ABSTRACT
Co-inoculation of selected nitrogen-fixing bacteria with plant growth-promoting bacteria is the promising way for the improvement of soybean production through enhancing plant growth, nodulation, and N2 fixation. Therefore, this experiment was conducted to study the effects of co-inoculation of Bradyrhizobium elkanii BLY3-8 with Streptomyces griseoflavus P4 on plant growth, nodulation, N2 fixation, N uptake, and seed yield of Rj4 soybean varieties. Two experiments with completely randomized design and three replicates were done in this study. N2-fixation ability of soybean was evaluated by acetylene reduction activity (ARA) and relative ureide method. In the first experiment, synergetic effect in N2 fixation and nodulation was occurred in co-inoculation treatment (BLY3-8 + P4) in Yezin-3 and Fukuyutaka. Based on these results, co-inoculation effect of BLY3-8 and P4 was assessed on Yezin-3 and Fukuyutaka varieties at three different growth stages, using Futsukaichi soil under natural environmental conditions. This study shows that co-inoculation of BLY3-8 and P4 significantly increased N2 fixation at V6 stage; plant growth, nodulation, N2 fixation, and N uptake at R3.5 stage; and shoot growth, N uptake, and seed yield at R8 stage, in Rj4 soybean varieties compared with the control. Significant difference in plant growth, nodulation, N2 fixation, N uptake, and yield between co-inoculation and control, not between single inoculation and control, suggests that there is a synergetic effect due to co-inoculation of BLY3-8 and P4.
1. Introduction
Soybean, in symbiosis with Bradyrhizobium, has the ability to fix nitrogen up to 300 kg N ha−1 under favorable conditions (Smith and Hume Citation1987). However, the symbiosis depends on the host specificity. This specificity might be also related to nodulation regulatory genes of soybean cultivars and nodulation types of rhizobia, especially in soybean. Ishizuka et al. (Citation1991a); (Citation1991b)) tested the compatibility and preference of Rj genotypes of soybean cultivars with specific Bradyrhizobium strains. The Bradyrhizobium strains are classified into nodulation types A, B, and C, based on their compatibility with Rj cultivars. Type A strains are preferred by the non-Rj genotype cultivars and nodulate with all Rj genotype cultivars. Type B strains are preferred by Rj4 cultivars and inhibit nodulation with the Rj2Rj3 gene harboring cultivars. Type C strains are preferred by Rj2Rj3 cultivars and restrict effective nodule formation with the Rj4 genotype cultivars. Therefore, selection of type A, B, and C for their specific soybean cultivars is a novel phenomenon because their positive effects depend on the combination of nodulation type of rhizobia and the host soybean genotype.
Different soybean cultivars possess different nodulation regulatory genes known as Rj genes. These Rj (rj) genes involved in the formation of nitrogen-fixing root nodule in soybean (Hayashi et al. Citation2012). In soybean, the Rj genes Rj2, Rj3, and Rj4 restrict the nodule formation by certain Bradyrhizobium strains (Caldwell Citation1966; Caldwell et al. Citation1966; Vest Citation1970; Vest and Caldwell Citation1972). The regulatory gene Rfg1 inhibits nodulation by the fast-growing strain Sinorhizobium fredii USDA257 (Trese Citation1995). The compatibility of Bradyrhizobium and Ensifer/Sinorhizobium species is dependent on the Rj (rj) gene (Hayashi et al. Citation2012). Therefore, inoculation of specific soybean cultivars with compatible strain is necessary to form functional nodules, which can fix the atmospheric nitrogen.
Soybean cultivars in Myanmar harboring non-Rj, Rj2Rj3, Rj3, or Rj4 genes were reported in a previous study (Htwe et al. Citation2015). Among them, Rj4 gene harboring soybean cultivars are widely grown in Myanmar and account for 60% of all cultivars. Devine and Kuykendall (Citation1996) reported that >60% of soybean in Southeast Asia have the Rj4 gene. Therefore, inoculation of type B strains, which prefer Rj4 genotype cultivars, is necessary to increase nodulation and N2 fixation in Myanmar where 60% of Rj4 gene harboring soybean cultivars are widely grown.
Streptomyces is a beneficial actinomycetes and a plant growth-promoting rhizobacterium with many effects on plant growth (Ardakani and Mafakheri Citation2011). Streptomyces griseoflavus P4 used in this experiment can induce production of the plant growth hormone indole acetic acid (Soe Citation2013). Moreover, P4 used in this study was reported that it is compatible with some bradyrhizobial strains, and combined use of P4 and compatible bradyrhizobial strains had a synergetic effect for plant growth, nodulation, and N2 fixation (Soe et al. Citation2012). Those facts are attractive to use this endophytic strain as a co-inoculant with indigenous Bradyrhizobium to increase plant growth and N2-fixation rates. Therefore, the present study was aimed to evaluate co-inoculation effects of B. elkanii type B strain with P4 on plant growth, nodulation, N2 fixation, N uptake, and seed yield of Rj4 soybean varieties.
2. Materials and methods
2.1. Inoculant preparation
B. elkanii BLY3-8 (type B) strain was obtained from a previous experiment (Htwe et al. Citation2015). B. diazoefficiens strain USDA110 (Type strain) was obtained from the Laboratory of Plant Nutrition, Kyushu University, Japan. Bradyrhizobium strains and S. griseoflavus P4 were cultured in A1E liquid media (Kuykendall Citation1987) and in IMA-2 medium (Shimizu et al. Citation2000), respectively, on a rotary shaker (100 rpm) at 30°C for 7 days.
2.2. Soybean varieties
Myanmar soybean varieties, Yezin-3 (Rj4) and Yezin-6 (non-Rj), were collected from Food Legumes Section, Department of Agricultural Research, Yezin, Myanmar. Their Rj genes were identified by Soe et al. (Citation2013). Yezin-3 and Yezin-6 are semideterminate types. Japanese soybean varieties, Fukuyutaka (Rj4) and Akisirome (non-Rj), were obtained from the Laboratory of Plant Nutrition, Kyushu University, Japan. Their Rj genes were described in Ishizuka et al. (Citation1991a). Fukuyutaka and Akisirome are determinate types.
2.3. Cultivation, crop management, plant sampling, and analysis of pot experiment under controlled environmental conditions
The 1 L pots were filled with vermiculite and 0.6 L of half-strength modified Hoagland nutrient (MHN) solution (Nakano et al. Citation1997). The pots were autoclaved at 120°C for 20 min. Surface sterilization of the seeds was done by soaking them in 2.5% sodium hypochlorite solution for 5 min, rinsing five times with 10 mL of 99.5% ethanol, and washing five times with sterilized MHN solution. Five surface sterilized seeds were sown in the pots. The liquid bacterial cultures were diluted with sterilized MHN solution (Nakano et al. Citation1997) to 105 cells mL−1. Each seed was inoculated with 5 mL of bacterial suspension. The plants were cultivated in an environmentally controlled room (25°C and 75% relative humidity) for 1 month. Completely randomized design was used with three replications. Watering was done as necessary using autoclaved deionized water. At the harvest time, N2 fixation was analyzed using acetylene reduction activity (ARA) as described by Haider et al. (Citation1991). After the assay, nodules were counted. Shoots, roots, and nodules were collected separately and oven dried at 70°C for 24 h to record dry weights. This experiment was conducted from January 2017 to May 2017.
2.4. Cultivation, crop management, plant sampling, and analysis of pot experiment under natural environmental conditions
Futsukaichi soil was collected from Kamigoka, Chikushino city, Fukuoka prefecture, Japan. Before cultivation, the rhizobial population of Futsukaichi soil was evaluated by the most probable number method (Vincent Citation1970) using Yezin-3 (Rj4) as the host plant. The estimated rhizobial population was 0.29 × 104 rhizobia per 1 g dried soil. For pot preparation, the a/5000 Wagner pot was filled with 3.7 kg (oven dry basis) of Futsukaichi soil. The physiochemical properties of Futsukaichi soil were analyzed by Myint et al. (Citation2011). This soil had a sandy loam with pH 6.11 (soil: water, 1:2.5) and with low content of total nitrogen (0.68 g kg−1). CaMg(CO3)2 was added to adjust soil pH (pH 6.1 to pH 6.5). Then, compound fertilizer (Kumiai Mame-kasei 300, Ryoto Fertilizer Co., Ltd., Ooita, Japan) containing 3% N, 10% P2O5, and 10% K2O was applied at the rate of 1.6 g pot−1 at the time of pot preparation. Water content was kept at 60% of the water-holding capacity at the time of sowing. In this experiment, seed inoculation method was used prior to seed sowing as shown in Htwe et al. (Citation2018). Four inoculated seeds were planted in one pot and covered with soil just after seed sowing. After 20 days after sowing, thinning was done to maintain one plant per pot. Pesticides were sprayed as necessary. Plant samples were collected from three growing stages: V6 (six unfolded trifoliate leaves), R3.5 (early pod-fill stage), and R8 (maturity stage). The growth stages refer to Fehr et al. (Citation1971).
At V6 stage, the plants were uprooted and washed to collect data. ARA measurement and collected data were the same as described above. Shoots, roots, and nodules were oven dried at 70°C for 72 h to record their dry weights. Dried shoot was grounded into a powder using a Cyclotec 1093 sample mill (100–120 mesh, Tecator AB, Hoedanaes, Sweden). Total nitrogen accumulation in the shoot was measured by using indophenol method (Cataldo et al. Citation1974) after extracting the nutrient contents using H2SO4–H2O digestion method (Ohyama et al. Citation1991).
The plants were cut just under the cotyledonary nodes and inserted into a silicon tube. The xylem sap was collected within 1 h after cutting at the R3.5. The sap samples were stored at −30°C before analysis. Then, amino acid (Moore and Stein Citation1954), nitrate (Cataldo et al. Citation1975), and ureide (Young and Conway Citation1942) were analyzed from root bled saps. The relative ureide index (RUI) of root bled sap was calculated using the following formula (Peoples et al. Citation1989);
The percentage of N derived from N2 fixation was calculated using the following formula (Herridge and Peoples Citation1990);
in which y is RUI (%) and x is the percentage of N derived from N2 fixation (% Ndfa), respectively. Determination of N accumulation and other data were collected as described above.
At R8, the plants were cut at the cotyledon nodes to determine seed yield and yield component parameters such as number of pods per plant, number of seeds per pod, hundred-seed weight, and seed yield. Determination of N accumulation was the same as described above. This experiment was conducted from July 2017 to November 2017.
2.5. Statistical analysis
Data were analyzed using the STATISTIX 8 software package (Analytical Software, Tallahassee, FL, USA) and the means were compared by Tukey’s HSD test with a P value < 0.05 taken to indicate statistical significance.
3. Results
3.1. Effects of single or co-inoculation on symbiotic effectiveness of different soybean varieties
The results of plant growth, nodulation, and N2 fixation are shown in . Shoot dry weight was significantly affected by single or dual inoculation of bradyrhizobial strains compared with control in Yezin-3, Yezin-6, and Akisirome, except Fukuyutaka variety. However, root dry weight was not significantly increased among the treatments in all tested varieties. Nodule number, nodule dry weight, and N2 fixation were significantly different among single or dual inoculation of N2-fixation bacteria in Yezin-3 and Fukuyutaka, but not in Yezin-6 and Akisirome soybean varieties. In both Rj4 soybean varieties (Yezin-3 and Fukuyutaka), inoculation with USDA110, USDA110 + P4, and BLY3-8 + P4 showed the higher nodulation and nitrogenase activity compared with single inoculation of BLY3-8. Moreover, co-inoculation treatment (BLY3-8 + P4) shows the higher nodulation and N2 fixation compared with single inoculation with BLY3-8, although USDA110 did not show the synergetic effect due to co-inoculation with P4. Considering all the varieties, synergetic effect in nodulation and N2 fixation were occurred due to co-inoculation compared with single inoculation of BLY3-8 in Rj4 soybean varieties (Yezin-3 and Fukuyutaka), but not in non-Rj soybean varieties (Yezin-6 and Akisirome). Positive effect in shoot growth was formed by single or co-inoculation in some varieties except Fukuyutaka soybean variety.
Table 1. Effect of single or dual inoculation of B. elkanii BLY3-8 strain and B. diazoefficiens USDA110 on nodule number (NN), nodule dry weight (NDW), shoot dry weight (SDW), root dry weight (RDW), and the acetylene reduction activity (ARA) of Yezin-3, Yezin-6, Fukuyutaka, and Akisirome soybean varieties at 30 days after sowing.
3.2. Effects of single or co-inoculation on symbiotic effectiveness of Yezin-3 (Rj4) and Fukuyutaka (Rj4) soybean varieties at different growth stages
The results of plant growth, nodulation, and N uptake at different growth stages are shown in for Yezin-3 and for Fukuyutaka. The results of N2 fixation for both varieties are shown in . At V6 growth stage, root dry weight, shoot dry weight, and N uptake were not significantly different among inoculated treatments in both varieties ( and ). Nodule numbers and nodule dry weight were significantly differed by inoculation in Fukuyutaka (), but not in Yezin-3 (). In Fukuyutaka soybean variety, co-inoculation BLY3-8 and P4 produced relatively higher nodule numbers and nodule dry weight than control, although it was not significantly different from single inoculation of BLY3-8 and P4 in nodule number (). N2 fixation in terms of C2H4 production was significantly different among treatments () in both varieties. Co-inoculation of BLY3-8 + P4 showed the highest nitrogenase activity compared with single inoculation P4 and control. Besides, single inoculation of BLY3-8 significantly enhanced N2 fixation compared with control in both varieties. These results indicate that single inoculation of BLY3-8 also promoted N2 fixation. Consequently, co-inoculation of both bacteria occurred the synergetic effect in N2 fixation.
Table 2. Effect of co-inoculation of B. elkanii BLY3-8 and S. griseoflavus P4 nodule number (NN), nodule dry weight (NDW), shoot dry weight (SDW), root dry weight (RDW), and shoot N uptake of Yezin-3 soybean variety at different growth stages.
Table 3. Effect of co-inoculation of B. elkanii BLY3-8 and S. griseoflavus P4 nodule number (NN), nodule dry weight (NDW), shoot dry weight (SDW), root dry weight (RDW), and shoot N uptake of Fukuyutaka soybean variety at different growth stages.
Figure 1. Effect of co-inoculation of B. elkanii BLY3-8 and S. griseoflavus on (A) acetylene reduction activity (ARA) at V6 stage, (B) relative ureide index (%) at R3.5 stage, (C) N derived from N fixation (%Ndfa) at R3.5 of Yezin-3 and Fukuyutaka soybean varieties. The histograms with the same letter at each variety are not significantly different at P < 0.05 (Tukey’s test). The bar on each histogram indicates standard deviation (SD).
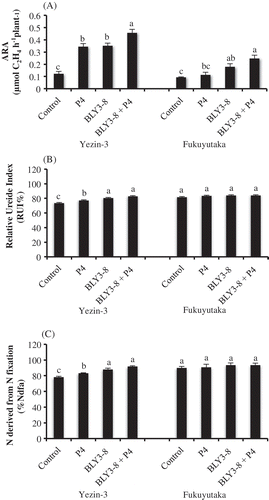
At R3.5 growth stage, nodule number, nodule dry weight, root dry weight, shoot dry weight, and N uptake were significantly different among treatments in both varieties ( and ). Co-inoculation treatment produced higher nodule number, nodule dry weight, root dry weight, and shoot dry weight compared with control, but not single inoculation treatments. These results highlight that synergetic effects for plant growth and nodulation was formed by co-inoculation of BLY3-8 and P4 in both varieties. RUI (%) and %Ndfa were significantly different among treatments ( and ) in Yezin-3 variety, but not in Fukuyutaka. Inoculation with BLY3-8 + P4 or BLY3-8 showed the higher RUI (%) and %Ndfa compared with single inoculation of P4 and control in Yezin-3. Moreover, single inoculation of P4 showed the significantly higher RUI (%) and %Ndfa than control. These results highlight that sole inoculation or dual inoculation of BLY3-8 and P4 increased plant growth, nodulation, and N2 fixation in Yezin-3 soybean variety.
At R8 growth stage, shoot biomass and shoot N uptake were significantly different in both soybean varieties ( and ). Inoculation of BLY3-8, P4, and BLY3-8 + P4 produced the higher shoot biomass than control. Moreover, BLY3-8 + P4 increased N uptake of shoot compared with control but not single inoculation treatments in both varieties.
The yield and yield component results are shown in . Yield component parameters, such as the number of pods per plant, number of seeds per pod, and 100-seed weight, were not significantly different among treatments in both varieties. However, seed yields were significantly different. Co-inoculation of BLY3-8 with P4 resulted in significantly higher seed yield than control in Yezin-3, but not single inoculation treatments. Significant difference in yield between co-inoculation and control, not between single inoculation and control, indicates that a synergetic effect is formed by combined application of both bacteria in Yezin-3. In Fukuyutaka soybean variety, single inoculation of BLY3-8, P4, and co-inoculation of BLY3-8 with P4 resulted in significantly higher seed yield than control. This result suggests that BLY3-8 and P4 are the effective bacteria, which are suitable for increasing soybean productivity. Although co-inoculation of both bacteria did not significantly increase seed yield, its co-inoculation did not show any detrimental effect in Fukuyutaka.
Table 4. Effect of co-inoculation of B. elkanii BLY3-8 and S. griseoflavus on seed yield and yield components of Yezin-3 and Fukuyutaka soybean varieties at maturity stage.
4. Discussion
Co-inoculation of rhizobia with plant growth-promoting bacteria rather than a single inoculation of rhizobia has become popular because co-inoculation improves soybean yield and contributes to sustainable agriculture (Hungria et al. Citation2015). Therefore, co-inoculation effects of BLY3-8 with P4 for N2 fixation were studied in pot experiments using sterilized vermiculite and standard Hoagland nutrient solution in the Phytotron under environmentally controlled condition. In this study, synergetic effect in nodulation and N2 fixation was occurred in co-inoculation treatment (BLY3-8 + P4) in Rj4 soybean varieties (Yezin-3 and Fukuyutaka), but not in non-Rj soybean varieties (). This supports the results of our previous study (Soe et al. Citation2012; Htwe and Yamakawa Citation2016), in which the dual inoculation of P4 with bradyrhizobial strains increased the nodulation and N2 fixation in some soybean varieties but not in other varieties.
Based on the results of the first experiment, a pot experiment was conducted to assess co-inoculation effects of BLY3-8 and P4, in Yezin-3 and Fukuyutaka varieties using Futsukaichi soil in screen house (natural environmental conditions). The combination of P4 with BLY3-8 resulted in significant increases in plant biomasses at R3.5 and R8 stages ( and ), number of nodules and nodule dry weight at V6 and/or R3.5 stages ( and ), N2 fixation at V6 and/or R3.5 stage (, B and C), N uptake at R3.5 and R8 stages ( and ), and seed yield () in both Yezin-3 and Fukuyutaka soybean varieties. These results support the findings of our group in which dual inoculation of bradyrhizobial strains and P4 increased plant growth, nodulation, N2 fixation, N uptake, and seed yield of soybeans (Soe et al. Citation2012; Soe and Yamakawa Citation2013; Htwe et al. Citation2018).
We previously reported that co-inoculation of Streptomyces griseoflavus P4 with a type A Bradyrhizobium strain, SAY3-7, had synergetic effects on the plant growth, nodulation, N2 fixation, N uptake, and seed yield of non-Rj soybean variety (Htwe et al. Citation2018). The present study showed that co-inoculation of a type B Bradyrhizobium strain with P4 strain synergistically enhanced the plant growth, nodulation, N2 fixation, N uptake, and seed yield of Rj4 soybean varieties. Differences between the present and previous studies were that the different types of Bradyrhizobium strain with P4 were investigated using different varieties in a field in the previous study and in a pot in the present study, under natural environmental conditions. However, our previous study (Htwe et al. Citation2018) and present study suggests that there is a synergetic effect due to co-inoculation of Bradyrhizobium strain and P4 because of the significant difference in plant growth, nodulation, N2 fixation, N uptake, and yield between co-inoculation and control, not between single inoculation of Bradyrhizobium strain and control.
According to the results of this study, it can be concluded that B. elkanii BLY3-8 (type B strain) together with P4 can be useful as effective inoculants for soybean cultivation of Myanmar where Rj4 gene harboring soybean cultivars are widely grown.
Acknowledgments
We are grateful to Ministry of Education, Culture, Sports, Sciences and Technology (MEXT) of Japan for financial support of this study.
Additional information
Funding
References
- Ardakani MR, Mafakheri S 2011: Designing a sustainable agroecosystem for Wheat (Triticum aestivum L.) production. J. Appl. Environ. Biol. Sci., 1(10), 401–413.
- Caldwell BE 1966: Inheritance of a strain-specific ineffective nodulation in soybeans. Crop Sci., 6, 427–428. doi:10.2135/cropsci1966.0011183X000600050010x
- Caldwell BE, Hinson K, Johnson HW 1966: A strain-specific ineffective nodulation reaction in the soybean Glycine max L. Merrill. Crop Sci., 6, 495–496. doi:10.2135/cropsci1966.0011183X000600050033x
- Cataldo DA, Haroon M, Schrader LE, Youngs VL 1975: Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal., 6, 71–80. doi:10.1080/00103627509366547
- Cataldo DA, Schrader LE, Youngs VL 1974: Analysis by digestion and colorimetric assay of total nitrogen in plant tissues high in nitrate. Crop Sci., 14, 854–856. doi:10.2135/cropsci1974.0011183X001400060024x
- Devine TE, Kuykendall LD 1996: Host genetic control of symbiosis in soybean (Glycine max L.). Plant Soil, 186, 173–187. doi:10.1007/BF00035072
- Fehr WR, Caviness CE, Burmood DT, Pennington JS 1971: Stage of development descriptions for soybeans, Glycine max (L) Merrill. Crop Sci., 11, 929–931. doi:10.2135/cropsci1971.0011183X001100060051x
- Haider J, Hussam AKMA, Ikeda M, Yamakawa T, Ishizuka J 1991: Effects of nitrate application on growth, nodulation and nitrogen fixation of nitrate-tolerant mutant soybean. Soil Sci Plant Nutr., 37, 521–529. doi:10.1080/00380768.1991.10415065
- Hayashi M, Saeki Y, Haga M, Harada K, Kouchi H, Umehara Y 2012: A review: rj (rj) genes involved in nitrogen-fixing root nodule formation in soybean. Breeding Sci., 61, 544–553. doi:10.1270/jsbbs.61.544
- Herridge DF, Peoples MB 1990: Ureide assay for measuring nitrogen fixation by nodulated soybean calibrated by 15N methods. Plant Physiol., 93, 495–503. doi:10.1104/pp.93.2.495
- Htwe AZ, Moh SM, Moe K, Yamakawa T 2018: Effects of co-inoculation of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 on plant growth, nodulation, nitrogen fixation, nutrient uptake, and yield of soybean in a field condition. Soil Sci. Plant Nutr, 1–8. doi:10.1080/00380768.2017.1421436
- Htwe AZ, Yamakawa T 2016: Low-density co-inoculation with Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 promotes plant growth and nitrogen fixation in soybean cultivars. American J. Plant Sci., 7, 1652–1661. doi:10.4236/ajps.2016.712156
- Htwe AZ, Yamakawa T, Sarr PS, Sakata T 2015: Diversity and distribution of soybean-nodulation bradyrhizobia isolated from major soybean-growing regions in Myanmar. African J. Microbiol. Res., 9(43), 2183–2196. doi:10.5897/AJMR2015.7702
- Hungria M, Nogueira MA, Araujo RS 2015: Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: A new biotechnological tool to improve yield and sustainability. American J. Plant Sci., 6, 811–817. doi:10.4236/ajps.2015.66087
- Ishizuka J, Suemasu Y, Mizogami K 1991a: Preference of Rj-soybean cultivars for Bradyrhizobium japonicum for nodulation. Soil Sci. Plant Nutr., 37, 15–21. doi:10.1080/00380768.1991.10415005
- Ishizuka J, Yokoyama A, Suemasu Y 1991b: Relationship between serotypes of Bradyrhizobium japonicum and their compatibility with Rj-cultivars for nodulation. Soil Sci. Plant Nutr., 37, 23–30. doi:10.1080/00380768.1991.10415006
- Kuykendall LD 1987: Isolation and identification of genetically marked strains of nitrogen-fixing microsymbionts of soybeans. In Practical Symbiotic Nitrogen Fixation Methodology, Elkan GH, Ed. pp. 205–220. Marcel Dekker, New York.
- Moore S, Stein WH 1954: A Modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem., 211, 907–913.
- Myint AK, Yamakawa T, Zenmyo T, Thao HTB, Sarr PS 2011: Effects of organic-manure application on growth, grain yield, and nitrogen, phosphorus, and potassium recoveries of rice variety Manawthukha in paddy soils of differing fertility. Commun. Soil Sci. Plant Anal., 42, 457–474. doi:10.1080/00103624.2011.542223
- Nakano Y, Yamakawa T, Ikeda M, Ishizuka J 1997: Nodulation of Rj-soybean varieties with Rhizobium fredii USDA193 under limited supply of nutrients. Soil Sci. Plant Nutr., 43, 929–932. doi:10.1080/00380768.1997.10414659
- Ohyama T, Ito M, Kobayashi K et al. 1991: Analytical procedures of N, P, K contents in plant and manure materials using H2SO4–H2O2 Kjeldahl digestion method. Jpn. Bull. Facul. Agric. Niigata Univ., 43, 110–120. (in Japanese with English summary).
- Peoples MB, Faizah AW, Rerkasem B, Herridge DF 1989: Methods for evaluating nitrogen fixation by nodulated legumes in the field. ACIAR Monograph., 11(7), 39–40.
- Shimizu M, Nakagawa Y, Sato Y, Furumai T, Igarashi Y, Onaka H, Yoshida R, Kunoh H 2000: Studies on endophytic actinomycetes (I) Streptomyces sp. isolate from rhododendron and its antifungal activity. J. Gen. Plant Patho., 66, 360–366. doi:10.1007/PL00012978
- Smith DL, Hume DJ 1987: Comparison of assay methods for N2 fixation utilizing white bean and soybean. Canadian J. Plant Sci., 67, 11–19. doi:10.4141/cjps87-002
- Soe KM 2013: Effect of co-inoculation of Myanmar bradyrhizobia with Streptomyces griseoflavus P4 on nodulation, nitrogen fixation and seed yield of Myanmar soybean cultivars. PhD Thesis. Laboratory of Plant Nutrition, Kyushu University, Japan.
- Soe KM, Bhromsiri A, Karladee D, Yamakawa T 2012: Effects of endophytic actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of different soybean varieties. Soil Sci. Plant Nutr., 58, 319–325. doi:10.1080/00380768.2012.682044
- Soe KM, Yamakawa T 2013: Low-density co-inoculation of Myanmar Bradyrhizobium yuanmingense MAS34 and Streptomyces griseoflavus P4 to enhance symbiosis and seed yield in soybean varieties. American J. Plant Sci., 4, 1879–1892. doi:10.4236/ajps.2013.49231
- Soe KM, Yamakawa T, Hashimoto S, Sarr P 2013: Phylogenetic diversity of indigenous soybean bradyrhizobia from different agro-climatic regions in Myanmar. ScienceAsia, 39, 574–583. doi:10.2306/scienceasia1513-1874.2013.39.574
- Trese AT 1995: A single dominant gene in McCall soybean prevents effective nodulation with Rhizobium fredii USDA257. Euphytica, 81, 279–282. doi:10.1007/BF00025618
- Vest G 1970: Rj3-A gene conditioning ineffective nodulation. Crop Sci., 10, 34–35. doi:10.2135/cropsci1970.0011183X001000010013x
- Vest G, Caldwell BE 1972: Rj4-A gene conditioning ineffective nodulation in soybean. Crop Sci., 12, 692–693. doi:10.2135/cropsci1972.0011183X001200050042x
- Vincent JM 1970: A Manual for the Practical Study of Root Nodule Bacteria. IBP Handbook No.15. Blackwell Scientific Publications, Oxford, UK.
- Young EG, Conway CF 1942: On the estimation of Allantoin by the Rimini–Schryver reaction. J. Biol. Chem., 142, 839–853.
