period of incubation, the germination percentage was determined. As a reference, seeds were incubated in 6 mL of distilled water.
2.4.1.1. Germination in distilled water, in soil solution from soil with and without the addition of rice bran
Experimental conditions: (i) Soil: soil A–E (). The ratios of rice bran to soil were nil (expressed as 0%), 0.3%, 0.6%, and 0.9% (w/w) for the incubation of flooded soil. (ii) Seed: [2014 (field), air-dried]. (iii) Lighting: 12-h light/12-h dark. (iv) Incubation periods: 3 d for both the incubation of flooded soil and the seed incubation. (v) The number of treatments was 21{[5 soils (A–E) × 4 rice bran contents (0%, 0.3%, 0.6%, and 0.9%)] + D.W.}. Number of tested seeds per treatment was 90 [30 per tube × 3 repetitions]. The germination percentages were expressed as the means of the values from the five soils.
2.4.1.2. Changes in the suppression and the promotion of germination during the flooded period
Experimental conditions: (i) Soil: soil B (). The ratios of rice bran to soil were 0% and 0.6% (w/w) for the incubation of the flooded soil. (ii) Seed: [2014 (field), air-dried]. (iii) Lighting during seed incubation: 12-h light/12-h dark. (iv) Incubation periods: After the incubation of flooded soil started, the soil solution was collected every day during the 4 d period of incubation. The incubation period of seed incubation was 3 d. (v) The number of treatments was 10 [1 soil (B) × 2 rice bran contents (0% and 0.6%) × 5 incubation periods of flooded soil (0, 1, 2, 3, and 4 d)]. Number of tested seeds per treatment was 90 [30 per tube × 3 repetitions].
2.4.2. Germination activity, dormant status, and exposure to light
The method of the incubation is shown in . Ten grams of air-dried soil () were mixed with 25 mL of distilled water (soil:water (w/v) = 1:2.5). The mixture was stirred several times with a spatula and immediately filtered. Thirty seeds of M. vaginalis and 6 mL of the filtrate were put into a 10-mL test tube. The tube was shaken to sink the seeds. The floated seeds were removed from the tube (same as below). The tube was incubated at 30°C.
Experimental conditions: (i) Soil: soil B (). (ii) Seed: [2012 (field); air-dried]; [2012 (field); in water]; [2014 (field); air-dried]; [2014 (field); in water]; [2015 (field), air-dried]; [2015 (field), in water]. (iii) Lighting: dark or 12-h light/12-h dark. (iv) Incubation period: 3 d. (v) The number of treatments was 24 [3 collection years of seeds (2012, 2014, and 2015) × 2 storage conditions of seeds (air-dried and in water) × 2 exposures of light during incubation (dark and 12-h light/12-h dark) × 2 (soil solution and D.W.)]. Number of tested seeds per treatment was 300 (30 per tube × 10 repetitions).
2.4.3. DO and Eh
2.4.3.1. Germination in soil solution and ascorbic acid solution
Preliminary experiment: [2015 (pot), air-dried] and [2015 (field), air-dried] were incubated to evaluate the characteristic of seeds from the pot.
Experimental conditions: i) Incubation in 10 mL of D.W. at 30°C. (ii) Seed: [2015 (pot), air-dried]; [2015 (field), air-dried]. (iii) Lighting: 12-h light/12-h dark. (iv) Incubation period: 3 d. (v) Number of tested seeds per treatment was 150 (30 per tube × 10 repetitions).
Main experiment: Soil solutions were obtained from the mixture of air-dried soil (10 g) and D.W. (25 mL) with the same procedure described in Section 2.4.2) (). The solution of ascorbic acid was prepared by dissolving it in D.W. The concentrations of ascorbic acid were 0.2, 0.4, 0.6, and 0.8 mM. The 6 mL of soil or the ascorbic acid solution were put into a 10 mL test tube followed by the addition of 30 seeds. The tubes were sealed with a flexible film and incubated at 30°C. As a reference, seeds were incubated in 6 mL of D.W.
Experimental conditions: (i) Soil and ascorbic acid solution: soils A–E (). Ascorbic acid: 0.2, 0.4, 0.6 and 0.8 mM. (ii) Seed: [2015 (pot), air-dried]. (iii) Lighting: 12-h light/12-h dark. (iv) Incubation period: 4 d. (v) The number of treatments was 10 [5 soil solutions + 4 ascorbic acid solutions and D.W.]. Number of tested seeds per treatment was 150 (30 per tube × 5 repetitions).
2.4.3.2. Changes in DO and Eh in soil and ascorbic acid solution
Soils A–E for determination of DO and soils B and C for that of Eh () were used in the experiment. After the mixture of 20 g of soil and 50 mL of D.W. was stirred several times with spatula, it was immediately filtered. This procedure was repeated several times and more than 300 mL of filtrate was obtained. The 0.2, 0.4, 0.6, and 0.8 mM ascorbic acid solutions were prepared by dissolving ascorbic acid in D.W.
For the determination of DO, 250 mL of soil solution or ascorbic acid solution were put into a 250-mL medium storage bottle. The bottle was closed with a screw cap and allowed to stand in an incubator at 30°C for 4 d. The DO of the solution was periodically measured using a DO meter (OM-51, HORIBA, Kyoto, Japan) equipped with an electrode (9551-20D, HORIBA, Kyoto, JAPAN). The experiment was performed without repetition. For the determination of Eh, 35 mL of soil solution or ascorbic acid solution was put into a 50-mL glass cylinder (40 mm in diameter and 130 mm in height). To measure Eh, a glass electrode (9300-10D, HORIBA, Kyoto, Japan) and an ORP (oxidation-reduction potential) meter (D-54, HORIBA, Kyoto, Japan) were used. The electrode was inserted into soil solution or ascorbic acid solution and allowed to stand in an incubator at 30°C for 4 d. The electrode was kept dipped in the solution throughout the incubation. The Eh was recorded every day and it was expressed as the mean of five repetitions.
2.4.4. pH
Three pH buffers of citrate, citrate-phosphate (McIlvaine’s), and phosphate (Sørensen’s) buffers were prepared according to modified methods (Stoll and Blanchard Citation1990). Citrate buffers of pH 4.0, 4.6, 5.2, and 5.6 were prepared using 10 mM of citric acid and 10 mM of trisodium citrate. Citrate–phosphate buffers of pH 4.6, 5.2, 5.8, and 6.4 were prepared using 20 mM of disodium hydrogen phosphate and 10 mM of citric acid. The phosphate buffers of pH 5.2, 5.8, 6.4, and 7.0 were prepared using 20 mM of disodium phosphate and 20 mM of potassium dihydrogen phosphate. Thirty seeds of M. vaginalis and 6 mL of each solution were put into a 10-mL test tube. The tube was incubated at 30°C for 4 d.
Experimental conditions: (i) Soil was not used in this experiment. (ii) Seed: [2014 (field), air-dried], [2015 (pot), air-dried] and [2015 (pot), in water]. (iii) Lighting: dark or 12-h light/12-h dark. (iv) Incubation period: 4 d. (v) The number of treatments was 48 [3 buffers (citrate, citrate-phosphate and phosphate) × 4 pH (from pH 4.0 to 7.0) × 4 seed treatments ([2014 (field), air-dried, exposure to light (+)], [2014 (field), air-dried, exposure to light (−)], [2015 (pot), in water, exposure to light (+)] and [2015 (pot), air-dried, exposure to light (+)]. Number of tested seeds per treatment was 210 (30 per tube × 7 repetitions).
3. Results and discussion
3.1. Rice bran
3.1.1. Germination in distilled water, in soil solution from soil with and without the addition of rice bran
In this experiment, 0.3%, 0.6%, or 0.9% rice bran was added to the soil. These values correspond to the application of 0.3, 0.6, or 0.9 t of bran to a field of 1 ha × 1 cm in accordance with the following calculations. In Japanese paddy fields, the bulk density of soil is around 1 Mg m−3 (g cm−3) (Shirato Citation2005). If the bulk density of soil is 1.00 g cm−3, the soil weight of a field with a plow layer 1 cm in depth is calculated to be 100 t h−1 (100 m × 100 m × 1 cm = 108 cm3 = 108 g = 100 t). When 1 t of rice bran is applied to this field and it is mixed with soil to 1 cm in depth, the ratio of rice bran to soil by weight is 1%. In this field, the application of 0.3%, 0.6%, and 0.9% of rice bran to soil corresponds to 0.3, 0.6, and 0.9 t ha−1, respectively. Miura et al. (Citation2015) demonstrated that the combination of mechanical weeding and the application of 1 t h−1 of rice bran into soil significantly reduced the amount of M. vaginalis under field conditions.
In this experiment, the germination of M. vaginalis tended to be as follows: soil solution without rice bran in soil (0%) ≈ 0.3% of rice bran in soil (0.3%) > D.W. ≈ 0.6% ≈ 0.9% (). First, these findings indicated that the solution from the soil without the addition of rice bran promoted germination. Second, the addition of more than 0.6% of rice bran suppressed the germination. Namely, the suppression due to rice bran counteracted the promotion due to the soil solution.
Figure 3. Effects of amount of rice bran (A) and incubation period of flooded soil (B) on germination. Experimental conditions: (A); soils: A–E, seed: [2014 (field), air-dried], lighting: present. Means denoted by the different letters are significantly different according to Tukey’s test (P < 0.05). Error bars indicate standard deviation of five soils. (B); soil: B, seed: [2014 (field), air-dried], lighting: present. Error bars indicate standard deviation of three repetitions.
![Figure 3. Effects of amount of rice bran (A) and incubation period of flooded soil (B) on germination. Experimental conditions: (A); soils: A–E, seed: [2014 (field), air-dried], lighting: present. Means denoted by the different letters are significantly different according to Tukey’s test (P < 0.05). Error bars indicate standard deviation of five soils. (B); soil: B, seed: [2014 (field), air-dried], lighting: present. Error bars indicate standard deviation of three repetitions.](/cms/asset/9e3f6f10-0551-4539-9c59-b0cd9bfb9348/tssp_a_1433957_f0003_b.gif)
3.1.2. Changes in the suppression and the promotion of germination during the flooded period
The germination rate without rice bran maintained throughout the incubation was greater than the rate in D.W. (). This finding indicates that the promotive factor of germination was not produced during the flooded condition but was originally present in the soil. Although the germination rate with 0.6% of rice bran at 0 d was equal to that without rice bran, the former decreased with the increase in the incubation period. This finding indicates that the suppressive factors with rice bran intensified with an increase in the flooded period. Tanaka et al. (Citation1990) reported that some phenolic acids were produced in the process of soil reduction and these suppress root elongation of rice. In our previous papers, we suggested that the accumulation of Fe2+ in soil solution was one of the factors that related to the growth suppression of paddy weeds (Nozoe et al. Citation2009; Nozoe et al. Citation2010). Organic acids (Tsutsuki and Ponnamperuma Citation1987) and Fe2+ (Tanji et al. Citation2003) increased with the addition of organic materials into soil. Therefore, it can be speculated that the decrease in germination with rice bran was associated with the increase in toxic substances such as organic acids and Fe2+.
In summary, (1) the promotive factor of the germination was not produced after flooding but was present in the soil originally, (2) it was soluble because the mixture of soil and distilled water was filtered immediately after mixing, as described in the Materials and Methods, and (3) it was stable since it was effective even under the flooded condition.
3.2. Germination activity, dormant status and exposure to light
It is reported that the germination of M. vaginalis with exposure to light was significantly greater than that under dark conditions (Yokota et al. Citation2014). Although the seeds of M. vaginalis remain in deep dormancy when kept under air-dried conditions, dormancy is broken gradually when dipped in cool water for more than 2 months (Wang et al. Citation1996). In this study, we express the air-dried and the water-dipped storage condition as ‘air-dried’ and ‘in water’, and the presence and absence of the exposure of light as ‘+’ and ‘–‘, respectively. Based on the germination experiment using the seeds collected in the field during 2012, 2014, and 2015, the germination rate in D.W. was as follows: iv (in water; +) > iii (air-dried; +) > ii (in water; –) > i (air-dried; –) (). This tendency was the same in seeds of all years. Those findings indicate that exposure to light is an environmental factor and the shallow dormancy of seed is a physiological factor important for the germination of the weed’s seed. The average germination percentage in D.W. was expressed as [average (i–iv)] in . The order of average (i–iv) was 2012 > 2014 > 2015 (germination activity decreased in this order). Therefore, the optimum conditions for germination were (2012 × iv) and the most inferior conditions were (2015 × i). Although most germination in soil solutions was significantly greater than in D.W., there were exceptions to this. For instance, the soil solution did not promote germination under either the optimum (2012 × iv) or the most inferior (2015 × i) condition. Based on these findings, the soil solution promotes germination when either of the following factors is insufficient: the exposure of light as an environmental factor and shallow dormancy and/or germination activity of seed as physiological factors. However, germination was not promoted when these conditions were sufficient [e.g., (2012 × iv)] or insufficient [e.g., (2015 × i)]. Presumably, the seed could germinate without the help of the soil solution under the optimum condition. On the contrary, the soil solution might not have enough power to promote the germination independently under the inferior condition.
Table 2. Effects of seed condition on the germination.
3.3. DO and Eh
3.3.1. Germination in soil solution and ascorbic acid solution
Preliminary experiment: [2015 (pot), air-dried] and [2015 (field), air-dried] were incubated in D.W. The means of germination (%) were 15.1 ± 7.6 (standard deviation of 10 repetitions) in the former and 1.1 ± 1.7 in the latter. The value of the former was significantly greater than that of the latter at 1% level. Although the seeds with the same genetic background grew in the same year, the finding obtained in the experiment indicates that environmental condition affected the germination. The nutrient status in the former was speculated to be better than the latter because the planting density in the pot (1 plant per pot) was smaller than that under the field condition. Namely, M. vaginalis could uptake the most nutrients in the pot. On the contrary, rice and M. vaginalis were forced to compete for the nutrients under the field condition. Actually, the plant size in pot was greater than that in field (). He et al. (Citation2014) reported that a higher N concentrations of mother plant led to less dormant seeds. This supports to explain the greater germination in the pot seed.
Main experiment: Generally, M. vaginalis can germinate under anaerobic conditions such as flooded soil. Submerged soil sometimes promoted the germination of M. vaginalis (Pons Citation1982). In this study, the ascorbic acid solution as well as the soil solution promoted germination (. Ascorbic acid is known to be a reducing agent (Murphy and Riley Citation1962).
Figure 4. Effects of difference in soil (A) and concentration of ascorbic acid (B) on germination. Experimental condition; seed: [2015 (pot), air-dried], lighting: present. Error bars indicate standard deviation of 10 repetitions. Means denoted by the different letters (A) are significantly different according to Tukey’s method (P < 0.05).
![Figure 4. Effects of difference in soil (A) and concentration of ascorbic acid (B) on germination. Experimental condition; seed: [2015 (pot), air-dried], lighting: present. Error bars indicate standard deviation of 10 repetitions. Means denoted by the different letters (A) are significantly different according to Tukey’s method (P < 0.05).](/cms/asset/d06519c8-44af-4f49-83a6-3e00e2fbf821/tssp_a_1433957_f0004_b.gif)
3.3.2. Changes in DO and Eh in soil and ascorbic acid solution
Takeuchi et al. (Citation2001) indicated that a low content of DO in medium promoted the germination of M. vaginalis. In this study, both the DO in soil solution and in the ascorbic acid solution were smaller than that in D.W. (A-1 and B-1). After recording the lowest values at the first day of incubation, DO tended to increase gradually. On the basis of the changes in DO (A-1 and B-1), integrated DO was calculated (A-2 and B-2). Integrated DO corresponds to the area of each treatment during the 4-d period of the incubation. There was a negative correlation between the integrated DO and the ascorbic acid contents (B-2). The values of germination ( and those of integrated DO (A-2 and B-2) were converted to A-1 and B to analyze the effect of DO on the germination. There was a significant and negative relationship between germination and integrated DO indicating that low DO in soil solution was one of the promoting factors of germination.
Figure 5. Changes in DO (A-1, B-1) and differences in integrated DO (A-2, B-2) of soil solution and ascorbic acid solution.
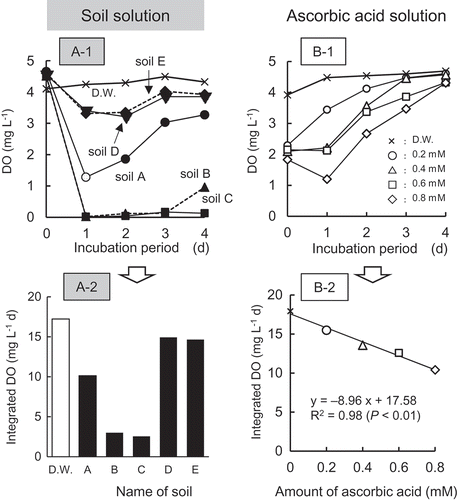
Figure 6. Relationship among germination, integrated DO and soil pH. Experimental condition; seed: [2015 (pot), air-dried], lighting: present. Symbols of A-2 and A-3 were same as those of A-1. Data of A-1 were converted from (germination) and 5A-2 (integrated DO); A-2 were from (germination) and (soil pH); A-3 were from A-2 (integrated DO) and (soil pH); and B were from (germination) and 5B-2 (integrated DO).
![Figure 6. Relationship among germination, integrated DO and soil pH. Experimental condition; seed: [2015 (pot), air-dried], lighting: present. Symbols of A-2 and A-3 were same as those of A-1. Data of A-1 were converted from Fig. 4A (germination) and 5A-2 (integrated DO); A-2 were from Fig. 4A (germination) and Table 1 (soil pH); A-3 were from Fig. 5A-2 (integrated DO) and Table 1 (soil pH); and B were from Fig. 4B (germination) and 5B-2 (integrated DO).](/cms/asset/e6f2e47b-2ce8-4abf-b499-c0df2dcfd3e1/tssp_a_1433957_f0006_b.gif)
Soil pH is one of the important factors that are responsible for the growth of weeds (Buchanan et al. Citation1975; Walker and Buchanan Citation1982). Thus, the effects of soil pH on germination and that in integrated DO were shown in A-2 and A-3, respectively. The data of A-2 were converted from 4A (germination) and (soil pH), and that of A-3 were from 5A-2 (integrated DO) and . Seemingly in this experiment, the soil pH had no significant relationship either with germination (A-2) or with integrated DO (A-3). The interaction between the pH and DO in soil solution will be discussed in the following section.
Generally, Eh begins to decrease immediately after the flooding of soil and finally decreases to less than −200 mV (Inubushi et al. Citation1984). This decrease was attributed to the sequential reduction of O2, nitrate (NO3−), manganese [Mn(IV)], ferric iron [Fe(III)], and sulfate (SO42−) in soil (Takai and Kamura Citation1966). In this study, the values of Eh were maintained at approximately +400 and +500 mV throughout the incubation (). This finding indicates that a negative value of Eh was not necessary for the reduction of O2 (A-1 and B-1) and consequently, for the promotion of germination (). It was reported that humic substances such as humic and fulvic acids have strong reducing powers (Scott et al. Citation1998). Actually, significant amounts of fulvic acid were identified in paddy soil (Dai et al. Citation2006) and in leachate from paddy soil (Maie et al. Citation2004). Therefore, chemical substances with reducing power might be associated with promotion.
Figure 7. Changes in Eh of soil solution (A) and ascorbic acid (B). Error bars indicate standard deviation of five repetitions.
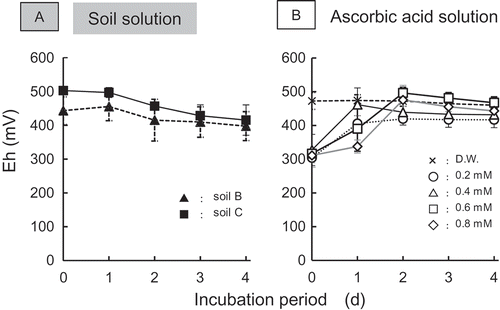
In summary, the small DO in soil solution promoted germination. Generally, O2 in soil is reduced with decrease in Eh after flooding (Tanji et al. Citation2003). In this study, however, low Eh was not a requirement for the promotion of germination because the reducing agents in soil presumably reduced the DO.
3.4. pH
Based on the experiment using buffer solution with pH 7.0–9.0, Kawaguchi et al. (Citation1997) reported that pH did not affect the germination of M. vaginalis. However, the pH of most Japanese paddy soil is less than 7.0. Actually in this study, the pH of soils ranged from 5.7 to 6.4 ().
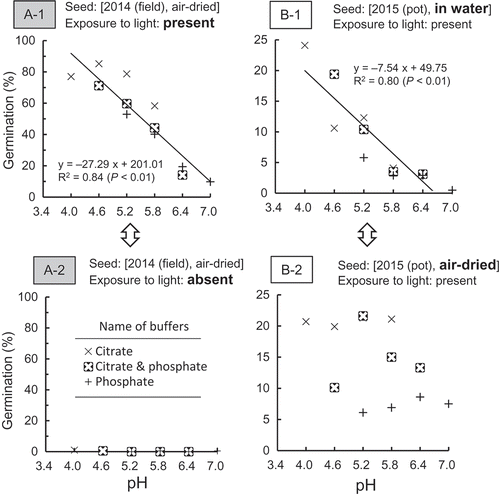
First, in this study, the seeds [2014 (field), air-dried] were incubated with and without exposure to light (A-1 and A-2). Germination with exposure to light increased with the decrease in pH (A-1). On the contrary, seed without exposure to light hardly germinated irrespective of pH (A-2), indicating that the increase in the germination at the lower pH was effective in the presence of light. Second, in the presence of light, the seeds [2015 (pot), air-dried] and [2015 (pot), in water] were incubated (B-1 and B-2). The condition for the germination of the former (B-1) was preferable to the latter (B-2) because the storage of seed in water broke dormancy (Wang et al. Citation1996). The germination of the former increased with the decrease in pH (B-1). On the contrary, the pH did not affect the germination of the latter (B-2). Those findings indicate that the increase in the germination at the lower pH was effective when seed dormancy was shallow. Finally, although both seeds [2014 (field)] (A-1) and [2015 (pot)] (B-2) were incubated under the same conditions (storage: air-dried; exposure to light: present), only the germination of the former increased with the decrease in pH. Presumably, the germination activity of the former was greater than that of the latter. On the basis of the findings, the low pH of soil solution increased germination as long as the pH ranged between 4.0 and 7.0. However, this tendency was not effective when conditions such as dormancy, the exposure to light, and germination activity were not enough for germination. Based on the finding obtained in this study, the promotion of the germination of M. vaginalis under low pH could be one of the factors that increase the population of this weed in organic farming. This increase of M. vaginalis could become a risk to reduce rice yields.
Figure 8. Effect of pH on germination in relation to exposure to light (A-1 and A-2) and storage condition (B-1 and B-2).
Although there was the negative correlation between the pH and the germination (A-1 and 8B-1), this relationship was not observed in the soil solution of the previous section (A-2). In relation to the regression lines, the slopes of soil and ascorbic acid solution were ‒1.55 (A-1) and ‒4.07 (), respectively. The milder slope in the soil solution suggests that some soil factor counteracted the promotion of germination by low DO. For instance, the low values of pH in soils D and E (A-3) were the promoting factors for the germination (A-1 and B-1). On the contrary, the high values of integrated DO in those (A-3) are opposite factors (). Therefore, there was a possibility that the soil pH and integrated DO were conflicted for the germination. It can be speculated that many factors including unknown ones were associated with the germination. Further study is needed to identify the factors and to elucidate the relationship among the factors.
In summary, four conclusions were made. (1) The solution of paddy soil promoted the germination of M. vaginalis. Environmental and physiological factors were closely related to this promotion. The exposure to light is environmental, and the high germination activity and the shallow dormancy of seed are physiological. The soil solution promoted germination when those factors were insufficient. (2) The low DO was one soil factor that promoted germination. Reducing agents such as fulvic acid in soil presumably reduced DO content. (3) Germination increased with decrease in pH of soil solution, as long as the pH ranged from 4.0 to 7.0. This finding indicates that pH was also one of the soil factors that promoted germination.
In a previous report, we indicated that the suppressive effects of rice bran on seed germination differed among soil types (Nozoe et al. Citation2012). Although site-specific management is necessary for the control of M. vaginalis, information with regard to the relationship between the soil properties and the promotion of the germination is barely available. For instance, soil pH and reducing power of soil have not been taken into account for the control of M. vaginalis. The results in the present study can provide new and useful indicators to suppress the growth of M. vaginalis in rice organic farming.
References
- Buchanan GA, Hoveland CS, Harris MC 1975: Response of weeds to soil pH. Weed Sci., 23, 473–477.
- Dai J, Ran W, Xing B, Gu M, Wang L 2006: Characterization of fulvic acid fractions obtained by sequential extractions with pH buffers, water, and ethanol from paddy soils. Geoderma, 135, 284–295. doi:10.1016/j.geoderma.2006.01.003
- De Datta SK, Bernasor PC 1973: Chemical weed control in broadcast-seeded flooded tropical rice. Weed Res., 13, 351–354. doi:10.1111/j.1365-3180.1973.tb01285.x
- He H, De Souza Vidigal D, Snoek LB, Schnabel S, Nijveen H, Hilhorst H, Bentsink L 2014: Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. J. Exp. Bot., 65, 6603–6615. doi:10.1093/jxb/eru378
- Inubushi K, Wada H, Takai Y 1984: Easily decomposable organic matter in paddy soil. IV. Relationship between reduction process and organic matter decomposition. Soil Sci. Plant Nutr., 30, 189–198. doi:10.1080/00380768.1984.10434682
- Kawaguchi S, Yoneyama K, Yokota T, Takeuchi Y, Ogasawara M, Konnai M 1997: Effects of aqueous extract of rice plants (Oryza sativa L.) on seed germination and radicle elongation of Monochoria vaginalis var plantaginea. Plant Growth Regul., 23, 183–189. doi:10.1023/A:1005986126035
- Maie N, Watanabe A, Kimura M 2004: Chemical characteristics and potential source of fulvic acids leached from the plow layer of paddy soil. Geoderma, 120, 309–323. doi:10.1016/j.geoderma.2004.02.007
- Miura S, Uchino A, Nozoe T et al. 2015: Weed suppression and rice production by mechanical weeding and rice bran application work in organic rice cultivation system. Bull. NARO. Agric. Res. Cent., 24, 55–69 (in Japanese with English summary). https://www.naro.affrc.go.jp/publicity_report/publication/files/narchokoku-24-4.pdf
- Murphy J, Riley JP 1962: Colorimetric method for determination of P in soil solution. Anal. Chim. Acta, 27, 31–36. doi:10.1016/S0003-2670(00)88444-5
- Nozoe T, Agbisit R, Fukuta Y, Rodriguez R, Yanagihara S 2008: Characteristics of iron tolerant rice lines developed at IRRI under field conditions. JARQ, 42, 187–192. doi:10.6090/jarq.42.187
- Nozoe T, Aoki D, Matsuoka H, Matsushima K, Miura S, Uchino A, Wan XC 2016: Relationship between physical property of soil and growth of Monochoria vaginalis under paddy condition of organic farming—analysis using settled soil volume in water of superficial layer. Plant Prod. Sci., 19, 238–245. doi:10.1080/1343943X.2015.1128105
- Nozoe T, Shinano T, Tachibana M, Uchino A 2010: Tolerance of rice (Oryza sativa L.) and Echinochloa weeds to growth suppression by rice straw added to paddy soil in relation to iron toxicity. Plant Prod. Sci., 13, 314–318. doi:10.1626/pps.13.314
- Nozoe T, Tachibana M, Uchino A, Yokogami N 2009: Effects of ferrous iron (Fe) on germination and root elongation of paddy rice and weed. Weed Biol. Manag., 9, 20–26. doi:10.1111/j.1445-6664.2008.00314.x
- Nozoe T, Uchino A, Okawa S, Yoshida S, Kanda Y, Nakayama Y 2012: Suppressive effect of rice bran incorporation in paddy soil on germination of Monochoria vaginalis and its relationship with electric conductivity. Soil Sci. Plant Nutr., 58, 200–205. doi:10.1080/00380768.2012.670864
- Pons TL 1982: Factors affecting weed seed germination and seedling growth in lowland rice in Indonesia. Weed Res., 22, 155–161. doi:10.1111/j.1365-3180.1982.tb00159.x
- Scott DT, McKnight DM, Blunt-Harris EL, Kolesar SE, Lovley DR 1998: Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol., 32, 2984–2989. doi:10.1021/es980272q
- Shirato Y 2005: Testing the suitability of the DNDC model for simulating long-term soil organic carbon dynamics in Japanese paddy soils. Soil Sci. Plant Nutr., 51, 183–192. doi:10.1111/j.1747-0765.2005.tb00022.x
- Stoll VS, Blanchard JS 1990: [4] Buffers: principles and practice. Meth. Enzymol., 182, 24–38. doi:10.1016/S0076-6879(09)63006-8
- Takai Y, Kamura T 1966: The mechanism of reduction in waterlogged paddy soil. Folia Microbiol. (Praha), 11, 304–313. doi:10.1007/BF02878902
- Takeuchi T, Kawaguchi S, Yoneyama K 2001: Inhibitory and promotive allelopathy in rice (Oryza sativa L.). Weed Biol. Manag., 1, 147–156. doi:10.1046/j.1445-6664.2001.00031.x
- Tanaka F, Ono S, Hayasaka T 1990: Identification and evaluation of toxicity of rice root elongation inhibitors in flooded soils with added wheat straw. Soil Sci. Plant Nutr., 36, 97–103. doi:10.1080/00380768.1990.10415714
- Tanji KK, Gaoa S, Scardacib SC, Chow AT 2003: Characterizing redox status of paddy soils with incorporated rice straw. Geoderma, 114, 333–353. doi:10.1016/S0016-7061(03)00048-X
- Tsutsuki K, Ponnamperuma FN 1987: Behavior of anaerobic decomposition products in submerged soils. Soil Sci. Plant Nutr., 33, 13–33. doi:10.1080/00380768.1987.10557549
- Walker RH, Buchanan GA 1982: Crop manipulation in integrated weed management systems. Weed Sci., 30 (S1), 17–24.
- Wang GT, Kusanagi T, Itoh K 1996: Environmental factors relating to dormancy breaking, germination and emergence of seeds in Monochoria korsakowii Regel et Maack and M. vaginalis (Burm. f.) Kunth. Weed Res. (Japan), 41, 247–254 (in Japanese with English summary). doi:10.3719/weed.41.255
- Watanabe H, Hoshino K, Adachi Y 2017: Effects of poultry manure on soil solution electrical conductivity and early growth of Monochoria vaginalis. Plant Prod. Sci., 20, 67–71. doi:10.1080/1343943X.2016.1246064
- Yokota T, Harada H, Yamada Y, Yoneyama K, Takeuchi Y 2014: Mechanism of the rice hull-induced germination of Monochoria vaginalis seeds in darkness. Weed Biol. Manag., 14, 138–144. doi:10.1111/wbm.12042