ABSTRACT
The aim of this experiment was to evaluate the impact of colonization with arbuscular mycorrhizal (AM) fungus Glomus constrictum on the biomass production, flower quality, chlorophyll content, macronutrients and heavy metals content of marigold (Tagetes erecta L.) planted under uncontaminated soil and watered with various rates of sewage water. Sewage water utilization significantly decreased biomass production, characters of flower, nutrient concentration and rates of mycorrhizal colonization of mycorrhizal (M) and non-mycorrhizal (NM) marigold as compared to control untreated plants especially at the higher rates, but the reduction rate was proportionally higher in non-AM treatments. Mycorrhizal plants had significantly greater yield, relative chlorophyll content, leaf area, flower quality and element (P, N, K and Mg) content compared to non-inoculated marigold plants irrigated with or without sewage water. Furthermore, AM inoculation had highly decreased heavy metal (Zn, Co, Mn, Cu) content in tissues as compared to equivalent non-inoculated plants grown under sewage water application. Growing marigold with AM inoculum can reduce toxicity of heavy metals and enhance biomass production and P uptake. The results support the view that AM have a protective function for the host plant, hence playing a potential function in soil polluted immobilization processes, and thus are of assessing the potential of phytoremediation of heavy metals in sewage water contaminated soil.
1. Introduction
Soils that pose serious environmental and health problems as a result of heavy metal (HM) contamination require considerable efforts to mitigate potential environmental risks. HMs are greatly toxic to horticulture and ornamental plants. Huang et al. (Citation2002) and Tang et al. (Citation2009) indicated that bioremediation is an economically non-destructive approach to manage contaminated soils. The objective of bioremediation research is to restore contaminated ecosystems and to determine the environmental fate and influence of pollutants on plant (Andres and Polle Citation2002). Arbuscular mycorrhizal (AM) fungi are necessary components of soil phytoremediation by enhancing biomass production and mineral uptake (Shen et al. Citation2006; Wang et al. Citation2008; Rashid et al. Citation2008; Tang et al. Citation2009). The function of AM for land remediation has been studied and shown to form vital valuable symbiotic relations with the roots of most (over 85%) plant species (Abdel-Fattah et al. Citation2009). In addition, AM enhances soil properties and plant resistance to both biotic and abiotic stresses (Audet and Charest Citation2006; Abdel-Fattah and Asrar Citation2011; Asrar and Elhindi Citation2011; Kanwal et al. Citation2015). Furthermore, Shen et al. (Citation2006) noticed reduction in the availability of HMs in the contaminated soils as a result of AM inoculation due to the significant increase in soil solution pH, which strongly supports the utilization of AM for the phytoremediation of contaminated soils.
Marigold (Tagetes erecta L.) plants is among the most important commercial ornamental, medicinal and industrial plants (Ai et al. Citation2017). These plants are commonly used as ornamental flowering plants in landscaping. Furthermore, they have several medicinal benefits, for example, marigold is used in the treatment of gastrointestinal parasites (Piña-Vázquez et al. Citation2017). The nematicidal effects of the acetone extract of marigold’s flowers was, also, reported (Galicia et al. Citation2008). Ai et al. (Citation2017) indicated the significant anthelmintic effects of marigold’s crude extracts on Caenorhabditis elegans.
Several methods exist to clean up the environment from contaminants; however, many of them are costly and often difficult to implement. With AM crop inoculation in contaminated soils, the established symbiotic relationship reportedly raises the plants’ tolerance to HMs (Soares and Siqueira Citation2008; Rashid et al. Citation2008; Chaturvedi et al. Citation2018; Hristozkova et al. Citation2017). Thus, a mutual symbiosis exists between AM fungi and roots of terrestrial plants by enhancing plant biomass, absorption of immobile elements like P, Zn and Cu (Sheng et al. Citation2009) and by decreasing metal toxicity to plants by reducing root to shoot HM translocation and shoot HM concentration (Smith and Read Citation2008). This HMs uptake restriction may be elucidated by the AM capability to retain and immobilize HMs in the fungi wall, or in substances like glomalin and chitin (Khan et al. Citation2000; González-Chávez et al. Citation2004), and by decreased metal translocation from root to shoots (Bi et al. Citation2003; Christie et al. Citation2004). AM also have the ability to enhance the plant’s capacity to uptake essential nutrients while partially excluding harmful non-essential ones, hence diluting the roots and shoot content of HM (Kaldorf et al. Citation1999; Aroca et al. Citation2013; Wu et al. Citation2014; Hashem et al. Citation2016).
Furthermore, the root inoculation by AM usually results in increased relative immobile metal microelements uptake, like Zn, Cu, Co and Mn (Heggo et al. Citation1990). However, the influences of AM by the host plant on acquisition of immobile metal nutrients are still poorly understood (Abdel-Salam et al. Citation2017). Moreover, there is scarce information about the vital role of AM to mitigate the toxicity in sewage water contaminated soils of some HMs. Therefore, the present experiment was conducted to determine the effect of AM inoculation on the growth rate and flower quality of marigold grown under a range of HM concentrations by irrigating with several sewage water application rates.
2. Material and methods
2.1. Experimental design
The present study was conducted at the Experimental Agricultural Research Station at Dirab (lat 24°25′N, long 46°34′E), 40 km southwest of Riyadh, Saudi Arabia. The trial was laid down in a 2 × 4 randomized complete block design with 2 levels of AM inoculation (inoculated and non-inoculated) and 4 levels of polluted sewage water (0%, 25%, 50% and 75% diluted with tap water) with 4 replicates (plants) for each one of the eight different (2 AM levels × 4 sewage water levels) treatment. Therefore, the total number of pots used in this experiment was 32 pots each contains one plant only. Inoculum was provided by stock mycorrhizal cultures isolated from the experimental soil obtained from the same site, where the soil had been polluted by sewage water.
2.2. Mycorrhizal fungus inoculum
The dried pot culture inoculum of Glomus constrictum included inoculated root fragments of sudangrass (Sorghum halepense L.) plants and soil/sand (rhizosphere). The inoculum contained spores and external hyphae mixed into the soil at a 1:9 ratios (w/w). Non-mycorrhizal (NM) control soil had a similar culture, but without mycorrhizal (M) fungi. AM fungal inocula, containing 15 g of rhizosphere soil (750 spores) and 0.4 g of infected root fragments of sudangrass with an infection level of 86.3%, were added to each pot and placed 5 cm deep below the marigold seedling at planting time. The NM treatment received the same amount of sterilized soil inoculum to obtain an equal microflora minus AM fungi.
2.3. Plant and growth conditions
Marigold seeds (T. erecta L.), cultivar ‘Jubilee’, were surface-sterilized in 7% sodium hypochlorite (NaOCl) for 7 min and germinated for 1 week on sterile wet filter paper in Petri dishes in a controlled growth chamber. Then, seedlings were grown into 8 × 8 × 9-cm3 pots including moist-autoclaved vermiculite soil, and transferred to a greenhouse to grow under controlled conditions (225 μmol m–2 s –1 light intensity, 25/20°C day/night temperatures, 70–75% relative humidity and 14-h photoperiod). Three weeks later, the plants were planted in pots of 25 cm diameter with a mixture of autoclaved (121°C, 25 min; 1.5 air pressure on 3 separate times) sandy loamy soil (3.5 kg soil pot–1). After 3 weeks of growing, plants were irrigated with the corresponding sewage water rate (0%, 25%, 50% and 75%, respectively). The added water volume assigned for each treatment was calculated based on the soil field capacity according to Abdel-Salam et al. (Citation2017). After 8 weeks of normal growth, pots received 20 mL of Hoagland’s solution without P, because high P levels restrict the growth of AM fungi in root tissues (De Miranda and Harris Citation1994). Under controlled conditions, plants were kept in a greenhouse and harvested 12 weeks after sowing. Top watering with distilled water was added daily in each pot to replace water lost throughout the growth period to maintain the soil moisture at 70%.
2.4. Soil preparation
A sandy loam soil was obtained from the top layer (0–30 cm) of the Experimental Agricultural Research Station at Dirab, Riyadh region, Saudi Arabia. Soil was homogenized using the grinding and sieving method as it is considered the most efficient process for soil homogenization (Schumacher et al. Citation1990). Soil was, then, autoclaved (121°C, 1.5 air pressure, 2 h) to eliminate viable AM fungal propagules. Soil physical and chemical properties are shown in .
Table 1. Physical and chemical properties of the soil used in this study to grow arbuscular mycorrhizal and non-arbuscular mycorrhizal inoculated marigold plants.
2.5. Sewage water
Four levels of polluted sewage water (0%, 25%, 50% and 75% diluted with tap water) were used in this study. The physical and chemical analysis of the polluted sewage water is shown in .
Table 2. Chemical analysis of sewage irrigation water used in this study to grow arbuscular mycorrhizal and non-arbuscular mycorrhizal inoculated marigold plants.
2.6. Plant measurements and analysis
2.6.1. AM root colonization
Part of roots for each treatment were cleaned with tap water, rinsed for 45 min in 7% KOH at 90°C, rewashed with tap water, acidified in 1% HCl and stained in 0.05% trypan blue in lactophenol. Mycorrhizal colonization levels, including frequency of colonization (F%), intensity of colonization (M%) and rate of arbuscules development (A%) of the stained roots, were determined according to Trouvelot et al. (Citation1986).
2.6.2. Plant biomass
At harvest, fresh plants were cleaned and weighted separately as root and shoot biomass. For each treatment, leaf area of the plants was estimated using a leaf area meter (Li-Cor, Lincoln, NV, USA). Roots and shoots were oven dried at 60°C for 72 h and then weighted. The growth performance included shoot height, flower fresh and dry weight (DW), as well as the number and diameter of flowers. The relative content of chlorophyll in the fresh leaves was determined with a SPAD-502 (Opti-Sciences, Hudson, NY, USA) portable chlorophyll apparatus. Mycorrhizae for the dependence (MD) of plants was estimated as (%) of the plant growth rate that was contributed by AM inoculation and measured according to the method of Menge et al. (Citation1978).
Tolerance indices (Ti) of M and NM plants to polluted sewage water were determined as described by Shetty et al. (Citation1995).
2.6.3. Nutrient and metals content analysis
After determination of DW, the oven dried plant samples of both shoots and roots were grounded and digested in HNO3 (70%) and H2O2 by the microwave accelerated reduction system (Mars 3, CEM Co. Ltd, USA). The metal concentrations (K+, Mg2+, Co2+, Cu2+, Mn2+ and Zn2+) in shoot and root parts were determined by an atomic absorption spectrophotometer (Analyst 300, Perkin- Elmer, Germany), according to Wolf (Citation1982), and calculated from the standard curve (10–100 μg mL−1) for each element. The P leaf concentration was determined by the ammonium vanadomolybdate method (Jackson Citation1973). Total N was determined using the Kjeldahl’s method (Nelson and Sommers Citation1973).
2.6.4. Statistical analysis
The results were statistically analyzed by 2-factor analysis of variance (ANOVA). Means were separated by Duncan’s multiple range test by the least significant difference (LSD, p ≤ 0.05) method using Costat software (Cohort, Berkeley, CA, USA).
3. Results
3.1. Plant biomass
shows that root and shoot DW and height of AM and non-AM inoculated marigold grown under 75% of sewage water were significantly lower than those of control plants. Although treatment with sewage water greatly lowered the growth parameters for both AM and non-AM plants, the AM inoculated plants had higher values for root DW and shoot height than the non-AM plants, when both were under contamination treatments. Thus, the Ti plant which had a negative relationship with the sewage water treatment level was significantly higher in AM plants than in non-AM ones.
Table 3. Fresh and dry weight of both shoot and root systems, shoot height and tolerance indices of mycorrhizal and non-mycorrhizal marigold plants grown under sewage water contaminated soil.
Leaf area, relative chlorophyll content and Mg level in both AM and non-AM plants was the greatest under the lowest level of sewage water treatment (25%) and then decreased as the sewage water concentration was further increased (). Under the contamination conditions, the content of relative chlorophyll, leaf area and Mg concentration of AM plants was higher than for non-AM plants, thus showing that high concentration of sewage water might inhibit leaf area and Mg concentration of marigold plants.
Table 4. Relative content of chlorophyll, leaf area and leaf magnesium content of mycorrhizal and non-mycorrhizal marigold plants grown in sewage water contaminated soil.
3.2. Flower parameters
shows that the flower fresh weight (FW) and DW, spike length and the diameter flower of both AM and non-AM plants under the treatment sewage water were significantly decreased when compared to the controls, especially under the higher contamination levels (50% and 75% concentrations). On the other hand, the reduction in those parameters due to contamination was more pronounced in non-AM plants than in AM ones. Under both control and contaminated conditions, the flowering parameters were greater in AM plants when compared to non-AM ones. The data presented in show that the level of marigold mycorrhizal dependency (flower FW) was positively related with the level of contamination, as this dependency level was significantly higher under sewage water than under control conditions.
Table 5. Flower diameter, flower fresh and dry weights and dependence on arbuscular mycorrhiza of mycorrhizal and non-mycorrhizal marigold plants grown in sewage water contaminated soil.
3.3. Mycorrhizal colonization levels
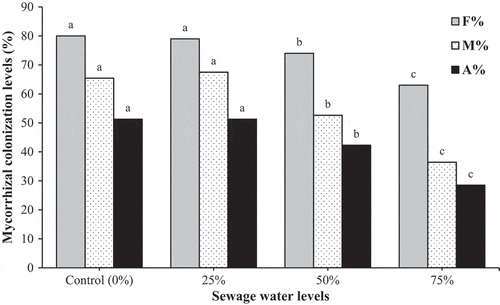
As shown in , the frequency (F%), intensity (M%) of colonization and arbuscules development (A%) on marigold roots were decreased with an increase in the sewage water contamination level, and this negative relationship was clearly pronounced under the maximal sewage water contamination level. AM colonization levels were not significantly different between controls and the 25% of sewage water contamination. In non-AM plants, no levels of AM colonization were detected.
Figure 1. Frequency, intensity of mycorrhizal colonization and arbuscular frequency in the root tissues of mycorrhizal marigold plants grown under sewage water contaminated soil. Non-mycorrhizal plants showed no colonization. There is no significant difference (P ≤ 0.05) between the mean values which are followed by the same letter (Duncan’s multiple range test). Each bar shows the mean of 4 replicates. F% = Frequency (%); M% = Intensity of mycorrhizal colonization (%); A% = Arbuscular frequency (%).
3.4. Plant nutrient contents
Data in show a positive relationship between the mycorrhizal inoculation and the root and shoot contents of P, N and K, as the content of those nutrients was higher in AM than in non-AM plants, regardless of sewage water treatments. On the other hand, the same table shows the plant contents of P, N and K as PNK levels were higher in control than in sewage water treated plants for both AM and non-AM plants. The reduction in the nutrients content was highly more pronounced under the higher contamination levels (50% and 75%) and was relatively greater in non-AM than in AM plants.
Table 6. Macronutrients concentrations in both shoot and root systems of mycorrhizal and non-mycorrhizal marigold plants grown under sewage water contaminated soil.
3.5. Metals content
The Zn, Cu, Co and Mn contents of shoots and roots of AM and non-AM plants were positively related with the level of sewage water in the soil (). The HMs content under contamination conditions was significantly lower in AM inoculated plants than in non-AM plants. However, AM marigold exhibited greater shoot and root Zn, Cu, Co and Mn concentrations when compared with non-AM plants when grown in uncontaminated (control) soils. Comparing root to shoot element levels in AM plants, the results revealed that the metals concentration in the roots was approximately twofold greater than in the shoots.
Table 7. Heavy metals concentrations in both shoots and roots of mycorrhizal and non-mycorrhizal marigold plants grown under sewage water contaminated soil.
4. Discussion
In agricultural lands, the use of sewage sludge or wastewater for irrigation may cause the accumulation of toxic elements, such as HMs, subsequently changing the physical and chemical properties of the exposed soil. Thus, pollution in irrigated water may be studied to determine its impact on the nutritional or morphological properties of plants (Tordoff et al. Citation2000; Soares and Siqueira Citation2008). The impacts of AM (in the context of phytoremediation) on plant growth and HM uptake may vary depending on a number of factors, such as plant species, fungus species and concentrations of HMs (Ahmed et al. Citation2006; Shen et al. Citation2006; Göhre and Paszkowski Citation2006; Repetto et al. Citation2006; Wang et al. Citation2008; Kamal et al. Citation2010).
In the current experiment, AM inoculation increased growth rate and flower quality of marigold planted in both sewage water and unpolluted soils compared to non-AM plants. Under the sewage water treatment, the positive growth effect of AM inoculation was more pronounced than in control soils. These results are in agreement with Shen et al. (Citation2006), who observed that AM inoculation on maize enhanced the growth rate, P content and the plant tolerance to Cd and Zn through either lowering its levels in the uptake process or through up-taking it onto the extrametrical mycelium of the AM fungi. Under HMs stress conditions, AM root colonization works as a sieve to non-essential or HM uptake and, at the same time, provides the plant with other essential nutrients (such as P), helps with water uptake (Perveen et al. Citation2012) and helps to protect plant roots against pathogens and toxic HMs (Perveen et al. Citation2012). Increasing the plant’s ability to uptake essential minerals, especially P, is one of the main techniques used by AM to alleviate stress conditions (Wang et al. Citation2005; Soares and Siqueira Citation2008). Thus, AM inoculation is considered a feasible and practical solution that may be used in the reclamation of sewage water contaminated soils as phytoremediation is a practical solution to the HMs stress (Turnau et al. Citation2006).
The drastic decline in the chlorophyll content of marigold plants depicted in our results due to sewage water stress supports the findings of Feng (Citation2006), who reported that polluted sewage water affects the synthesis of chlorophyll enzymes, decreasing plant photosynthesis and limiting plant growth. Our findings showed greater chlorophyll synthesis, possibly resulting in enhanced photosynthesis from the inoculation with AM in polluted soils. AM induced improvement in chlorophyll pigments was also cited by Malekzadeh et al. (Citation2012) and Hashem et al. (Citation2016). AM fungus improves de novo synthesis of proteins and chlorophyll due to its effect on the Mg uptake, which plays an important role in the synthesis of chlorophyll (Sheng et al. Citation2008) and because it slows the chlorophyll degradation process (Tang et al. Citation2009).
In the present experiment, the AM marigold root colorization rate was negatively related to the level of soil contamination with sewage water, in agreement with the results reported by Tang et al. (Citation2009) in relation to diesel levels in the soil, but our results differed from those of Gong et al. (Citation2002), who reported that the colonization was not affected by the soil contamination with organic matter. On the other hand, Audet and Charest (Citation2006) and Wang et al. (Citation2008) reported high AM colonization rates on various HM contaminated locations. AM under HM stress might be explained by the different origin and species of AM and by the particular plants species used in the experiments related to those reports (Soares and Siqueira Citation2008).
The data from our study showed that the plant (shoots and roots) contents of Mg, Zn, Cu and Co were significantly decreased in AM treated plants under contaminated conditions, when compared to NM treated plants. The same results were reported by Soares and Siqueira (Citation2008) and Lee and George (Citation2005) as they observed that, under mycorrhizal colonization, the maize shoot contents of Zn and Cd significantly decreased even when the soil concentrations of these metals were high. The plausible reasons for the AM positive HMs lowering effect in plants tissues include a positive effect on the plant general growth rate and biomass production, which dilutes the HM concentration in the tissues (Cavagnaro Citation2008; Soares and Siqueira Citation2008) by the enhanced concentration of HM uptake on the AM mycelium (Repetto et al. Citation2006) and through retention of HMs in the glomalins produced by the AM (González-Chávez et al. Citation2004). Therefore, as reported by Weissenhorn et al. (Citation1995), the AM inoculation was effective to slow or stop the uptake of HMs.
AM colonization under soil contamination reduced all element (Zn, Co, Cu and Mn) concentrations in both shoots and roots when compared to NM marigold. In addition, the element concentrations in the shoot were lower than in the root tissues. The same observations were cited by Lee and George (Citation2005), indicating the formation of lower mobile metal-phosphate compounds. Furthermore, AM inoculation increased metal translocation from the polluted soil to roots. Soares and Siqueira (Citation2008) and Tang et al. (Citation2009) also reported that accumulated metals were maintained in the roots, hence protecting the plants. Thus, the concentration of HMs in root tissues of AM inoculated plants may help with the bioremediation of polluted soils. However, the effectiveness of AM colonization of polluted soils depends on the particular chemical metal element and is affected by the particular soil conditions (Göhre and Paszkowski Citation2006; Wang et al. Citation2007).
5. Conclusion
It is concluded from the results that the AM fungi G. constrictum inoculation protected the marigold plants from metal toxicity and also improved nutrient uptake. The obtained results indicate that AM inoculated plants grew faster, exhibited enhanced mineral nutrition and had greater biomass than non-AM plants. The function of AM inoculated plants ranges from stress mitigation to bioremediation in soils polluted with HMs and its objective is to enhance soil characteristics and plant performance in semiarid and/or HM polluted sites.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research, King Saud University, Saudi Arabia, for the funding of this research through the Research Group No. RGP –1436-020.
Additional information
Funding
References
- Abdel-Fattah GM, Asrar A-WA 2011: Arbuscular mycorrhizal fungal application to improve growth and tolerance of wheat (Triticum aestivum L.) plants grown in saline soil. Acta Physiologiae Plantarum, 34 (1), 267–277. doi:10.1007/s11738-011-0825-6.
- Abdel-Fattah GM, El-Dohlob SM, Hafez EE, Rashad YM 2009: An ecological view of arbuscular mycorrhizal status in some Egyptian plants. J. Environ. Sci. 37, 123–136.
- Abdel-Salam E, Alatar A, El-Sheikh MA 2017: Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. doi:10.1016/j.sjbs.2017.10.015.
- Ahmed FR, Sadeque KK, Alexander I 2006: Influences of Arbuscular Mycorrhizal Fungus Glomus mosseae on growth and nutrition of lentil irrigated with arsenic contaminated water. Plant and Soil, 283 (1–2), 33–41. doi:10.1007/s11104-005-0415-8.
- Ai Y, Zhang C, Sun Y, Wang W, Yanhong H, Bao M 2017: Characterization and functional analysis of five MADS-Box B class genes related to floral organ identification in Tagetes erecta. PloS One, 12 (1), e0169777. doi:10.1371/journal.pone.0169777.
- Andres S, Polle A 2002: Plant responses to abiotic stresses: heavy metal‐induced oxidative stress and protection by mycorrhization. J. Exp. Bot., 53 (372), 1351–1365. doi:10.1093/jxb/53.372.1351.
- Aroca R, Ruiz-Lozano JM, Zamarreño ÁM, Paz JA, García-Mina JM, Pozo MJ, López-Ráez JA 2013: Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol., 170 (1), 47–55. doi:10.1016/j.jplph.2012.08.020.
- Asrar A-WA, Elhindi KM 2011: Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J. Biol. Sci., 18 (1), 93–98. doi:10.1016/j.sjbs.2010.06.007.
- Audet P, Charest C 2006: Effects of AM colonization on “wild tobacco” plants grown in zinc-contaminated soil. Mycorrhiza, 16 (4), 277–283. doi:10.1007/s00572-006-0045-x.
- Bi YL, Li XL, Christie P 2003: Influence of early stages of arbuscular mycorrhiza on uptake of zinc and phosphorus by red clover from a low-phosphorus soil amended with zinc and phosphorus. Chemosphere, 50 (6), 831–837. doi:10.1016/s0045-6535(02)00227-8.
- Cavagnaro TR 2008: The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: a review. Plant and Soil, 304 (1–2), 315–325. doi:10.1007/s11104-008-9559-7.
- Chaturvedi R, Favas P, Pratas J, Varun M, Paul MS 2018: Assessment of edibility and effect of arbuscular mycorrhizal fungi on Solanum melongena L. grown under heavy metal(loid) contaminated soil. Ecotoxicol. Environ. Saf. 148, 318–326. doi:10.1016/j.ecoenv.2017.10.048.
- Christie P, Xiaolin L, Chen B 2004: Arbuscular mycorrhiza can depress translocation of zinc to shoots of host plants in soils moderately polluted with zinc. Plant and Soil, 261 (1/2), 209–217. doi:10.1023/b:plso.0000035542.79345.1b.
- De Miranda JCC, Harris PJ 1994: The effect of soil phosphorus on the external mycelium growth of arbuscular mycorrhizal fungi during the early stages of mycorrhiza formation. Plant and Soil, 166 (2), 271–280. doi:10.1007/bf00008340.
- Feng G 2006: Effects of single pollution of the different density mercury, lead and chromium on physiological and biochemical indexes of mungbean. J. Shanxi Agric. Univ. 3, 382–387.
- Galicia AHH, Mendoza DP, Salinas SDO, López AME, Liébano HE, López AU, Guadalupe VC 2008: In vitro nematocidal activity of plant extracts of Mexican flora against Haemonchus contortus fourth larval stage. Annals NY. Academy Sci., 1149 (1), 158–160. doi:10.1196/annals.1428.075.
- Göhre V, Paszkowski U 2006: Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta, 223 (6), 1115–1122. doi:10.1007/s00425-006-0225-0.
- Gong CN, Li PJ, Chen SH, Xiao BY, Han JY, Zhang CG 2002: Effects of different arbuscular mycorrhizal fungi on soil tolerance of Trifolium subterraneum L. CHINESE JOURNAL of APPLIED and ENVIRONMENTAL BIOLOGY. 8, 648–652.
- González-Chávez MC, Carrillo-González R, Wright SF, Nichols KA 2004: The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut., 130 (3), 317–323. doi:10.1016/j.envpol.2004.01.004.
- Hashem AE, Abd_Allah F, Alqarawi AA, Al Huqail AA, Egamberdieva D, Wirth S 2016: Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci., 23 (2), 272–281. doi:10.1016/j.sjbs.2015.11.002.
- Heggo A, Angle JS, Chaney RL 1990: Effects of vesicular-arbuscular mycorrhizal fungi on heavy metal uptake by soybeans. Soil Biology and Biochemistry, 22 (6), 865–869. doi:10.1016/0038-0717(90)90169-z.
- Hristozkova M, Geneva M, Stancheva I, Iliev I, Concepción A-A 2017: Symbiotic association between golden berry (Physalis peruviana) and arbuscular mycorrhizal fungi in heavy metal-contaminated soil. J. Plant Prot. Res. 57 (2), 173–184. doi:10.1515/jppr-2017-0024.
- Huang Y, Jang XY, Tao S 2002: Contribution of mycorrhizal fungi to biodegradation of POPs in soil. Soil Environ. Sci. 11, 221–228.
- Jackson NE 1973: Soil Chemical Analysis. 1st. Prentice Hall, New Delhi.
- Kaldorf M, Kuhn AJ, Schröder WH, Hildebrandt U, Bothe H 1999: Selective element deposits in maize colonized by a heavy metal tolerance conferring arbuscular mycorrhizal fungus. J. Plant Physiol., 154 (5–6), 718–728. doi:10.1016/s0176-1617(99)80250-8.
- Kamal S, Prasad R, Varma A 2010: Soil microbial diversity in relation to heavy metals. In Soil Heavy Metals, edited by. Sherameti I, Varma A, pp. 31–63, Springer Berlin Heidelberg, Berlin, Heidelberg.
- Kanwal S, Bano A, Malik RN 2015: Effects of arbuscular mycorrhizal fungi on wheat growth, physiology, nutrition and cadmium uptake under increasing cadmium stress. Int. J. Agron. Agric. Res. 5, 30–42.
- Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ 2000: Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere, 41 (1–2), 197–207. doi:10.1016/s0045-6535(99)00412-9.
- Lee YJ, George E 2005: Contribution of mycorrhizal hyphae to the uptake of metal cations by cucumber plants at two levels of phosphorus supply. Plant and Soil, 278 (1–2), 361–370. doi:10.1007/s11104-005-0373-1.
- Malekzadeh P, Farshian SH, Ordubadi B 2012: Interaction of arbuscular mycorrhizal fungus (Glomus intraradices and Glomus etunicatum) with tomato plants grown under copper toxicity. Afr. J. Biotechnol. 11, 46. doi:10.5897/ajb11.3461.
- Menge JA, Johnson ELV, Platt RG 1978: Mycorrhizal dependency of several citrus cultivars under three nutrient regimes. New Phytologist, 81 (3), 553–559. doi:10.1111/j.1469-8137.1978.tb01628.x.
- Nelson DW, Sommers LE 1973: Determination of total nitrogen in plant material. Agron. J., 65 (1), 109. doi:10.2134/agronj1973.00021962006500010033x.
- Perveen S, Samad A, Nazif W, Shah S 2012: Impact of sewage water on vegetables quality with respect to heavy metals in Peshawar, Pakistan. Pakistan J. Bot. 44 (6), 1923–1931.
- Piña-Vázquez DM, Mayoral-Peña Z, Gómez-Sánchez M, Salazar-Olivo LA, Arellano-Carbajal F 2017: Anthelmintic effect of Psidium guajava and Tagetes erecta on wild-type and Levamisole-resistant Caenorhabditis elegans strains. J. Ethnopharmacol. 202, 92–96. doi:10.1016/j.jep.2017.03.004.
- Rashid A, Ayub N, Ahmad T, Gul J, Khan AG 2008: Phytoaccumulation prospects of cadmium and zinc by mycorrhizal plant species growing in industrially polluted soils. Environ. Geochem. Health., 31 (1), 91–98. doi:10.1007/s10653-008-9159-8.
- Repetto O, Massa N, Gianinazzi-Pearson V, Dumas-Gaudot E, Berta G 2006: Cadmium effects on populations of root nuclei in two pea genotypes inoculated or not with the arbuscular mycorrhizal fungus Glomus mosseae. Mycorrhiza, 17 (2), 111–120. doi:10.1007/s00572-006-0082-5.
- Schumacher BA, Shines KC, Burton JV, Papp ML 1990: Comparison of three methods for soil homogenization. Soil Sci. Soc. America J., 54 (4), 1187–1190. doi:10.2136/sssaj1990.03615995005400040046x.
- Shen H, Christie P, Xiaolin L 2006: Uptake of zinc, cadmium and phosphorus by arbuscular mycorrhizal maize (Zea mays L.) from a low available phosphorus calcareous soil spiked with zinc and cadmium. Environ. Geochem. Health., 28 (1–2), 111–119. doi:10.1007/s10653-005-9020-2.
- Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y 2009: Influence of arbuscular mycorrhizae on the root system of maize plants under salt stress. Can. J. Microbiol., 55 (7), 879–886. doi:10.1139/w09-031.
- Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y 2008: Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza, 18 (6–7), 287–296. doi:10.1007/s00572-008-0180-7.
- Shetty KG, Hetrick BAD, Schwab AP 1995: Effects of mycorrhizae and fertilizer amendments on zinc tolerance of plants. Environ. Pollut., 88 (3), 307–314. doi:10.1016/0269-7491(95)93444-5.
- Smith SE, Read D 2008: Growth and carbon economy of arbuscular mycorrhizal symbionts. In Mycorrhizal symbiosis, Eds. Smith SE, Read D, pp. 117-144, Elsevier, Amsterdam.
- Soares CRFS, Siqueira JO 2008: Mycorrhiza and phosphate protection of tropical grass species against heavy metal toxicity in multi-contaminated soil. Biol. Fertil. Soils, 44 (6), 833–841. doi:10.1007/s00374-007-0265-z.
- Tang M, Chen H, Huang JC, Tian ZQ 2009: AM fungi effects on the growth and physiology of Zea mays seedlings under diesel stress. Soil Biology and Biochemistry, 41 (5), 936–940. doi:10.1016/j.soilbio.2008.11.007.
- Tordoff GM, Baker AJM, Willis AJ 2000: Current approaches to the revegetation and reclamation of metalliferous mine wastes. Chemosphere, 41 (1–2), 219–228. doi:10.1016/s0045-6535(99)00414-2.
- Trouvelot A, Kough JL, Gianinazzi-Pearson V 1986: Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhiza, edited by. Gianinazzi-Pearson V, Giainazzi S, pp. 217–221, INRA Publications, Paris.
- Turnau K, Orlowska E, Ryszka P, Zubek S, Anielska T, Gawronski S, Jurkiewicz A 2006: Role of mycorrhizal fungi in phytoremediation and toxicity monitoring of heavy metal rich industrial wastes in southern Poland. In Soil and Water Pollution Monitoring, Protection and Remediation, edited by. Irena Twardowska HE, Allen MMH, Stefaniak S, pp. 533–551, Springer Netherlands, Dordrecht.
- Wang F, Lin X, Yin R 2005: Heavy metal uptake by arbuscular mycorrhizas of Elsholtzia splendens and the potential for phytoremediation of contaminated soil. Plant and Soil, 269 (1–2), 225–232. doi:10.1007/s11104-004-0517-8.
- Wang FY, Lin XG, Yin R 2007: Role of microbial inoculation and chitosan in phytoextraction of Cu, Zn, Pb and Cd by Elsholtzia splendens – a field case. Environ. Pollut., 147 (1), 248–255. doi:10.1016/j.envpol.2006.08.005.
- Wang Z-H, Zhang J-L, Christie P, Xiao-Lin L 2008: Influence of inoculation with Glomus mosseae or Acaulospora morrowiae on arsenic uptake and translocation by maize. Plant and Soil, 311 (1–2), 235–244. doi:10.1007/s11104-008-9677-2.
- Weissenhorn I, Mench M, Leyval C 1995: Bioavailability of heavy metals and arbuscular mycorrhiza in a sewage-sludge-amended sandy soil. Soil Biology and Biochemistry, 27 (3), 287–296. doi:10.1016/0038-0717(94)00179-5.
- Wolf B 1982: A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun. Soil Sci. Plant Anal., 13 (12), 1035–1059. doi:10.1080/00103628209367332.
- Wu Q-S, Zou Y-N, Elsayed Fathi A-A 2014: Mycorrhizal Association and ROS in Plants A2 - Ahmad, Parvaiz. In Oxidative Damage to Plants, edited by. Ahmad P. pp. 453–475. Academic Press, San Diego.
