ABSTRACT
Effective management of the nutrients and enzyme activity in the soil is necessary for maximum crop growth and productivity. However, the excessive use of chemical fertilizers (CFs) not only adversely affects the soil nutrient status and soil physicochemical properties but also aids pollution to the ecosystem. The objective of present study was to investigate the effect of single as well as combined applications of phosphate-solubilizing bacteria and agrochemicals on important soil enzyme activities and their impact on the growth of kasumbha (safflower). Pseudomonas putida (P. putida;106 cells/mL) was applied as seed inoculation prior to sowing, and CFs were applied as full, half, and quarter doses during sowing to modulate the growth of kasumbha host plants. P. putida in combination with half dose of CFs (PH) increased the soil urease and phosphatase activities, while P. putida combined with quarter dose of CFs (PQ) augmented the soil invertase activities. Moreover, the PQ treatment exhibited the maximum colony-forming units of P. putida. Leaf chlorophyll, carotenoids, protein contents, and root lengths were increased by PH treatment. Whereas, shoot length and leaf area were improved by PH and PQ treatments, respectively. Leaf protease activity was enhanced by P. putida in combination with full dose of CFs and PQ treatments, while leaf phosphate contents were significantly improved by PQ treatment. It can be concluded that P. putida in combination with half as well as quarter doses of CFs is a promising approach for the improvement of soil enzyme activities and growth of kasumbha and replacing 50% of the use of CFs.
1. Introduction
Soil enzymes play a key role in the energy transfer through decomposition of soil organic matter (SOM) and nutrient cycling and hence play an important role in improving the soil fertility status. These enzymes catalyze many vital reactions necessary for the life processes of soil microorganisms and help in stabilization of soil structure (Rao et al. Citation2017). Soil enzymes are necessary catalysts for decomposition of SOM and nutrient cycling and strongly influence the soil fertility, energy transformation, environmental quality, and agronomic productivity. Among soil enzymes, urease performs the most important role in hydrolyzing the urea that has been leached down. Urea hydrolysis is a complicated process influenced by multiple factors (Lei et al. Citation2017). Two steps are the most important when urea undergoes transformation: the first step is the urea transformation into carbonate and ammonia in the presence of urease enzyme; in the second step, ammonia is converted to nitrite and then to nitrate ions which are readily available for the direct use of plant (Maithani et al. Citation2017). Phosphatase enzyme plays an important role in phosphate solubilization and release of inorganic phosphate and provides phosphorus nutrition to the plants (Beheraa et al. Citation2017). Although phosphorous is the second most important and essential nutrient element after nitrogen (N), it is unavailable to plants as it is commonly present in its fixed form (Satyaprakash et al. Citation2017). Invertase is another important soil enzyme, which plays an important role in carbon cycling and catalyzes the hydrolysis of sucrose into glucose and fructose (Wang et al. Citation2013). In soil, the invertase activity mainly depends upon the presence of SOM (Shi et al. Citation2008).
Soil enzymes are involved in the biogeochemical cycles (nutrient cycles) and are known as the determinant of the soil microbial activity (Schimel and Schaeffer Citation2012). Microorganisms improve soil health by ameliorating the soil enzyme activity (Gong et al. Citation2017). Inoculation of suitable microorganisms is a key procedure in organic matter biodegradation and nutrient transformation.
Plant growth-promoting rhizobacteria (PGPR) circulate the nutrients and reduce the excessive use of chemical fertilizers (CFs) to some extent (Azimi et al. Citation2013). Pseudomonas spp. possess many traits that make them well suited as biocontrol, growth-promoting agents and for improving soil health by the action of various enzymes. Similarly, phosphate solubilization and indole acetic acid production are important characteristic of Pseudomonas putida (P. putida; Meliani et al. Citation2017).
Kasumbha is a drought stress-tolerant, industrial oil seed crop and economically important to many countries of the World. Seeds of kasumbha contain 30%–35% oil contents, 24% protein, and also some fibers. Kasumbha oil contains omega-6-fatty acid which improves the blood circulation and maintains the cholesterol level. It can also be used for coloring of food, varnishes, paint, and dying purposes (Chaudhary and Ahmad Citation2017). Although the growth conditions are favorable for the cultivation of the crop in Pakistan, it is still an ignored crop in the country and its cultivation is restricted only to a limited area. The rationale for using kasumbha in the present study was to preserve the crop from extinction, create awareness about its importance. and improve its growth with sustainable measures. Furthermore, as it is a drought-tolerant crop, it can also be cultivated in drought stress areas of the country. Based on the aforementioned discussion, the current study was conducted to evaluate the effect of P. putida Khsr4 isolated from Sonchus arvensis L. growing in Khewra salt range (Naz and Bano Citation2010) and mineral fertilizers to improve the soil enzyme activity and growth of both varieties of kasumbha under controlled conditions.
2. Materials and methods
2.1. Plant material and growing conditions
Certified seeds of kasumbha cultivars Thori (spineless) and Saif-32 (spiny) were obtained from National Agricultural Research Centre, Islamabad. Seeds were surface-sterilized with 10% chlorox for 2–3 min and subsequently rinsed thoroughly three times with sterile distilled water.
Different seed treatments comprising the P. putida Khsr4 (Naz and Bano Citation2010) and CFs were tested in pot experiments under greenhouse conditions. The surface-sterilized seeds were soaked in the Luria Bertani (LB) broth culture of P. putida Khsr4 (isolated from Sonchus arvensis L. growing in Khewra salt range) at the rate of 106 cells/mL for 6 h prior to sowing. Texture of the soil used for the experiment was clay loam. A mixture of autoclaved soil and sand at 2:1 (w/w), with organic matter content (0.43%) and available nutrients total N (0.023%), phosphate (3.3 mg/kg), and potassium (100 mg/kg), was added to sterilize plastic pots (11×8 cm2). Ten seeds were sown in each pot and thinned to six plants per pot after germination. The recommended doses of CFs were applied in the form of urea and diammonium phosphate (DAP) at the rate of 60 and 30 kg ha−1, respectively, as aqueous solutions at the time of sowing. A completely randomized design was followed with eight treatments (listed in ) and three replications per treatment. Plants were harvested after 30 days of sowing to measure growth attributes, and soil sampling was done for soil enzyme activity analysis.
Table 1. Detail of treatments used in the study.
2.2. Soil analysis
Soil urease activity was measured following the method of Douglas and Bremner (Citation1970). Urease concentration was estimated from a urea-N standard curve (0–10 µg/mL) prepared on the day of analysis. The unit used for the measurement of soil urease activity was units/g of soil which indicates that one unit will liberate 1.0 mmole of NH3 from urea per minute per gram of soil.
Soil phosphatase activity was determined by the procedure of Tabatabai and Bremner (Citation1969) using disodium p-nitrophenyl phosphate as a substrate. Soil phosphatase activity was measured in units/g of soil which corresponds to the amount of p-nitrophenol (1 μmol) liberated per gram of soil per min.
Soil invertase activity was measured by the method of Zhou and Zhang (Citation1980). The unit used for the measurement of soil invertase activity was units/g of soil which indicates the release of 1 µg glucose per gram of soil per min.
Soil (1 g) was suspended in autoclaved distilled water and colony-forming unit (CFU) of P. putida was determined by plating 0.1 mL of 10-7 serial dilution on LB agar medium. Number of P. putida colonies/g soil was calculated from the CFUs obtained on plates using the formula proposed by James (Citation1978).
2.3. Plant analysis
Extraction of chlorophyll was done according to the method of Hiscox and Israelstam (Citation1979) with some modifications. Leaf tissue (0.05 g) was soaked in 2.5 mL of dimethyl sulfoxide in the test tubes and heated at 65°C for 1 h in water bath. Then, the absorbance of each sample was measured by a spectrophotometer at 663 nm and 645 nm for chlorophyll a and b, respectively, and then the total chlorophyll was calculated. Carotenoid amount in the leaves was estimated by conventional spectrophotometry using equations of Lichtenthaler and Wellburn (Citation1983) and Wellburn (Citation1994).
Protein content of the leaves was determined following the method of Lowry et al. (Citation1951) using bovine serum albumin (BSA) as standard. Fresh leaves (0.1 g) were ground in 1 mL of phosphate buffer of pH 7.5 with a mortar and pestle, and the homogenate was centrifuged at 3000 rpm for 10 min at room temperature. The supernatant was transferred to the test tubes and distilled water was added to make a total volume of 1 mL. Alkaline copper sulfate reagent (1 mL) was added, and after shaking for 10 min, 0.1 mL of the Folin’s reagent was added and the mixture was incubated for 30 min. The absorbance of each sample (three replicates/treatment) was recorded at 650 nm against a blank (1.0 mL of 0.5 M sodium hydroxide). The concentration of soluble protein was determined with reference to a standard curve using BSA.
Protease activity of seedling was measured following the method of McDonald and Chen (Citation1965). The unit of protease activity was defined as the amount of enzyme required to produce an increase in optical density at 660 nm of 0.1/h at 30°C and pH 7.0 under the assay condition defined.
Leaf area was measured according to Ahmed and Morsy (Citation1999).
Phosphorus contents of leaves were determined following the molybdo-phosphate method of Watanabe and Olsen (Citation1965). Leaf material was oven dried at 60ºC for 48 h. Dried leaf tissues (0.05–0.08 g) were ground and digested in a mixture of concentration H2SO4 (5 mL) and 30% H2O2 (5 mL). The digested mixture was analyzed colorimetrically for total phosphorus contents.
2.4. Statistical analysis
The data were analyzed statistically by factorial analysis of variance using Statistix software version 8.1. Comparisons among mean values of treatments were made by least significant difference to test significant differences at p < 0.05 (Steel and Torrie Citation1980).
3. Results
Soil urease activity was markedly improved by all the treatments in both the varieties (). Maximum increase (889%) was recorded in treatment containing P. putida in combination with quarter dose of CFs (PQ) followed by P. putida in combination with half dose of CFs (PH; 878%) as compared to the untreated control in cv. Thori. While in cv. Saif-32, maximum significant increase (266%) was recorded in PH treatment followed by PQ (226%) over the untreated control. The results for the varietal differences showed that urease activity was more pronounced in cv. Thori as compared to cv. Saif-32.
Figure 1. Effect of P. putida and chemical fertilizers on soil (A) urease, (B) phosphatase, (C) invertase activity, and (D) colony-forming unit of P. putida.
All means which share a common letter are similar; otherwise differ significantly at p < 0.05. C: Control (without inoculation and without chemical fertilizers), CFF: Chemical fertilizers full dose, CFH: Chemical fertilizers half dose, CFQ: Chemical fertilizers quarter dose, P: Seed soaking with P. putida prior to sowing, PF: P. putida+full dose of chemical fertilizers, PH: P. putida+half dose of chemical fertilizers, PQ: P. putida+quarter dose of chemical fertilizers.
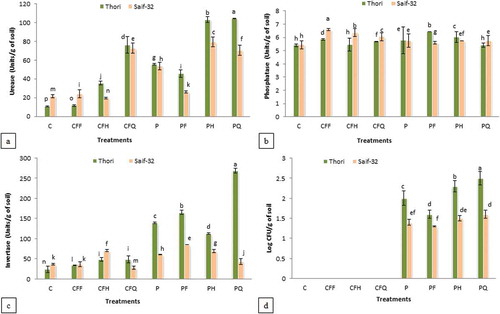
All the treatments were found to be effective in increasing the soil phosphatase activity in both the varieties (). However, phosphatase activity was higher in cv. Saif-32 as compared to cv. Thori, where 20% increase was shown by the full dose of CFs (CFF) treatment over the control.
Soil invertase activity was increased by all the treatments in cv. Thori, and maximum increase (1016%) was recorded by PQ treatment as compared to the control (). In cv. Saif-32, all the treatments showed increase in invertase activity except quarter dose of CF (CFQ). Maximum increase (133%) was recorded in P. putida in combination with full dose of CFs (PF) treatment as compared to the control. Soil collected from cv. Thori rhizosphere showed even more increase in invertase activity as compared to cv. Saif-32.
The survival efficiency of P. putida in the form of CFUs was higher (25% and 14%) in PQ treatment as compared to the P. putida alone (P) in rhizospheric soil of cv. Thori and Saif-32, respectively (). When the varietal differences were compared, the cv. Thori showed better survival efficiency over cv. Saif-32.
Maximum increase (145%) in chlorophyll contents of kasumbha leaves was recorded in PH treatment in cv. Saif-32 (). While in cv. Thori, an increase of 31% was recorded in CFQ treatment.
Figure 2. Effect of P. putida and chemical fertilizers on leaf (A) chlorophyll, (B) carotenoid, (C) protein, and (D) protease activity of safflower.
All means which share a common letter are similar; otherwise differ significantly at p < 0.05. C: Control (without inoculation and without chemical fertilizers), CFF: Chemical fertilizers full dose, CFH: Chemical fertilizers half dose, CFQ: Chemical fertilizers quarter dose, P: Seed soaking with P. putida prior to sowing, PF: P. putida+full dose of chemical fertilizers, PH: P. putida+half dose of chemical fertilizers, PQ: P. putida+quarter dose of chemical fertilizers.
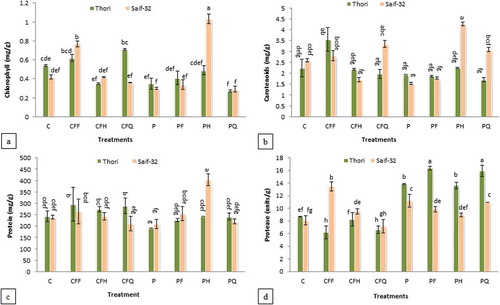
Results in indicated that the maximum increase (59%) in carotenoid contents in cv. Thori was observed in CFF treatment. While in cv. Saif-32, the maximum increase (64%) was recorded in PH treatment as compared to the control.
showed that maximum increase (22%) in leaf protein was recorded in CFF treatment in cv. Thori, while other treatments did not show significant increase. In cv. Saif-32, the treatment PH showed 68% increase in leaf protein contents as compared to the control.
Protease activity was increased by all the treatments in both the varieties except CF-treated plants (). Maximum increase (88%) in protease activity was shown by the PF treatment followed by PQ treatment (83%) in cv. Thori. In cv. Saif-32, CFF treatment showed an increase of 65% compared to the control.
Results in indicated that all the treatments improved the root length in both varieties. The PQ treatment showed maximum increase (148%) in cv. Thori and PF treatment (98%) in cv. Saif-32 as compared to the control.
Figure 3. Effect of P. putida and chemical fertilizers on (A) root length, (B) shoot length, (C) leaf area, and (D) leaf phosphate contents of safflower.
All means which share a common letter are similar; otherwise differ significantly at p < 0.05. C: Control (without inoculation and without chemical fertilizers), CFF: Chemical fertilizers full dose, CFH: Chemical fertilizers half dose, CFQ: Chemical fertilizers quarter dose, P: Seed soaking with P. putida prior to sowing, PF: P. putida+full dose of chemical fertilizers, PH: P. putida+half dose of chemical fertilizers, PQ: P. putida+quarter dose of chemical fertilizers.
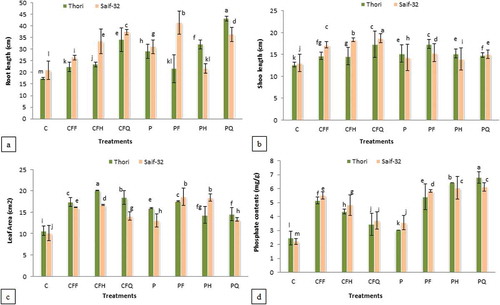
Shoot length was also improved by all the treatments in both varieties; however, maximum significant increase was recorded in cv. Saif-32 (). In cv. Thori, PF and CFQ treatments showed 36% increase, while in case of cv. Saif-32, treatment CFQ showed 43% increase in shoot length as compared to the control.
Results in showed that all the treatments increased the leaf area in both the varieties; however, maximum increase (88%) was recorded in cv. Thori by the half dose of CF (CFH) treatment. In cv. Saif-32, the PF treatment exhibited maximum increment (84%) in leaf area followed by PH treatment (81%).
Results in indicated that all the treatments ameliorate the leaf phosphate contents in both varieties. Maximum increase (176%) in leaf phosphate contents was recorded in PQ treatments as compared to control in both varieties. Cultivar Thori showed more increase in leaf phosphate contents over Saif-32.
4. Discussion
The plant rhizosphere is a major soil ecological environment for plant–microbe interactions involving colonization of different microorganisms in and around the roots of the growing plants. Soil enzymes play crucial role in the fertility of the soil. Urease has been involved in urea hydrolysis and increases the utilization rate of nitrogen fertilizer (Maithani et al. Citation2017). It was observed from the results of current study that PH and PQ increased the urease activity. Madhaiyan et al. (Citation2010) had demonstrated the significant increase in soil urease activity by PGPR inoculation. Gong et al. (Citation2017) investigated that soil urease activity was increased with increase in soil microbial populations. Our results are in agreement with the reported findings in which the CFUs and urease activity are positively correlated with each other, which mean that increase in microbial population increased the soil urease activity which, ultimately leads to improve the soil fertility status (Bansal et al. Citation2014).
It was observed that PGPR markedly increased the invertase activity in rhizosphere of both cv. Thori and Saif-32. The PQ treatment showed maximum increase in invertase activity in the rhizospheric soil of cv. Thori. Soil invertase is an important indicator of soil quality and varies with land type (Liu et al. Citation2002). Hui et al. (Citation2004) reported that inorganic fertilizers increased the soil invertase and urease activities, which were further positively correlated with increased microbial activities in the rhizosphere. Previous studies showed that microorganisms such as bacteria, yeast, fungi etc. secreted invertase into the medium (Shah et al. Citation2013).
Then, observations were made that CFF and PF treatments significantly enhanced the soil phosphatase activity over the control in both varieties. Soil phosphatase activity has often been anticipated as an indicator of the soil potential for organic phosphorus mineralization and biological activity (Huang et al. Citation2011). Krey et al. (Citation2011) reported that the phosphatase activities were increased to the greatest extent in the unfertilized soil after application of P. fluorescens. Hussain et al. (Citation2013) reported that inoculation of Pseudomonas in the presence of farm yard manure improved the rhizosphere phosphatase activity by mineralization of organic phosphate into available phosphate. P. putida plays an essential role in carbon–nitrogen cycling in the environment particularly carbon, nitrogen, phosphorus, and sulfur. In this way, P. putida not only improves the soil nutrient status but also improves soil enzyme activity and ultimately the soil fertility status and plant growth. The CFUs were increased in PH and PQ treatments, which is due to the fact that the respective treatments might have provided suitable environment for the growth of microbes. The higher doses of CFs decrease the soil pH which is not favorable for proper growth of microbes. Similar results were reported by Nakano (Citation2007) in Brassica rapa.
To expand our knowledge of the P. putida–kasumbha interaction in response to different doses of CFs, leaf chlorophyll, carotenoid, and protein contents were analyzed. Among different treatments, PH and CFF were stimulatory for leaf chlorophyll, carotenoid, and protein contents. It is evident from the results that lower doses of CF are required to augment the stimulatory effects of PGPR. Kuan et al. (Citation2016) found that PGPR inoculation could contribute about 70% of the total nitrogen requirement of the host plant which plays an important role in protein build-up process of the plant. Nitrogen fixation was the first mechanism proposed to explain the improved plant growth following inoculation with PGPR.
One of the fundamental components in plant development is the protein turnover. Proteolytic enzymes are necessary for protein turnover. During seed germination, breakdown of reserved food material takes place and proteases help in the degradation of proteins to make them available as a source of reduced nitrogen that assist in the seedling establishment (Alencar et al. Citation2012). During the present investigation, PF and PQ treatments effectively improved the protease activity of seedling. Gianfreda (Citation2015) supported our findings that PGPR secretes extra-cellular enzymes like proteases in the rhizosphere, which might have caused increased protease activity in kasumbha seedling.
Previous research showed that various Pseudomonas species were found to increase root and shoot length and also increased fresh and dry weight of the wheat seedling at p = 0.05 over the untreated control (Iqbal and Hasnain Citation2013). In our study, the maximum increase in root length was recorded in PQ, and shoot length was improved by CFQ, CFH, and PF treatments. A significant increase in plant height, root length, shoot dry weight, root dry weight, and grain yield were observed by Hussain et al. (Citation2013) in response to inoculation with the selected Pseudomonas species. The increase in growth parameters like shoot length and leaf area; contents of pigment fractions in seedling leaves; carbohydrate, total nitrogen, and total phosphate contents; and protease and amylase activities in germinated seedlings have been reported by bacterial inoculation (Karakurt and Aslantas Citation2010; Osman et al. Citation2010). Phosphorus is an important nutrient that is usually present in soil in insoluble form. Its deficiency is overcomed by the application of CFs or using phosphate-solubilizing bacteria which solubilizes the insoluble phosphate and helps the plant in nutrient uptake. In the present study, maximum increase in phosphate contents was recorded in PQ and PH treatments. Many of our colleagues reported that application of CFs and PGPR augmented the phosphorus uptake by the plant and increased the phosphate contents. Kruczek (Citation2005) reported that the application of mineral fertilizers from 17.4 kg phosphate ha−1 to 56.7 kg phosphate ha−1 increased the phosphate contents of the plant. Microbial inoculants have been reported in increasing the nutrient availability for the inoculated plant. Previous reports have indicated positive impacts of microbes on phosphate solubilization and improved uptake of fixed soil phosphate for the plant (Aseri et al. Citation2008). The mechanism resulting in increased availability of inorganic phosphate appears to be through the action of organic acids synthesized by the inoculants (Rodriguez and Fraga Citation1999).
In conclusion, this study has demonstrated the improved soil enzyme activities, overall soil health, and growth of kasumbha by the combined applications of P. putida with lower doses (half and quarter) of CFs. The variety Thori performed even better as compared to Saif-32 in the pot experiment under controlled conditions. The application of P. putida not only reduced the excessive use of CFs to about 50%–70% but also improved the soil fertility status and plant growth and leads toward sustainable and environmental-friendly agriculture.
Declaration of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to the Higher Education Commission of Pakistan for providing funding (No. 106-1822-Bm6-05) to accomplish this piece of work successfully.
Additional information
Funding
References
- Ahmed FF, Morsy MH 1999: A new method for measuring leaf area in different fruit species. Mania J. Agric. Develop., 19, 97–105.
- Alencar NLM, Innecco R, Gomes-Filho E, Gallão MI, Alvarez-Pizarro JC, Prisco JT, DE Oliveira AB 2012: Seed reserve composition and mobilization during germination and early seedling establishment of Cereus Jamacaru D.C. ssp. Jamacaru (Cactaceae). Anais da Academia Brasileira de Ciências (Annal. Brazilian Acad. Sci.), 84(3), 823–832. doi:10.1590/S0001-37652012000300024
- Aseri GK, Jain N, Panwar J, Rao AV, Meghwal PR 2008: Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.) in Indian Thar Desert. Scientia Hort., 117, 130–135. doi:10.1016/j.scienta.2008.03.014
- Azimi SM, Farnia A, Shaban M, Lak M 2013: Effect of different biofertilizers on seed yield of barley (Hurdeom vulgar L.), Bahman cultivar. Int. J. Adv. Biol. Biomed. Res., 1(5), 538–546. http://www.ijabbr.com
- Bansal OP, Singh G, Katiyar P 2014: Effect of untreated sewage effluent irrigation on heavy metal content, microbial population and enzymatic activities of soils in Aligarh. J. Environ. Biol., 35(4), 641–647.
- Beheraa BC, Yadav H, Singh SK, Sethi BK, Mishra RR, Kumari S, Thatoi H 2017: Paper alkaline phosphatase activity of a phosphate solubilizing Alcaligenes faecalis, isolated from mangrove soil. Biotechnol. Res. Innov., doi:10.1016/j.biori.2017.01.003
- Chaudhary MAM, Ahmad S 2017: Safflower – a potential industrial crop in Pakistan. Technology Times. https://www.technologytimes.pk/?p=18432
- Douglas LA, Bremner JM 1970: Colometric determination of microgram quantities of urea. Anal. Lett., 3, 79–87. doi:10.1080/00032717008067782
- Gianfreda L 2015: Enzymes of importance to rhizosphere processes. J. Soil Sci. Plant Nut., 15(2), 283–306. doi:10.4067/S0718-95162015005000022
- Gong X, Wei L, Yu X, Li S, Sun X, Wang X 2017: Effects of rhamnolipid and microbial inoculants on the vermicomposting of green waste with Eisenia fetida. PLoS ONE, 12(1), e0170820. doi:10.1371/journal.pone.0170820
- Hiscox JD, Israelstam GF 1979: A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot., 57, 1332–1334. doi:10.1139/b79-163
- Huang W, Liu J, Zhou G, Zhang D, Deng Q 2011: Effects of precipitation on soil acid phosphatase activity in three successional forests in southern China. Biogeosciences, 8, 1901–1910. doi:10.5194/bg-8-1901-2011
- Hui Z, Weijiong L, Yongzhen N 2004: Bio-organic and inorganic fertilizer on soil microbial activity. Rural Eco-Environ., 20(1), 37–40.
- Hussain MI, Asghar HN, Akhtar MJ, Arshad M 2013: Impact of phosphate solubilizing bacteria on growth and yield of maize. Soil Environ., 32(1), 71–78.
- Iqbal A, Hasnain S 2013: Auxin producing Pseudomonas strains: biological candidates to modulate the growth of Triticum aestivum beneficially. Amer. J. Plant Sci., 4, 1693–1700. doi:10.4236/ajps.2013.49206
- James GC 1978: Natalic Sherman Rockland Community College, State University of New York, pp. 75–80. The Benjamin/cummins Publishing Co. Inc.
- Karakurt H, Aslantas R 2010: Effects of some plant growth promoting rhizobacteria (PGPR) strains on plant growth and leaf nutrient content of apple. J. Fruit Ornam. Plant Res., 18, 101–110.
- Krey T, Caus M, Baum C, Ruppel S, Eichler-Löbermann B 2011: Interactive effects of plant growth–promoting rhizobacteria and organic fertilization on P nutrition of Zea mays L. and Brassica napus L. J. Plant Nut. Soil Sci., 174, 602–613. doi:10.1002/jpln.200900349
- Kruczek A 2005: Phosphorus utilization from fertilizer and accumulation of mineral components in the initial stage of maize development. Polish J. Environ. Stud., 14(4), 467–475.
- Kuan KB, Othman R, Rahim KA, Shamsuddin ZH 2016: Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS One, 11(3), 1–19. e0152478. doi:10.1371/journal.pone.0152478
- Lei T, Sun XH, Guo XH, Ma JJ 2017: Quantifying the relative importance of soil moisture, nitrogen, and temperature on the urea hydrolysis rate. Soil Sci. Plant Nut., 63(3), 225–232. doi:10.1080/00380768.2017.1340813
- Lichtenthaler HK, Wellburn AR 1983: Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans., 11, 591–592. doi:10.1042/bst0110591
- Liu KL, Lai CM, Helen W 2002: Soil enzyme activities as indicators of agricultural soil quality. 17th World Congress of Soil Science, Bangkok, Thailand, 14th-20th August, 2002. Symposium No. 32, Paper No. 1386. p. 8.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1951: Protein measurement with Folin phenol reagent. J. Biol. Chem., 193, 265–276.
- Madhaiyan M, Poonguzhali S, Kang BG, Lee YJ, Chung JB, Sa TM 2010: Effect of co-inoculation of methylotrophic Methylobacterium oryzae with Azospirillum brasilense and Burkholderia pyrrocinia on the growth and nutrient uptake of tomato, red pepper and rice. Plant Soil, 328, 71–82. doi:10.1007/s11104-009-0083-1
- Maithani S, Pal M, Maitya A, Pradhan M 2017: Isotope selective activation: a new insight into the catalytic activity of urease. RSC Adv., 7, 31372–31376. doi:10.1039/C7RA05489K
- McDonald CE, Chen LL 1965: The lowry modification of the Folin reagent for determination of proteinase activity. Anal. Biochem., 10, 175–177. doi:10.1016/0003-2697(65)90255-1
- Meliani A, Bensoltane A, Benidire L, Oufdou K 2017: Plant growth-promotion and IAA secretion with Pseudomonas fluorescens and Pseudomonas putida. Res Rev. J. Bot. Sci., 6(2), 16–24.
- Nakano Y 2007: Effects of Effective Microorganisms™ on the growth of Brassica rapa. Brigham Young University of Hawaii, USA. http://www.em-la.com/archivos-de-usuario/base_datos/em_growth_brassica_rapa.pdf
- Naz I, Bano A 2010: Biochemical, molecular characterization and growth promoting effects of phosphate solubilizing Pseudomonas sp. Isolated from weeds grown in salt range of Pakistan. Plant Soil, 334, 199–207. doi:10.1007/s11104-010-0372-8
- Osman MEH, El-Sheekh MM, El-Naggar AH, Gheda SF 2010: Effect of two species of cyanobacteria as biofertilizers on some metabolic activities, growth, and yield of pea plant. Biol. Fertil. Soil., 46, 861–875. doi:10.1007/s00374-010-0491-7
- Rao CS, Grover M, Kundu S, Desai S 2017: Soil Enzymes, 3rd ed., pp. 2100–2107. Central Research Institute for Dry land Agriculture, Indian Council of Agricultural Research (ICAR), Hyderabad, India Encyclopedia of Soil Science. doi:10.1081/E-ESS3-120052906,2100–2107
- Rodriguez H, Fraga R 1999: Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv., 17, 319–339. doi:10.1016/S0734-9750(99)00014-2
- Satyaprakash M, Nikitha T, Reddi EUB, Sadhana B, Vani SS 2017: Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. App. Sci., 6(4), 2133–2144. doi:10.20546/ijcmas.2017.604.251
- Schimel JP, Schaeffer 2012: Microbial control over carbon cycling in soil. Front Microbiol. doi:10.3389/fmicb.2012.00348
- Shah HS, Patel CM, Parikh S 2013: Production of invertase from bacteria by using waste jiggery. Microbes, 3, 19–23.
- Shi ZJ, Lu Y, Xu ZG, Fu SL 2008: Enzyme activities of urban soils under different land use in the Shenzhen city, China. Plant Soil Environ., 54(8), 341–346. doi:10.17221/415-PSE
- Steel RGD, Torrie GH 1980: Principles and Procedures of Statistics, 2nd ed., pp. 172–177. McGraw Hill Book Co. Inc, Singapore.
- Tabatabai MA, Bremner JM 1969: Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem., 1, 301–307. doi:10.1016/0038-0717(69)90012-1
- Wang Q, Xiao F, He T, Wang S 2013: Responses of labile soil organic carbon and enzyme activity in mineral soils to forest conversion in the subtropics. Annal. Forest Sci., 70(6), 579–587. doi:10.1007/s13595-013-0294-8
- Watanabe FS, Olsen SR 1965: Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Proc. Soil Sci. Soc., 29, 677–678. doi:10.2136/sssaj1965.03615995002900060025x
- Wellburn AR 1994: The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol., 144, 307–313. doi:10.1016/S0176-1617(11)81192-2
- Zhou LK, Zhang ZM 1980: Measurements of soil enzyme. Chin. J. Soil Sci., 5, 37–38.
