ABSTRACT
Plant growth requires mineral nutrients from soil environment. It is important for plant to develop its root to import mineral nutrient from environment. Both inorganic nitrogen source and plant hormone auxin influence the root system architecture (RSA). Previous study indicated that polar auxin transport is partly involved in ammonium-inhibition of primary root in an Arabidopsis ecotype, Columbia 0 (Col-0); however, the effect of auxin in other ecotypes remains unclear. The purpose of this study is to describe and examine the effect of TIBA, an inhibitor for the auxin polar transport, in ammonium supply-dependent changes of root development. Two Arabidopsis ecotypes, Col-0 and Landsberg erecta 2 (Ler-2), were used due to their different response to ammonium supply. The changes of the RSA in response to ammonium supply on vertical agar medium containing three levels of TIBA and four levels of ammonium were determined in two ecotypes. The primary root length of Ler-2 was markedly shortened by increasing ammonium concentration in the medium, while that of Col-0 was relatively insensitive to ammonium. Conversely, the lateral root length of Ler-2 was increased by ammonium supply. Both primary and lateral root of Col-0 were more sensitive to TIBA than those of Ler-2. ANOVA indicated the significant interaction of TIBA and ammonium in Col-0; however, no interaction in Ler-2. These results suggested the genetic diversity in the interactive effect of auxin and ammonium.
KEY WORDS:
1. Introduction
Plant growth requires mineral nutrients from soil environment. In order to import nutrients efficiently, plant develops its roots to maximize their surface area. Plant available inorganic nitrogen is nitrate and ammonium (Marschner Citation1995). Supply of nitrate or ammonium promotes the development of root (Drew and Saker Citation1975). Besides nitrogen nutrition, auxin also influences the root system architecture (RSA, Krouk et al. Citation2011). Auxin acts as a common integrator for lateral root formation (Lavenus et al. Citation2013) and inhibitor for primary root elongation (Rahman et al. Citation2007). The approaches with system biology reveal the importance of auxin signal transduction in lateral root development promoted by nitrate supply (Vidal et al. Citation2010, Citation2013; Gifford et al. Citation2013). Compared with nitrate, the detailed mechanism of ammonium-dependent root development remains unclear. Ammonium supply inhibits primary root elongation (Liu et al. Citation2013) but triggers lateral root development in ammonium transporter-dependent manner (Lima et al. Citation2010). These previous studies suggested a possible involvement of auxin in ammonium-dependent RSA changes.
Reverse genetic approach indicated that auxin transport is involved in the ammonium-dependent root development (Liu et al. Citation2013). The previous work pointed that polar auxin transport partially contributes to the ammonium-dependent inhibition of primary root elongation in one Arabidopsis ecotype Columbia 0 (Col-0, Liu et al. Citation2013). However, other works suggested the genetic diversity of Arabidopsis ecotypes in the response to ammonium (Sarasketa et al. Citation2014; Yasuda et al. Citation2017). Arabidopsis ecotypes showed diversity in ammonium tolerance (Sarasketa et al. Citation2014). Ammonium accumulation in shoot seems to be important since shoot biomass of the Arabidopsis ecotypes grown under ammonium nutrition showed negative correlation to the ammonium concentration in shoot (Sarasketa et al. Citation2014). The article encouraged the importance of the root and the quality of the root system in ammonium tolerance (Sarasketa et al. Citation2014). Recently, the diversity of root ammonium uptake in Arabidopsis ecotypes was reported (Yasuda et al. Citation2017). Arabidopsis ecotype Landsberg erecta 2 (Ler-2) showed higher shoot: root ratio in ammonium nutrition, which indicates Ler-2 had a high tolerance of ammonium (Yasuda et al. Citation2017). Isotope tracer study indicated that ammonium transport at higher concentration of Ler-2 was double the capacity of Col-0 (Yasuda et al. Citation2017). In addition, cytosolic glutamine synthetase 1;2, one of the key enzyme for primary ammonium assimilation in root, was highly increased by ammonium supply in the root of Ler-2 (Yasuda et al. Citation2017).
Although the previous article partly contributed to the revealing of the importance of the root in ammonium tolerance of different Arabidopsis ecotypes (Yasuda et al. Citation2017), however, the quality of the root system in response to ammonium supply remains to be determined. In addition, it is necessary to reevaluate the interaction of auxin and ammonium in root system in other Arabidopsis ecotypes.
The purpose of this work is to compare the responses of RSA to ammonium supply in two Arabidopsis ecotypes, Col-0 and Ler-2. In order to investigate the effect of the inhibitor for the auxin polar transport in ammonium supply-dependent changes of root development, the changes of the RSA in response to ammonium supply on vertical agar medium containing three levels of the inhibitor for polar auxin transport and four levels of ammonium were evaluated. In this paper, the different interaction of ammonium and auxin on RSA in two Arabidopsis ecotypes was discussed.
2. Materials and methods
2.1. Plant material
Seeds of the Arabidopsis thaliana ecotypes Col-0 (CS1092) and Ler-2 (CS8581) were used. They were provided by the Arabidopsis Biological Resource Center, Ohio University, USA.
2.2. Root measurement
The medium was prepared based on MGRL medium (Fujiwara et al. Citation1992). It was modified to eliminate nitrate from the nitrogen-free medium and was supplemented with 10 μM KNO3 and 1% agar for the plant culture medium (Wako Pure Chemical Industries, Ltd., Osaka, Japan). The modified MGRL medium was then supplemented with both ammonium and a polar auxin transport inhibitor, 2,3,5-triiodobenzoic acid (TIBA). Ammonium chloride was given as following concentration, 0, 0.5, 1, or 5 mM. TIBA was supplemented as three levels, 0, 0.1, or 1 µM. Plants were cultured in a growth cabinet controlled at 22°C with a 60% relative humidity under a 16 h/8 h light/dark cycle, as previously reported (Ishiyama et al. Citation2004). The plants were grown for 14 days.
Roots were scanned at a resolution of 300 dpi using a scanner (ES-10000G; Seiko Epson Corporation, Nagano, Japan) with transmission mode. The background noise in the images was cleaned with photo retouching software, Adobe Photoshop (Adobe Systems Incorporated, CA, USA). WinRHIZO version Pro2007d (Regents Instruments Inc., Canada) was used to determine the structure and the length of the roots.
2.3. Statistics
All data sets were analyzed using Microsoft Excel add-in software (Social Survey Research Information Co., Ltd., Tokyo, Japan). Correlation and partial correlation coefficients were tested between RSA and the biomass of the plants. A p value less than 0.05 was considered as a significant correlation. A three-way ANOVA was used to detect whether the supply of ammonium and TIBA influenced the following parameters: (1) root length and structure (total root length, primary root length, lateral root length, and lateral root numbers) and (2) the root growth of ecotype (Col-0 and Ler-2).
3. Results
3.1. Three-way interaction of RSA of Arabidopsis ecotype, ammonium, and TIBA
RSA is developmentally controlled by several factors. Auxin is one of the factors that regulate RSA. Previous works pointed that auxin response modules are involved in nitrate-dependent lateral root development (Gifford et al. Citation2008; Vidal et al. Citation2013) and also in primary ammonium assimilation in roots (Saito et al. Citation2017). Other article indicated the regulation of lateral root development by ammonium supply (Lima et al. Citation2010). This study focused on the interactive effect of ammonium supply and auxin polar transport on RSA and aimed to identify the relation between auxin polar transport and the different response in Arabidopsis ecotypes to ammonium supply. Three statistical factors were tested: Arabidopsis ecotypes (Col-0 and Ler-2), ammonium concentration (0, 0.5, 1, and 5 mM), and TIBA concentration (0, 0.1, and 1 mM). Four factors for RSA were measured: TRL (total root length), PRL (primary root length), LRL (lateral root length), and LRN (lateral root number). Neither TIBA nor ammonium dramatically changed the growth of shoot in this work. The Arabidopsis plants were at cotyledon stage. The length of hypocotyl was approximately 1.5 mm and that of leaf blade was approximately 1 mm.
First of all, three-way ANOVA was conducted to test the significant variations in RSA due to ecotype, ammonium, and TIBA and their interaction (). Three-way ANOVA showed a significant three-way interaction in TRL and LRN, but not in PRL and LRL (). Since TRL and LRN showed significant three-way interaction, simple interaction effect should be tested for TRL and LRN, and two-way interaction should be investigated for PRL and LRL. Simple interaction effect is the effect of each pair of factors at each level of the third factor. The following effects could be tested in this work. The interaction effect of Arabidopsis ecotypes and ammonium, for each level of TIBA. The interaction effect of Arabidopsis ecotypes and TIBA, for each level of ammonium. The interaction effect of ammonium and TIBA, for each Arabidopsis ecotypes. The total number of second-order interaction effect would be nine effects (3 + 4 + 2). In order to focus the effect, and also to look at further interactions more closely, the level of ammonium and TIBA should be fixed. For this purpose, the changes of RSA including all ammonium or TIBA concentrations in Col-0 and Ler-2 were plotted (). illustrates the changes of RSA in different concentrations of ammonium or TIBA in two Arabidopsis ecotypes. TRL and PRL in Col-0 were always significantly longer than those in Ler-2 at all three concentrations of TIBA (). At 0 µM TIBA, PRL in Col-0 was almost triple the length of that in Ler-2 (). Increase of TIBA concentration in medium significantly shortened TRL, in both Col-0 and Ler-2. At 1 µM TIBA, TRLs in both ecotypes were significantly smaller than those at 0 and 0.1 µM TIBA (). TRL in both Col-0 and Ler-2 at 1 µM TIBA was significantly smaller than TRL at 0 and 0.1 µM TIBA. PRL in Col-0 was also sensitive to the increase of TIBA in the medium, while that in Ler-2 was insensitive (). TRL in Col-0 was maximal at either 0.5 or 1 mM ammonium, and minimal at 5 mM ammonium (). Conversely, TRL in Ler-2 was gradually decreased with the rise of ammonium (). TRL in Col-0 is longer than that in Ler-2 at 0.5, 1, and 5 mM ammonium supply (). The increase of ammonium concentration in the medium considerably decreased the PRL in Ler-2, while PRL in Col-0 was not much sensitive to ammonium increase (). PRL in Col-0 was almost quadruple the length of PRL in Ler-2 at 0.5 and 1 mM ammonium condition (). Conversely, LRL in Col-0 was significantly shorter than that in Ler-2 at 0, 0.5, 1 mM ammonium condition ( and ). LRL () and LRN () were sharply decreased in both ecotypes at 1 µM TIBA, while they were not changed at 0.1 µM TIBA. At 0.5 and 1 mM ammonium, LRL () and LRN () were maximal in Col-0 and Ler-2. LRL in Ler-2 was double the length of that in Col-0 ().
Table 1. Results of three-way ANOVA showing F-values and level of significance for total root length, primary root length, lateral root length, and lateral root numbers of two ecotypes of Arabidopsis thaliana at different concentrations of ammonium and TIBA.
Figure 1. Root system architecture of Col-0 and Ler-2 grown on the vertical agar plate culture containing various concentration of ammonium and TIBA.
TRL: Total root length (A and B); PRL: primary root length (C and D); LRL: lateral root length (E and F), LRN: lateral root number per plant (G and H). Arabidopsis ecotypes were grown for 14 days on a vertical agar plate. The MGRL medium (Fujiwara et al. Citation1992) contained no nitrogen, but was supplemented with 10 µM KNO3, and was used as a basic medium. The medium was supplemented with either 0, 0.5, 1, or 5 mM NH4Cl, and with either 0, 0.1, or 1 µM TIBA. Bars indicate mean values (n = 30–56), and significant differences between Col-0 and Ler-2 indicated as following; *p < 0.05 or **p < 0.01. The values were compared with one-way ANOVA followed by Bonferroni, and significant differences at p < 0.05 within each line are indicated by different letters.
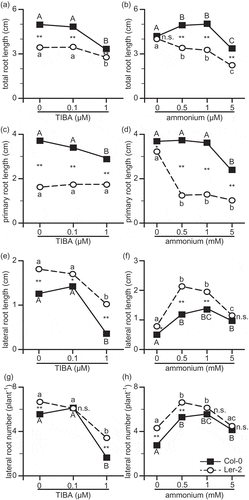
3.2. Interactions of ammonium and TIBA showed a difference in total root length of Arabidopsis ecotypes
showed that there was most obvious difference in RSA of Col-0 and Ler-2 in ammonium concentration at 0.5 mM and in TIBA concentration at 0 µM. Since significant three-way interaction was found in TRL (), simple interaction effect of Arabidopsis ecotypes and TIBA was tested at 0.5 mM ammonium () and simple interaction effect of Arabidopsis ecotypes and ammonium was tested at 0 µM TIBA (). There was significant simple interaction effect in TIBA and ecotype at 0.5 mM ammonium (), and also in ammonium and ecotype at 0 µM TIBA (). Then next, simple–simple main effect was tested to investigate the effect of TIBA on each ecotype at 0.5 mM ammonium (). There was no significant difference in TRL between Col-0 and Ler-2 at 1 µM TIBA (); however, TRL in Col-0 was significantly longer than that in Ler-2 at either 0 or 0.1 µM TIBA (). TRL in Col-0 was sensitive to 1 µM TIBA, while that in Ler-2 was not influenced (). Then next, simple–simple main effect was tested to investigate the effect of ammonium on each ecotype at 0 µM TIBA (). Tests of simple–simple main effects indicated significance in both ecotype and ammonium (). Although there was no significant difference in TRL between Col-0 and Ler-2 at 0 mM ammonium, TRL in Col-0 was significantly longer than that in Ler-2 at 0.5, 1, and 5 mM ammonium (). TRL in Col-0 was increased at 0.5 and 1 mM ammonium, while that in Ler-2 was gradually shortened according to the increase of ammonium (). Since TRL of Col-0 and Ler-2 showed different response to ammonium and TIBA ( and ), their interaction was finally tested (). There was significant interaction between TIBA and ammonium in TRL of Col-0 (). Both TIBA and ammonium showed significant simple main effects in both Col-0 and Ler-2; however, conversely, TRL in Ler-2 did not show significant interaction in ammonium × TIBA ().
Table 2. Results of two-way ANOVA showing F values and level of significance for total root length, primary root length, lateral root length, and lateral root numbers of two ecotypes of Arabidopsis thaliana at different concentrations of ammonium and TIBA.
Figure 2. TIBA-dependent change of root system architecture in Col-0 and Ler-2 at 0.5 mM ammonium.
TRL: Total root length (A); PRL: primary root length (B); LRL: lateral root length(C); LRN: lateral root number per plant (D). Col-0 and Ler-2 were grown for 14 days on a vertical agar plate. The MGRL medium (Fujiwara et al. Citation1992) contained no nitrogen, but was supplemented with 10 µM KNO3, and was used as a basic medium. The medium was supplemented with 0.5 mM NH4Cl, and with either 0, 0.1, or 1 µM TIBA. Bars indicate mean values (n = 9–13), and significant differences between Col-0 and Ler-2 indicated as following; *p < 0.05 or **p < 0.01. The values were compared with one-way ANOVA followed by Bonferroni, and significant differences at p < 0.05 within each line are indicated by different letters.
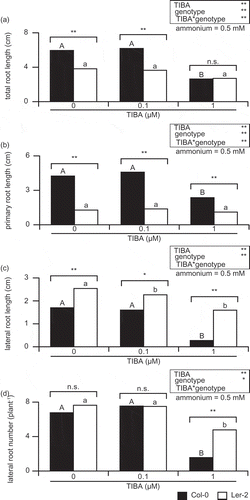
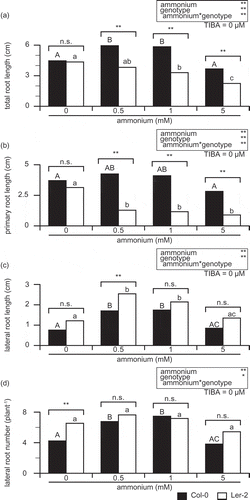
Figure 3. Ammonium-dependent change of root system architecture in Col-0 and Ler-2 at 0 µM TIBA.
TRL: Total root length (A); PRL: primary root length (B); LRL: lateral root length (C); LRN: lateral root number per plant (D). Col-0 and Ler-2 were grown for 14 days on a vertical agar plate. The MGRL medium (Fujiwara et al. Citation1992) contained no nitrogen, but was supplemented with 10 µM KNO3, and was used as a basic medium. The medium was supplemented with either 0, 0.5, 1, or 5 mM NH4Cl. Bars indicate mean values (n = 11–14), and significant differences between Col-0 and Ler-2 indicated as following; *p < 0.05 or **p < 0.01. The values were compared with one-way ANOVA followed by Bonferroni, and significant differences at p < 0.05 within each line are indicated by different letters.
3.3. Interactions of ammonium and TIBA in RSA in Arabidopsis ecotypes
The analysis of TRL indicated the interaction of TIBA and ammonium in Arabidopsis ecotype, then next, their effects were tested in the three components (PRL, LRL, and LRN) of RSA. There was no three-way interaction in PRL (); however, there were significant two-way interactions; TIBA × ammonium, TIBA × ecotype, and ammonium × ecotype, respectively (). Since TIBA × ecotype was significant, PRL of Col-0 and Ler-2 was compared at each TIBA concentrations. In order to test the simple main effect, ammonium concentration was examined at 0.5 mM (). Simple main effects tests showed significance in both ecotype and TIBA (). PRL in Col-0 was always longer than that in Ler-2 at all TIBA concentrations tested (). PRL in Ler-2 is about one-third as long as that in Col-0 at 0 µM TIBA ().
Ammonium × ecotype interaction in PRL was significant at 0 µM TIBA (). There were significant simple main effects in ecotype and TIBA (). While there was no significant difference in PRL between Col-0 and Ler-2 at 0 mM ammonium, TRL in Col-0 was significantly longer than that in Ler-2 at 0.5, 1, and 5 mM ammonium (). Then next, the interaction between ammonium and TIBA on PRL in each ecotype was tested (). In Col-0, the interaction of ammonium × TIBA was significant (). Both TIBA and ammonium showed significant simple main effects on PRL in Col-0 (). In Ler-2, ammonium × TIBA interaction was not significant (), and simple main effects tests indicated significance from ammonium but not from TIBA ().
Three-way ANOVA showed no significant three-way interaction in LRL (). There was a significant interaction only in ammonium × ecotype, neither TIBA × ammonium nor TIBA × ecotype affected LRL significantly (). A test for ammonium × ecotype interaction in LRL was conducted at 0 µM TIBA (). While there was significant simple main effects in ecotype and ammonium, there was no significant interaction of them (). LRL in Ler-2 was significantly longer than that in Col-0 at 0.5 mM ammonium (), whereas there was no significant difference in LRL between Col-0 and Ler-2 at 0, 1, and 5 mM ammonium ().
Three-way ANOVA showed a significant three-way interaction in LRN (). There was significant interaction in TIBA × ammonium and TIBA × ecotype, but not in ammonium × ecotype (). At 0.5 mM ammonium, there was no significant interaction between TIBA and ecotype, while there was significant simple main effects in TIBA and ecotype, respectively (). Only at 1 µM TIBA, there was significant difference in LRN between Col-0 and Ler-2 (). LRN in Ler-2 reduced by 37% and that in Col-0 reduced by 77%, compared to 0 µM TIBA (). Ammonium × TIBA interaction in LRN was investigated in each ecotype (). There was significant ammonium × TIBA interaction in LRN in both Col-0 and Ler-2 (). Simple main effects tests indicated the significant effects of both ammonium and TIBA on ecotypes ().
4. Discussion
Ammonium supply-dependent changes to RSA should be considered with cultural conditions because the nitrate in the medium influences them. When the medium contains 20–40 mM nitrate, primary root elongation is not inhibited by 10–20 mM ammonium (Qin et al. Citation2008; Barth et al. Citation2010). Conversely, when the medium contains little nitrate, 10 mM ammonium inhibits root elongation and plant growth (Qin et al. Citation2008), whereas 0.1–1 mM ammonium does not dramatically influence the root elongation (Qin et al. Citation2008). The present study used a medium containing only 10 µM nitrate. Therefore, it was possible to see the effect of ammonium at lower concentration in this work. Variation of ammonium concentration along a linear transect sampled at 4 m intervals was estimated in previous work (Miller et al. Citation2007). Transect positions were located in winter barley field, grass on waste ground, stubble after winter wheat, waste ground, two drilled winter wheat crops. The concentration of ammonium ranged 0.1–0.8 mM. There was a marked difference in RSA between Col-0 and Ler-2 at 0.5 mM ammonium concentration, it is in general agreement with previous work.
The RSA response to ammonium under nitrate deficiency showed natural variation; therefore, results in this study provide evidence that the root structure and function constitute adaptations to a higher ammonium environment. The primary root length of Ler-2 was shorter than that of Col-0, whereas the lateral root number of Ler-2 tended to be higher than that of Col-0 (–), indicating that Ler-2 could possess a larger root apex in an ammonium condition. Indeed, the root apex is associated with ammonium use in the environment since ammonium absorption is higher at the root apex (Bloom et al. Citation2002) and AMT is located at the root apex (Loque et al. Citation2006). The ammonium supply stimulates root branching (Lima et al. Citation2010). Increases in the root tip may lead to a higher ammonium uptake capacity. Ammonium efflux at the elongation zone is critical for minimizing the severity of ammonium toxicity (Li et al. Citation2010). Since the development of the elongation zone is dependent on the initiation of the lateral root, a higher number of lateral roots in Ler-2 might account for the adaptation to an ammonium condition.
Auxin is strongly involved in root development (Nacry et al. Citation2005). Auxin polar transport is inhibited by TIBA (Geldner et al. Citation2001). In this work, a relation between TIBA and ammonium in RSA was investigated. Since there was a genetic diversity in Arabidopsis ecotypes for responding to ammonium nutrition, the effect of TIBA and ammonium was investigated in two selected ecotypes, Col-0 and Ler-2.
It is apparent that the interaction of ammonium and TIBA showed a genetic diversity. There was significant interaction between TIBA and ammonium in PRL (). Ammonium showed significant simple main effects on PRL in both Col-0 and Ler-2, while TIBA showed them only in Col-0 (). Conversely, there was no significant interaction between TIBA and ammonium in LRL (). Although both ammonium and TIBA showed significant simple main effects on LRL in Col-0 and Ler-2, they are not interacted in Ler-2 (). Previous work revealed the involvement of auxin in ammonium-supply-induced inhibition of primary root in Col-0 (Liu et al. Citation2013).
Auxin transporters, AUX1 and PIN2, were downregulated by ammonium supply (Liu et al. Citation2013). Since the Arabidopsis mutants for auxin transport showed the ammonium-inhibited primary root growth, it was suggested that there is another mechanism for the regulation of primary root growth in ammonium supply behind AUX1 and PIN2 (Liu et al. Citation2013). This work showed the different interaction between ammonium and auxin in root growth in Col-0 and Ler-2, suggesting the different regulatory mechanism for the auxin-dependent response to ammonium supply in intraspecies. The findings in this work support and expand prior work. However, the genetic factors determining the difference between Col-0 and Ler-2 remain to be elucidated in future.
Acknowledgments
A lot of thanks to Mrs. Ikumi Sakurada-Enomoto and Mrs. Kaya Matsuoka for technical assistance.
Additional information
Funding
References
- Barth C, Gouzd ZA, Steele HP, Imperio RM 2010: A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J. Exp. Bot., 61, 379–394. doi:10.1093/jxb/erp310
- Bloom AJ, Meyerhoff PA, Taylor AR, Rost TL 2002: Root development and absorption of ammonium and nitrate from the rhizosphere. J. Plant Growth Reg., 21, 416–431. doi:10.1007/s00344-003-0009-8
- Drew MC, Saker LR 1975: Nutrient supply and the growth of the seminal root system in barley: II. Localized, compensatory increases in lateral root growth and rates of nitrate uptake when nitrate supply is restricted to only part of the root system. J. Exp. Bot., 26, 79–90. doi:10.1093/jxb/26.1.79
- Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S 1992: Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol., 99, 263–268.
- Geldner N, Friml J, Stierhof YD, Jurgens G, Palme K 2001: Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature., 413, 425–428. doi:10.1038/35096571
- Gifford ML, Banta JA, Katari MS, et al. 2013: Plasticity regulators modulate specific root traits in discrete nitrogen environments. Plos Genetics., 9, doi:10.1371/journal.pgen.1003760
- Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD 2008: Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA., 105, 803–808. doi:10.1073/pnas.0709559105
- Ishiyama K, Inoue E, Watanabe-Takahashi A, Obara M, Yamaya T, Takahashi H 2004: Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in Arabidopsis. J. Biol. Chem., 279, 16598–16605. doi:10.1074/jbc.M313710200
- Krouk G, Ruffel S, Gutierrez RA, et al. 2011: A framework integrating plant growth with hormones and nutrients. Trends Plant Sci., 16, 178–182. doi:10.1016/j.tplants.2011.02.004
- Lavenus J, Goh T, Roberts I, et al. 2013: Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci., 18, 455–463. doi:10.1016/j.tplants.2013.04.006
- Li Q, Li BH, Kronzucker HJ, Shi WM 2010: Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ., 33, 1529–1542. doi:10.1111/j.1365-3040.2010.02162.x
- Lima JE, Kojima S, Takahashi H, Von Wiren N 2010: Ammonium triggers lateral root branching in Arabidopsis in an ammonium transporter1;3-dependent manner. Plant Cell., 22, 3621–3633. doi:10.1105/tpc.110.076216
- Liu Y, Lai N, Gao K, Chen F, Yuan L, Mi G 2013: Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS ONE., 8, e61031. doi:10.1371/journal.pone.0061031
- Loque D, Yuan L, Kojima S, et al. 2006: Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J., 48, 522–534. doi:10.1111/j.1365-313X.2006.02887.x
- Marschner H 1995: Mineral Nutrition of Higher Plants, Academic Press, London.
- Miller AJ, Fan XR, Orsel M, Smith SJ, Wells DM 2007: Nitrate transport and signaling. J. Exp. Bot., 58, 2297–2306. doi:10.1093/jxb/erm066
- Nacry P, Canivenc G, Muller B, et al. 2005: A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol., 138, 2061–2074. doi:10.1104/pp.105.060061
- Qin C, Qian W, Wang W, et al. 2008: GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA., 105, 18308–18313. doi:10.1073/pnas.0806168105
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI 2007: Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J., 50, 514–528. doi:10.1111/j.1365-313X.2007.03068.x
- Saito M, Konishi N, Kanno K, Yamaya T, Kojima S 2017: Transcriptional repressor IAA17 is involved in nitrogen use by modulating cytosolic glutamine synthetase GLN1;2 in Arabidopsis roots. Soil Sci. Plant Nutr., 63, 163–170. doi:10.1080/00380768.2017.1314178
- Sarasketa A, Gonzalez-Moro MB, Gonzalez-Murua C, Marino D 2014: Exploring ammonium tolerance in a large panel of Arabidopsis thaliana natural accessions. J. Exp. Bot., 65, 6023–6033. doi:10.1093/jxb/eru342
- Vidal EA, Araus V, Lu C, et al. 2010: Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA., 107, 4477–4482. doi:10.1073/pnas.0909571107
- Vidal EA, Moyano TC, Riveras E, Contreras-Lopez O, Gutierrez RA 2013: Systems approaches map regulatory networks downstream of the auxin receptor AFB3 in the nitrate response of Arabidopsis thaliana roots. Proc. Natl. Acad. Sci. USA., 110, 12840–12845. doi:10.1073/pnas.1310937110
- Yasuda T, Konishi N, Kojima S 2017: Ammonium uptake capacity and response of cytosolic glutamine synthetase 1;2 to ammonium supply are key factors for the adaptation of ammonium nutrition in Arabidopsis thaliana. Soil Sci. Plant Nutr., 63, 553–560. doi:10.1080/00380768.2017.1395292
