ABSTRACT
The effects of pH on the adsorption of silicate and phosphate, either singly or in competition, by two acid soils were investigated. Both soils adsorbed two to three times more P than Si and adsorption isotherms at pH 5.0, 5.5, 6.0 and 6.5 showed that increasing pH greatly increased Si adsorption but decreased that of P. Silicate adsorption was very low below pH 5.0, increased rapidly up to pH 9–10 before decreasing again. Adsorption of P was at a maximum at pH 2.0, decreased slowly up to pH 7.0 and then more rapidly above pH 7.0. When Si and P were added at equimolar concentrations, the presence of P decreased Si adsorption between pH 6.0 and 8.0 while the presence of Si decreased P adsorption in the pH region 6.0 and 11. Addition of calcium silicate at rates equivalent to 300, 600 and 1200 kg Si ha−1 resulted in a progressive increase in soil pH. Separate samples of soil were treated with Ca(OH)2 to give the same pH values so that the effect of Si could be identified. The highest rate of Si (1200 kg ha−1 which gave a pH of 6.5) caused a significant decrease in P adsorption (as determined by adsorption isotherms) and an increase in resin-extractable P but the lower rates had little effect. Addition of P to the soil as calcium phosphate at rates equivalent to 30, 60 and 100 kg P ha−1 all caused a decrease in Si adsorption capacity and an increase in CaCl2-extractable Si. It was concluded that the strategy of adding Si to lower P requirements in acid soils is not likely to be effective while addition of fertilizer P may well lower Si adsorption and promote Si desorption and its increased mobility.
1. Introduction
Silicate fertilizers are routinely applied to sugarcane and rice throughout the world and positive yield responses are common on soils with low Si status (Liang et al. Citation2015; Haynes Citation2017a). Yield responses have also been recorded in a wide range of other crops (Liang et al. Citation2015). The chemistry of the availability of Si in agricultural soils, and its interaction with that of other nutrients, is still, however, poorly understood (Haynes Citation2014; Haynes Citation2017b). The interaction between applied Si and applied P has been discussed for many years (e.g., Taylor Citation1961; Suehisa et al. Citation1963) but it is still a matter of controversy. Both silicate and phosphate are specifically adsorbed to soil colloids and their adsorption is thought to be at least partially competitive (Obihara and Russell Citation1972; Sandim et al. Citation2014). A tempting remedy for the management of soils with high phosphate fixing capacity is, therefore, to apply cost-effective materials that release silicate (e.g., slags) which is then adsorbed thus reducing subsequent P adsorption and therefore lowering fertilizer P requirements (Roy et al. Citation1971; Smyth and Sanchez Citation1980).
Although some workers have noted increased P extractability following additions of Si (Smyth and Sanchez Citation1980; Lee et al. Citation2004; Lee and Kim Citation2007; Sandim et al. Citation2014), others have observed little or no effect (Haynes Citation1984; Ma and Takahashi Citation1990). In addition, the majority of materials used as fertilizer Si sources (e.g., wollastonite, blast furnace, steel and other slags) are also liming materials so they raise soil pH as well as supplying soluble Si (Haynes and Zhou Citation2018). Thus, a measured increase in P availability following additions of silicate fertilizers might be attributable to an increase in soil pH favoring phosphate desorption and therefore its increased extractability (Smyth and Sanchez Citation1980; Ma and Takahashi Citation1990) rather than the effects of competitive adsorption. An added complicating factor in the interaction between added Si and P is that typical rates of applied Si (300–1200 kg Si ha−1) are often an order of magnitude greater than those of P (e.g., 30–100 kg ha−1). In addition, the effects of added P on Si adsorption/availability are not well known.
The purpose of this study was to investigate the effects of pH on adsorption of silicate and phosphate, either singly or in competition, by two acid Si-deficient soils from the sugarcane belt in Australia and investigate how addition of fertilizer Si effects P extractability and vice versa.
2. Materials and methods
2.1. Materials used
The first soil used was excavated (0–10 cm) from the control plots of a Bureau of Sugar Experimental Stations (BSES) Si field trial near Bundaberg, Queensland. The gleyed, podzolic soil was classified as a Redoxic Hydrosol (Isbell Citation2002) or by US Soil Taxonomy as an Aquic Kandiustult. The second soil was excavated (0–10 cm) from a field trial near Tully, northern Queensland. It was a humic gleyed soil from an alluvial plain and was classified as a Redoxic Hydrosol (Isbell Citation2002) or as a Typic Tropaquept by Soil Taxonomy. The mineralogy of both soils is dominated by kaolinite with some illite and smectite present. The soils were sieved < 2 mm prior to use.
2.2. Soil analysis
Organic C content was measured by combustion using a C, H, N analyzer (Carlo Erba, Italy). Electrical conductivity and pH were analyzed in a 1:5 (v/v) water extract using a four-electrode conductivity probe and a glass electrode, respectively. Citrate/dithionate-extractable Fe and Al were analyzed as described by Rayment and Lyons (Citation2011) using a 1:50 w/v extraction ratio for 16 h. BSES-available P was extracted with 0.005 M sulfuric acid (1:200 w/v for 16 h) and P was analyzed colorimetrically by the molybdenum blue method (Rayment and Lyons Citation2011). Resin-extractable P was measured as described by Kuo (Citation1996). Exchangeable bases were extracted with 1 M ammonium acetate (pH 7) and Ca, Mg, K and Na in the extracts were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Rayment and Lyons Citation2011). Silicon was extracted from soils with 0.01 M CaCl2 (1:10 ratio for 16 h) (Berthelsen et al. Citation2003) and Si in extracts was determined colorimetrically by the molybdenum blue method (Kilmer Citation1965).
2.3. Adsorption isotherms
Calcium hydroxide was added to the two soil samples to give final pH values of 5.5, 6.0 and 6.5 and they were incubated at 70% of water holding capacity and incubated for 8 weeks at 25°C. The samples were then air-dried. Silicate and phosphate adsorption isotherms were constructed at 25°C with increasing concentrations of silicate or phosphate on unamended pH of 5, and the pH 5.5, 6.0 and 6.5.
To construct Si and P adsorption isotherms, triplicate soil samples (1g) were weighed into 50-mL centrifuge tubes and 20 mL of 0.01 M NaCl (containing increasing concentrations of Si or P as sodium silicate or sodium phosphate) was added (0.01 M NaCl rather than 0.01 M CaCl2 was used as the electrolyte to avoid formation/precipitation of Ca silicate compounds). Initial concentrations of Si in the solution were 0, 0.2, 0.4, 0.8, 1.2, 1.6 and 2.0 mM and those for P were 0, 0.4, 0.8, 1.2, 1.6, 2.4 and 3.0 mM. The mixture was shaken for 1 h and pH checked and adjusted, if necessary, to the desired pH (from 5.0, 5.5, 6.0 and 6.5) using HNO3 or NaOH. This was required because sodium silicate solutions are highly alkaline and tend to raise equilibrium solution pH. The mixture was then shaken on an end-over-end shaker for an additional 15 h. After centrifugation, Si and/or P in the supernatant was measured colorimetrically as outlined above. The concentration of adsorbed Si and/or P was calculated by difference between the initial and equilibrium elemental concentrations. Adsorption data (equilibrium Si or P concentration versus quantity adsorbed) were fitted to the Langmuir and Freundlich equations.
2.4. Effect of pH on Si and P adsorption
Silicate and phosphate adsorption at 1.6 mM silicate, 1.6 mM phosphate (noncompetitive adsorption) or 1.6 mM silicate plus 1.6 mM phosphate (competitive adsorption) was measured over the pH range 2–12 at 25°C. Triplicate soil samples (2 g) were weighed into 50-mL centrifuge tubes and 10 mL of 0.01 M NaCl was added. The mixture was shaken for 1 h and then adjusted to the desired pH (approximately 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12) using HNO3 or NaOH. Once the pH had stabilized, 10 mL sodium silicate solution (in 0.01 M NaCl and at 3.2 mM of Si, P or Si and P) was added and the pH was adjusted again. The mixture was shaken on an end-over-end shaker for 16 h and during that period the pH was checked and adjusted as necessary. After centrifugation, Si and P in the supernatants were measured as outlined above.
2.5. Effect of increasing rates of calcium silicate and phosphate
The effects of realistic rates of applied Si and P on the extractability of native soil P and Si and on subsequent P or Si adsorption were examined using soil 1 (which had a low Si and P status). The experiment was replicated three times in a completely randomized design. Calcium silicate was added to the soil at rates of 300, 600 and 1200 mg Si kg−1 (equivalent to about 300, 600 and 1200 kg Si ha−1 to a depth of 10 cm). After thorough mixing, soils were rewetted to 70% water holding capacity and incubated for 8 weeks. At the end of incubation, soils were air-dried. Based on preliminary studies, another set of soils was treated with Ca(OH)2 to give the same final pH values as the calcium silicate-treated samples. This was done so that the effect of calcium silicate addition on pH and added Si could be differentiated. Resin-extractable P was measured on the calcium silicate and calcium hydroxide-treated samples (CaCl2-extractable P was initially determined but values were extremely low and a trend with treatment was not identifiable) and phosphate adsorption isotherms were also constructed (as described above).
For increasing additions of P, calcium phosphate was added at rates of 30, 60 and 100 mg P kg−1 and the same incubation procedure and duration were used as for calcium silicate amendment (see above). Based on preliminary studies, another set of soils was treated with Ca(OH)2 to give the same final pH values as the calcium phosphate-treated samples. At the end of incubation, CaCl2-extractable Si was measured and Si adsorption isotherms were constructed as outlined previously.
2.6. Statistical analysis
The statistical significance of experimental treatments relating to the effects of added Si on P adsorption/extractability and vice versa was determined by subjecting the data to analysis of variance analysis using Minitab software package and differences were calculated at the 5% level using Tukey’s test.
3. Results and discussion
3.1. Soil properties
The two soils used in this study () are typical of those on the coastal sugarcane belt in Queensland, Australia. They are highly leached, acid tropical soils with similar mineralogy and a pH of 5.0. The soils had lower dithionate extractable Al than Fe levels (). Values for both parameters were slightly higher in soil 2 than soil 1 (). Both soils would be considered to have a low Si status (i.e., CaCl2-extractable Si < 10 mg kg−1) (Berthelsen et al. Citation2003). Soil 1 had a low P status (i.e., BSES-extractable P level <50 mg kg−1) (Schroeder et al. Citation2006) but soil 2 had a high P status. Thus, for optimum sugarcane yields, soil 1 requires Si and P applications and soil 2 Si applications only. Normally, lime is recommended to sugarcane soils if the pH is below 5.5 (soils 1 and 2) and/or when exchangeable Ca is below 15 mmolc kg−1 (Soil 1) so both soils warrant liming (Schroeder et al. Citation2009).
Table 1. Some chemical properties of the two soils used.
3.2. Adsorption isotherms
Both phosphate and silicate are specifically adsorbed to the surfaces of variable charge soil colloids such as Fe and Al hydrous oxide surfaces by ligand exchange reactions (Bowden et al. Citation1980) and both can form inner sphere bidentate surface complexes with such surfaces (Arai and Sparks Citation2001; Heimstra et al. Citation2007). Anion selectivity can be dependent on pH, nature of adsorbing surfaces, ionic strength and adsorbate/adsorbent ratios, but in general the order of affinity has been reported to be arsenite = phosphate = chromate ≥ arsenate > selenite = molybdate > silicate > sulfate = fluoride > selenate > chloride ≥ nitrate (Parfitt Citation1978; McBride Citation2000). Thus, phosphate generally has a greater affinity for adsorption surfaces than silicate and it is clear from that at equimolar equilibrium P or Si concentrations, adsorption of P was considerably greater than that of Si. Thus, for the study soils, adsorption surfaces had a greater affinity for P than Si. Soil 2 adsorbed slightly more Si and P than soil 1 at the same equilibrium Si and P concentrations () and this is related to its greater Fe and Al oxide content as indicated by the higher dithionate-extractable Fe and Al values ().
Figure 1. Silicate and phosphate adsorption isotherms at pH 5.0, 5.5, 6.0 and 6.5 for the two study soils. Standard errors of the mean shown.
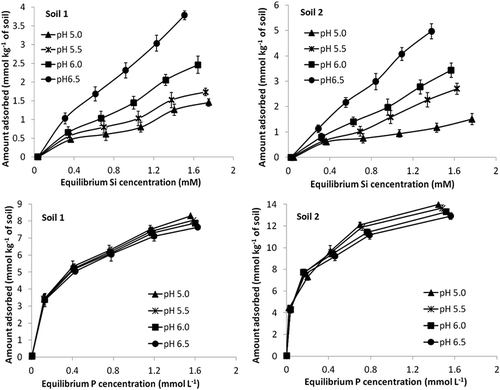
Two main factors interact to determine the effect of pH on anion adsorption. First, the surface charge on metal oxide surfaces arises from deprotonation and protonation of positive OH20.5+ and negative OH0.5– potential determining ions and as pH increases in solution the surface charge becomes increasingly negative (McBride Citation2000). As a result, there is greater electrostatic repulsion and therefore a decrease in electrostatic potential thus disfavoring specific anion adsorption. Second, adsorption is favored most strongly near the pK values of the acids because the proportion of negatively charged ions increases rapidly near the pKa values (Bowden et al. Citation1980). As shown in , phosphate adsorption was at a maximum at a pH of about 2. This is attributable to the pK1 for H3PO4 being at pH = 2. Adsorption decreased relatively slowly until about pH 7 (). Phosphate is thought to be preferentially adsorbed by surfaces as HPO42– rather than H2PO4– (Bowden et al. Citation1980) and as the pH rises from 2 to 7, the concentration of divalent ion increases 10-fold for each unit in pH (pK2 = 7). This partially offsets the decrease in electrostatic potential resulting in a slow decrease in adsorption. Above pH 7, the increase in concentration of divalent ion slows to zero and the decrease in surface potential continues so adsorption decreases more rapidly. In addition, at higher pH values, oxyanion adsorption is also inhibited due to increasing competitive effects of OH– for adsorption surfaces.
Figure 2. Effect of pH on the concentration of Si and P in equilibrium solutions after addition of Si and/or P at an initial concentration of 1.6 mM. Equilibrium was with either monoelement (Si or P) or multielement (Si + P) solutions. Standard errors of the mean shown.
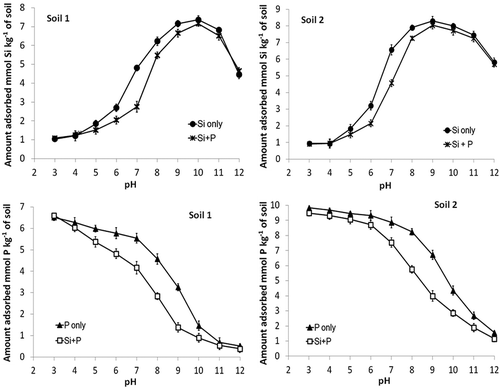
Since the pK1 for silicic acid (H4SiO4) is at pH 9.8, at typical soil pH values (i.e., 5–7), Si is present in soil solution predominantly as uncharged silicic acid. Nonetheless, Si is preferentially adsorbed as the oxyanion H3SiO4– (Hingston et al. Citation1972; Bowden et al. Citation1980) and since the proportion of Si present as H3SiO4– in the solution increases with increasing pH up to about 9.8, so too does Si adsorption (). Above that pH, electrostatic repulsion between silicate and the negatively charged surfaces, plus competitive effects of OH–, results in a decrease in adsorption (). It is evident that within the pH range commonly encountered in soils Si adsorption is much more affected by increasing pH than that of P since there is a pronounced increase in Si adsorption but only a slow decrease in P adsorption ().
While adsorption isotherms for P reached a pronounced maximum, Si adsorption isotherms had a much less well-defined maximum particularly at higher pH values (). That is, while at pH 5.0 and 6.0 Si adsorption isotherms were curvilinear and showed a maximum adsorption, at pH 6.0 and 6.5 isotherms were almost linear. This is because, as already noted, as pH increases up to pH 9.8, so too does maximum adsorption capacity. Thus, as pH increases, maximum Si adsorption capacity increases and becomes much greater than at the equilibrium solution Si concentrations used here (i.e., greater than 0–1.8 mM). Other workers have also reported linear Si adsorption isotherms (Ma and Takahashi Citation1991; Lee et al. Citation2004; Lee and Kim Citation2007). For this reason, Si adsorption isotherms fitted much more closely to the Freundlich than Langmuir model (R2 values were much greater for the Freundlich model) (). This is because the Langmuir model assumes monolayer adsorption onto a fixed number of sites and that a maximum adsorption capacity will be reached (Apak Citation2002). Others have also shown that Si adsorption by soils is well described by the Freundlich equation (Wada and Inoue Citation1974; Huang et al. Citation2006) and that the Freundlich model describes Si adsorption better than the Langmuir one (Huang et al. Citation2006). By contrast, P adsorption was equally well described by the Langmuir and Freundlich models (R2 values were similar) (). Since Si adsorption increased with increasing pH, the Freundlich constant Kf (related to adsorption capacity) and Langmuir qmax increased with increasing pH from 5.0 to 6.5 for both soils (). For P adsorption, both Langmuir qmax and Freundlich Kf constants decreased with increasing pH reflecting decreasing P adsorption with increasing pH. Values for the Freundlich constant 1/n (related to adsorption intensity) for both Si and P adsorption were less than 1.0 indicating bonding energies decreased with increasing surface adsorption densities (Apak Citation2002).
Table 2. Langmuir and Freundlich isotherm constants and coefficients of determination (R2) for adsorption of Si and P onto the soils at pH 5.0, 5.5, 6.0 and 6.5.
3.3. Simultaneous addition of Si and P
When Si and P were added simultaneously at equimolar concentrations, the negative effect of the presence of one on adsorption of the other was greatly affected by pH (). Adsorption of Si was most affected by the presence of P in the pH region 6–8. In this region adsorption of Si is increasing greatly while that of P is decreasing slowly. Adsorption of P was decreased by the presence of Si in the pH range 6–11. This occurred even though, as shown previously, adsorption surfaces of the two soils had a greater affinity for P than Si. This can occur because in the pH region 6–11 adsorption of Si is near maximum while that of P is decreasing rapidly. Such results suggest that in order to effectively decrease phosphate adsorption by previous application of silicate, the soil pH would need to be relatively high (e.g., > 6.0).
3.4. Effects of soil-applied Si and P
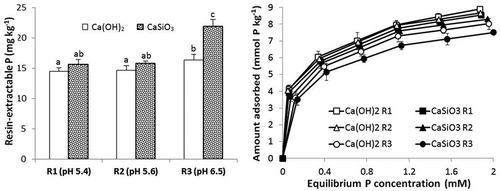
When realistic rates of Si were added to the two soils (i.e., equivalent to 300, 600 and 1200 kg Si ha−1), the pH increased from 5.4 to 6.5 (). This increase occurs because when calcium silicate dissolves it releases both Si and OH– ions:
Figure 3. Concentrations of resin-extractable P and P adsorption isotherms for soil 1 treated with three rates of Si (R1, R2 and R3 = 300, 600 and 1200 mg Si kg−1 respectively) as CaSiO3 which gave soil pH values of 5.4, 5.6 and 6.5 or an appropriate amount of Ca(OH)2 to give the same pH values. Standard errors of the mean for values on adsorption isotherm shown. Values of extractable P followed by the same letter are not significantly different P ≤ .05.
CaSiO3 + 3H2O ⇌ Ca2+ + H4SiO4 + 2OH–
The increased pH will tend to lower P adsorption and therefore increase P availability. This effect was, however, small as shown by both adsorption isotherms and extractable P concentrations when pH was raised using calcium hydroxide additions rather than those of calcium silicate. The effect of added Si on P adsorption was also small except at the high rate where the pH was 6.5. This corroborates earlier results where addition of Si only lowered P adsorption at pH values above pH 6.0 (). That is, it occurs in the pH region where P adsorption is decreasing while that of Si is increasing greatly. Similarly, the quantity of resin-extractable native soil P was markedly increased at the high rate of applied calcium silicate but not greatly affected by the lower two rates (). Thus, at pH 6.5 the high concentration of added silicate favored P desorption and an increase in P availability.
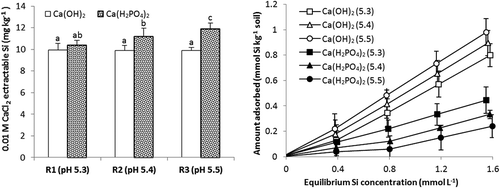
When calcium phosphate was added to soils, there was a very small increase in pH (). Others have also noted small increases in soil pH after P applications (Smyth and Sanchez Citation1980) which are attributable to the dissolution of calcium phosphate releasing OH– ions and/or release of OH– ions during phosphate adsorption. However, the small increase in pH had no significant effect on Si adsorption or Si extractability (). Nevertheless, all three rates of calcium phosphate caused a marked decrease in Si adsorption. In the pH region of the study soils (pH 5.3–5.5), adsorption of phosphate is strong and that of silicate is weak so that such a strong effect of added P is not unexpected. In addition, addition of P induced desorption of native silicate and an increase in CaCl2-extractable Si ().
Figure 4. Concentrations of CaCl2-extractable Si and Si adsorption isotherms for soil 1 treated with three rates of P (R1, R2 and R3 = 30, 60 and 100 mg P kg−1 respectively) as Ca(H2PO4)2 which gave soil pH values of 5.3, 5.4 and 5.5 or an appropriate amount of Ca(OH)2 to give the same pH values. Standard errors of the mean for values on adsorption isotherm shown. Values of extractable Si followed by the same letter are not significantly different P ≤ .05.
The results presented here suggest that in acid soils, such as those used here, applications of Si will have little effect in reducing P adsorption and therefore reducing fertilizer P requirements, unless the soil pH is raised to 6.0 or above. Since most Si fertilizer materials (e.g., calcium silicate or calcium/magnesium silicates in slags) act as liming materials as well Si sources (Haynes et al. Citation2013), such an effect is possible. Nonetheless, sugarcane is a characteristically acid-tolerant (and Al-tolerant) crop (Quinan and Wood Citation1989) so that soils are not normally limed unless they are below pH 5.5 (Schroeder et al. Citation2009) and not to a pH greater than about 5.7 (Noble et al. Citation1997). Thus, in general, Si applications are unlikely to significantly affect P availability. The effect of pH may, however, help explain the divergent results that have been previously reported with regard to the effect of added Si on P availability. That is, where pH is raised to above 6, some effect may be expected.
By contrast to the situation for added Si, applications of P (even at rates that are about one-tenth of those at which Si is commonly applied) can reduce Si adsorption and promote its desorption over a wide pH range. Thus, fertilizer P applications will tend to favor an increase in Si availability. Where there is no strong sink for soil Si such as plant uptake, this could even favor leaching loss of Si from the topsoil. While adsorbed P is generally considered strongly adsorbed, immobile and not immediately plant-available (and becomes less so over time) (Barrow Citation1980), adsorbed Si is considerably more mobile (Haynes Citation2017a). Indeed, Si is generally considered relatively easily leached and it seems likely that particularly in the acidic pH range of most sugarcane soils (where Si adsorption capacity is low) adsorption of silicate acts as a retention mechanism against leaching.
4. Conclusions
Soil surfaces generally have a greater affinity for P than Si and adsorption of P is greater than that of Si. Within the normal pH range of soils (i.e., 5–7), adsorption of Si increases greatly with increased pH while that of P decreases slowly. Applications of P, as calcium phosphate, at realistic field rates will tend to decrease silicate adsorption and promote desorption of silicate. By contrast, applications of Si, as calcium silicate, at realistic field rates will have little effect on P adsorption/desorption unless high rates of calcium silicate are applied so pH is raised to above 6.0–6.5 and concentrations of added silicate are high (or initial soil pH is already high). In most situations, the strategy of adding Si to lower P requirements in acid soils is not likely to be effective while addition of fertilizer P may well lower Si adsorption and promote Si desorption and in some cases even leaching losses of Si.
Acknowledgments
We thank Dr. G. Kingston and other members of BSES Limited for excavating and supplying the soil samples.
References
- Apak R 2002: Adsorption of heavy metal ions on soil surfaces and similar substances. In Encyclopedia of Surface and Colloid Science, Ed. Hubbard AT, pp. 385–417. Marcel Dekker, New York.
- Arai Y, Sparks DL 2001: ATR-FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrate-water interface. J. Colloid Interface Sci., 241, 317–326. doi:10.1006/jcis.2001.7773
- Barrow NJ 1980: Evaluation and utilization of residual phosphorus in soils. In The Role of Phosphorus in Agriculture, Ed. Khasawneh FE, Sample EC, Kamprath EJ, pp. 333–359. American Society of Agronomy, Madison, WI.
- Berthelsen S, Noble AD, Kingston G, Hurney AP, Rudd A, Garside AL 2003: Improving yield and ccs in sugarcane through the application of silicon based amendments. Final Report SRDC Project CLW009, Sugar Research and Development Corporation, May 2003.
- Bowden JW, Posner AM, Quirk JP 1980: Adsorption and charging phenomena in variable charge soils. In Soils with Variable Charge, Ed. Theng BKG, pp. 147–166. New Zealand Society of Soil Science, Lower Hutt.
- Haynes RJ 1984: Effect of lime, silicate and phosphate applications on the concentrations of extractable aluminium and phosphate in a spodosol. Soil Sci, 138, 8–14. doi:10.1097/00010694-198407000-00002
- Haynes RJ 2014: A contemporary overview of silicon availability in agricultural soils. J. Plant Nutr. Soil Sci., 177, 831–844. doi:10.1002/jpln.v177.6
- Haynes RJ 2017a: Significance and role of Si in crop production. Adv. Agron., 146, 83–166.
- Haynes RJ 2017b: The nature of biogenic Si and its potential role in Si supply in agricultural soils. Agric, Ecosyst. Environ., 245, 100–111. doi:10.1016/j.agee.2017.04.021
- Haynes RJ, Belyaeva ON, Kingston G 2013: Evaluation of industrial wastes as sources of fertilizer silicon using chemical extractions and plant uptake. J. Plant Nutr. Soil Sci., 176, 238–248. doi:10.1002/jpln.201200372
- Haynes RJ, Zhou Y-F 2018: Effect of pH and added slag on the extractability of Si in two Si-deficient sugarcane soils. Chemosphere, 193, 431–437. doi:10.1016/j.chemosphere.2017.10.175
- Heimstra T, Barnett MO, Van Riemsdijk WH 2007: Interaction of silicic acid with goethite. J. Colloid Interface Sci., 310, 8–17. doi:10.1016/j.jcis.2007.01.065
- Hingston FJ, Posner AM, Quirk JP 1972: Anion adsorption by goethite and gibbsite. I. The role of the proton in determining adsorption envelopes. J Soil Sci, 23, 177–192. doi:10.1111/j.1365-2389.1972.tb01652.x
- Huang L-Y, Li H-X, Zhang X-M, Lu W-S, Liu Y-J 2006: Silicate adsorption in paddy soils of Guangdong Province, China. Pedosphere, 16, 654–659. doi:10.1016/S1002-0160(06)60099-4
- Isbell RF 2002: The Australian Soil Classification. CSIRO, Collingwood, Victoria.
- Kilmer VJ 1965: Silicon. In Methods of Soil Analysis, Part 1. Chemical Methods, Ed. Black CA, pp. 959–962. American Society of Agronomy, Madison, WI.
- Kuo S 1996: Phosphorus. In Methods of Soil Analysis, Part 3. Chemical Methods, Ed. Sparks DL, pp. 869–919. American Society of Agronomy, Madison, WI.
- Lee YB, Hoon C, Hwang JY, Lee IB, Kim PJ 2004: Enhancement of phosphate desorption by silicate in soils with salt accumulation. Soil Sci. Plant Nutr., 50, 493–499. doi:10.1080/00380768.2004.10408505
- Lee YB, Kim PJ 2007: Reduction of phosphate adsorption by ion competition with silicate in soil. Korean J. Environ. Agric., 26, 286–293. doi:10.5338/KJEA.2007.26.4.286
- Liang YC, Nikolic M, Belanger R, Gong H, Song A 2015: Silicon in Agriculture. From Theory to Practice. Springer, Dordrecht.
- Ma J, Takahashi E 1990: The effect of silicic acid on rice in a P-deficient soil. Plant Soil, 126, 121–125. doi:10.1007/BF00041377
- Ma J, Takahashi E 1991: Effect of silicate on phosphate availability for rice in a P-deficient soil. Plant Soil, 133, 151–155. doi:10.1007/BF00009187
- McBride MD 2000: Chemisorption and precipitation reactions. In Handbook of Soil Science, Ed. Sumner ME, pp. B265–B302. CRC Press, Boca Raton.
- Noble AD, Bramley RGV, Wood AW, Hurney AP 1997: Sugarcane and soil acidity – why should we be worried? Proc. Aust. Soc. Sugar Cane Technol., 19, 87–99.
- Obihara CH, Russell EW 1972: Specific adsorption of silicate and phosphate by soils. J. Soil Sci., 23, 105–117. doi:10.1111/j.1365-2389.1972.tb01646.x
- Parfitt RL 1978: Anion adsorption by soils and soil minerals. Adv. Agron., 30, 1–50.
- Quinan DJ, Wood AW 1989: The effects of lime applications on acid soils in the Hambledon mill area. Proc. Aust. Soc. Sugar Cane Technol., 11, 70–75.
- Rayment GE, Lyons DJ 2011: Soil Chemical Methods – Australasia. CSIRO Publishing, Collingwood, Australia.
- Roy AC, Ali MY, Fox RL, Silva JA 1971: Influence of calcium silicate on phosphate solubility and availability in Hawaiian Latosols. In Proc. of the International Symposium on Soil Fertility Evaluation, New Delhi, February, 1971, ed Biswas TD, 757–765, Indian Society of Soil Science, New Delhi.
- Sandim AS, Bull LT, Furim AR, Lima GS, Garcia JLN 2014: Phosphorus availability in oxidic soils treated with lime and silicate applications. R. Bras. Ci. Solo, 38, 1215–1222. doi:10.1590/S0100-06832014000400018
- Schroeder BL, Panitz JH, Wood AW, Moody PW, Salter B 2009: Soil-specific nutrient management guidelines for sugarcane production in the Bundaberg district. BESE Technical Publication TE07004, BSES, Bundaberg, Australia.
- Schroeder BL, Wood AW, Moody PW, Panitz JH, Agnew JR, Sluggett RJ, Salter B 2006: Delivering nutrient management guidelines to growers in the central region of the Australian sugar industry. Proc. Aust. Soc. Sugar Cane Technol., 28, 142–154.
- Smyth JJ, Sanchez PA 1980: Effects of lime, silicate and phosphorus applications to an Oxisol on phosphorus sorption and ion retention. Soil Sci. Soc. Am. J., 44, 500–505. doi:10.2136/sssaj1980.03615995004400030012x
- Suehisa RH, Younge OR, Sherman DS 1963: Effects of silicates on phosphorus availability to Sudan Grass grown on Hawaiian soils. In Hawaii Agricultural Experiment Station Technical Bulletin No 51, Ed. Hawaii Agricultural Experiment Station, University of Hawaii, Manoa, Hawaii.
- Taylor AW 1961: Siliceous materials in plant nutrition. Review of the effects of siliceous dressings on nutrient status of soils. J. Agric. Food Chem., 9, 163–165. doi:10.1021/jf60114a022
- Wada K, Inoue A 1974: Adsorption of monomeric silica by volcanic ash soils. Soil Sci. Plant Nutr., 20, 5–15. doi:10.1080/00380768.1974.10433224
