ABSTRACT
We studied microbe-plant interactions of white lupin, a cluster root-forming plant, under low P and N conditions to examine increased nutrient acquisition by plants either by a shift to a more specialized microbial community or changes in microbial enzyme production. White lupin plants were grown in rhizoboxes filled with either P- or N-deficient soil; fertilized soil was used as control. After cultivation of plants in a glasshouse for 41 d, plant growth (shoot and roots) and P and N accumulation in shoots were measured. Microbial functions were analyzed by P- and N-cycling enzymes. The microbial community structure was estimated by fingerprinting (denaturing gradient gel electrophoresis) and sequencing techniques. P deficiency induced the released citrate and acid phosphomonoesterases from cluster roots and stimulated the production of microbe-derived alkaline phosphomonoesterase in the rhizosphere. P deficiency decreased microbial diversity in the cluster root rhizosphere. Increased relative abundance of Burkholderiales in the rhizosphere of P deficient plants might be responsible for the degradation of different organic P fractions such as phytates. N deficiency induced an increase of the number of nodules and P concentration in shoot as well as roots of white lupin. We clarified that high release of citrate from cluster roots might be the preferred mechanisms to meet the P demand of nodulated plants under N deficiency. In addition, the high abundance of Rhizobiales and Rhodospirillales in the rhizosphere of cluster roots showed that the importance of N-fixing microorganisms under N deficiency. The contribution of rhizosphere microorganisms due to similar activities of N-cycling enzymes under the two different N treatments is less important for N nutrition of plants. Further understanding of the regulation of cluster roots under N-deficiency will provide new information on the interactions between P and N nutrition.
1. Introduction
Plants have developed strategies to adapt to phosphorus (P) starvation because P is easily immobilized in soils. White lupin (Lupinus albus L.) can grow well under low-P conditions (Lambers et al. Citation2013; Dissanayaka et al. Citation2015, Citation2017) because of its higher ability to release carboxylates and acid phosphatase (AcPase) and its mobilization of sparingly soluble P and organic P, respectively (Gardner et al. Citation1983; Neumann and Martinoia Citation2002; Wasaki et al. Citation2003). A unique root structure, so-called cluster roots, formed under low-P conditions contributes to uptake P from sparingly available forms in their growing soil. Cluster roots are highly dense clustered roots arising from secondary roots and induced under poor nutrient conditions. Cluster roots were first described for Fabaceae and Proteaceae (Viminaria juncea, Hakea oblique, L. albus; Lamont Citation1972a, Citation1972b; Trinick Citation1977), and subsequently in many additional plant families (Shane and Lambers Citation2005). Such roots are known to be extremely effective for nutrient acquisition from low-fertility soils. P-deficient white lupin and Fe-deficient Macadamia integrifolia release a significant amount of organic acids from cluster roots (Hagström et al. Citation2001; Hue Citation2009). Root-secreted carboxylates can solubilize P bound with metal ions such as Fe-P, Ca-P, and Al-P by the chelating function.
Functions of cluster roots are differentiated into developmental stages. For example, malate exudation is higher in the younger stage than in mature or senescent stages. However, citrate secretion is highest in the mature stage (Neumann and Martinoia Citation2002). Because of the difference in substrate availability, the microbial community composition is expected to change during cluster root development (Marschner et al. Citation2002; Wasaki et al. Citation2005). Nevertheless, Weisskopf et al. (Citation2006) reported that the root-secreted carboxylates were protected against microbial consumptions by the decrease of pH in the rhizosphere of the young stage and secretion of antifungal compounds, based on physiological differences among developmental stages. On the other hand, microbe effects on plant nutrient supplies are suggested. White lupin can acquire phytate-P (Adams and Pate Citation1992), although root-secreted AcPase showed little relative activity to phytic acid (Li and Tadano Citation1996; Miller et al. Citation2001). This fact suggests that microorganisms in the white lupin rhizosphere are involved in phytate-P acquisition by plants. Unno et al. (Citation2005) isolated phytate utilizing bacteria from the White lupin rhizosphere. Their co-cultivation experiment revealed that some isolates supplied phytate-P to inoculated plants. However, the relation between the microbial community structures and P dynamics in the white lupin rhizosphere are still not well understood.
Nitrogen (N) deficiency causes an increase of cluster roots of white lupin and Proteaceae plants (Schmidt et al. Citation2003; Paungfoo-Lonhienne et al. Citation2009). Epidermal cells of cluster roots of Hakea actities (Proteaceae) were able to take up green fluorescence protein directly by endocytosis (Paungfoo-Lonhienne et al. Citation2008). The uptake of low-molecular-weight organic nitrogen compounds by cluster roots is a rare phenomenon described in the past only for selected plant species (e.g. Leucadendron laureolum, Proteaceae, and white lupin) (Hawkins et al. Citation2005). In contrast, Rath et al. (Citation2010) found no gene in N-deficient lupin targeting enzymes involved in the uptake of organic N or N mineralization. Consequently, it remains unknown which plants produce cluster roots under N deficiency and which functions these cluster roots have during N acquisition. Aside from direct uptake of organic N by cluster roots, it is possible that microbe–root interactions also contribute to improved N acquisition of plants.
Substrates released from cluster roots have a strong impact on the microbial community structure in the rhizosphere. Therefore, it is expected that P and N availability in the soil might change the structure and function of microbial communities. Information related to the phylogeny and function of soil microbial communities during cluster root development is rare (Marschner et al. Citation2002; Wasaki et al. Citation2005; Weisskopf et al. Citation2006, Citation2011). We know only from the study of Weisskopf et al. (Citation2011) that cultivation-based as well as DNA extraction-based communities were increasingly dominated by different Burkholderia species during the development of white lupin cluster roots.
We want to close the knowledge gap by characterizing the soil microbial community in the rhizosphere of cluster roots of white lupin under P- and N-deficiency. We hypothesize that nutrient deficiency reduces microbial diversity in the rhizosphere toward a more specialized microbial community supporting nutrient uptake of plants. Additionally, we wanted to ascertain whether this potential specialized microbial community in the rhizosphere produces preferentially extracellular enzymes involved in the decomposition of organic compounds.
2. Materials and methods
2.1. Plant and soil materials
Long-term experimental fields of Hokkaido University established at 1914 were used as soil materials for this study. A layer was collected from three plots as follows: control (NPK fertilized), −P (NK fertilized), and −N (PK fertilized) plots. Air-dried soils were used for rhizobox cultivation after sieving with 2 mm. The soil was fertilized as follows: 100 mg-N kg−1 soil as NH4NO3, 100 mg-P kg−1 soil as Ca(H2PO4)2, and 150 mg-K kg−1 soil as K2SO4. The −P and −N treatments were not P and N fertilized, respectively.
Surfaces of white lupin (L. albus L. cv. Kievskij mutant) seeds were sterilized with 70% ethanol for 5 min and 5% sodium hypochlorite for 10 min. Seeds were soaked in flowing tap water for 48 h to stimulate germination and sown on perlite. At 5 d after germination, two seedlings were transferred to each rhizobox (20 × 40 × 2.2 cm; constructed based on Dinkelaker and Marschner Citation1992) filled with fertilized soils. Plants in the rhizoboxes were cultured in a glass house for 41 d. Soil moisture was kept at 70% water holding capacity, supplying deionized water every day. Before harvesting plant materials, bulk and rhizosphere soil were collected according to Wasaki et al. (Citation2005). All collected samples were frozen immediately with liquid nitrogen and were stored at −80°C for additional analysis. Numbers of cluster roots and nodules were counted during this procedure. Plants were harvested at 27 and 41 days after transplanting (DAT) after separation into shoots and roots. After washing of the roots, all plant materials were dried at 80°C.
2.2. Nutrient analyses of plants
Dried plant materials were weighed and were finely ground. About 80 mg of each sample was digested with H2SO4-H2O2. The P and N concentrations of digested samples were measured, respectively, using the vanado-molybdate yellow method and Kjeldahl method (Watanabe et al. Citation1998). The K concentration was measured using an atomic absorption spectrometer.
2.3. Soil analysis
Available P concentration in soil was analyzed as Truog-P (Truog Citation1930). Available NH4+ and NO3− concentrations were extracted from 5 g of soil with 50 mL of 2 M KCl and were analyzed using an autoanalyzer (AACS-III; Bran + Luebbe, Norderstedt, Germany).
Activities of AcPase and alkaline phosphatase (AlPase), β-acetylglucosaminidase, Leu- and Tyr-peptidase in soils were measured using fluorogenic substrates and a microplate reader (Genios plus; Tecan, Männedorf, Switzerland) according to Wasaki et al. (Citation2005).
The citrate concentration was measured using an F-kit for citrate (Roche Diagnostics Corp., Basel, Switzerland) with scaling down for application to a microplate reader (Genios plus). Briefly, an aliquot of 0.2 g of soil sample was weighed and added to the same amount of ultrapure water in a microcentrifuge tube. Citrate in soil was extracted with well-mixing and a centrifuge at 12,000 × g and 4°C for 5 min. Supernatant of 100 µL was used for measurement. A 67 µL of Solution I in the kit mixed with 33 µL of ultrapure water and 100 µL of the soil extract in the wells of 96-hole microplates. After 5 min incubation, the absorbance at 360 nm was measured. Then, 1.3 µL of Solution II in the kit was added to all wells. The absorbance at 360 nm was measured again. The citrate concentration was calculated from the difference of absorbance according to the manufacturer’s instructions.
2.4. Molecular fingerprinting and sequencing
Soil samples collected at 41 DAT were well crushed under cooling with liquid nitrogen using a Multi-Beads Shocker (Yasui Kikai Corp., Tokyo, Japan). An ISOIL kit (Nippon Gene Co. Ltd., Toyama, Japan) was used for soil DNA extraction according to the manufacturer’s instructions. PCR–DGGE (denaturing gradient gel electrophoresis) analysis was performed using F984GC and R1378 as a primer set (Heuer et al. Citation1997) for bacterial fingerprinting in soils according to Wasaki et al. (Citation2005) with minor modification; taq polymerase hot start version (Takara Bio Inc., Otsu, Japan) was used as a thermostable DNA polymerase. After electrophoresis on a denaturant gradient gel, the DNA fragments were visualized with SYBR Green I as an intercalater and a gel imager (LumiVision 400EX; Aisin Seiki Co. Ltd., Kariya, Japan). Cluster analysis for the fingerprinting was conducted using the Quantity One software (Bio-Rad Laboratories, Hercules, CA, USA).
For the determination of the bacterial composition, PCR was performed using F984 and R1378 primers for soil samples at same conditions. Clone libraries were prepared using pGEM-T vector (Promega Corp., Madison, WI, USA). The clones were sequenced after preparation of sequencing templates with a TempliPhi kit (GE Healthcare, Pittsburgh, PA, USA) using Sanger’s dideoxy method. All sequences were classified using a ribosomal DNA database through a server at Michigan State University (Wang et al. Citation2007). Shannon’s indices were calculated from the data of clone libraries.
2.5. Statistical analysis
All quantitative analyses were performed in triplicate. Significant differences were inferred using general analysis of variance (ANOVA; P < 0.05). SPSS (ver. 16; SPSS Inc., Chicago, IL, USA) was used for ANOVA and cluster analysis for PCR–DGGE band patterns.
3. Results
3.1. Growth and mineral uptake of white lupin
A rhizobox experiment was used to elucidate the effects of P- and N-deficiency on soil and plant performance, cluster root formation, small scale function, and diversity of rhizosphere microorganisms. Available N and P in soil were, respectively, low in N- and P-deficient treatments (). Ammonium concentrations of all treatments decreased over time (). Reduced P and N concentrations in the soil did not change the shoot growth of white lupin after 27 d and 41 d, although under P-deficiency root biomass were reduced significantly ().
Table 1. N and P availabilities in the soils before and after the cultivation. Values represent mean of three replicates ± SE (standard error). Different letters in a given column under each date denote significant differences (P ≤ 0.05).
Table 2. Growth and mineral concentration of white lupin plants. Values represent mean of three replicates ± SE (standard error). Different letters in a given column under each date and organ denote significant differences (P ≤ 0.05).
The N concentration of shoots was significantly lower in −N treatment than in the control and −P treatments at 27 DAT, but it returned to comparable levels at 41 DAT (). Low mineral N contents of plant shoots of white lupin corresponded with the low number of nodules at 27 d ( and ). The numbers of nodules increased specifically under N deficiency over time (). The P concentration of plants was significantly lower in −P treatment at both harvests than in the two other treatments (). Potassium concentrations were similar among all treatments ().
Table 3. Number of nodules and cluster roots formed in white lupin roots. Values represent mean of three replicates ± SE (standard error). Different letters in a given column under each date denote significant differences (P ≤ 0.05).
3.2. Cluster root formation and exudation of organic acids
The P deficiency tended to increase the total number of cluster roots in comparison to the control treatment (). The number of matured clusters was significantly higher in −P than in controls at both harvests. At 41 DAT, the number of senescent cluster roots was also higher in N-deficient plants than in the control treatment (). The elder cluster roots, i.e. mature and senescent ones, were the major component of cluster roots formed under nutrient deficiency. The number of nodules was especially high in N deficiency at 41 DAT ().
Although citrate concentration in the bulk soil was very low, citrate concentrations up to 0.474 µmol g−1 of soil were found in the rhizosphere of white lupin (). Citrate concentration increased mainly in the mature stages of cluster root development under −P treatment ().
Table 4. Citrate concentration in rhizosphere soil at 41 DAT (µmol g−1). Values represent mean of three replicates ± SE (standard error). Different letters in a given column denote significant differences (P ≤ 0.05).
3.3. Enzyme activities associated to cluster roots
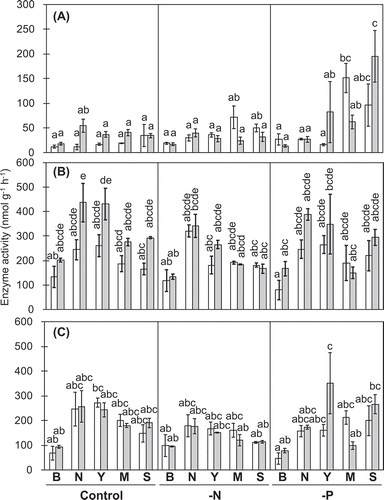
In general, enzyme activities involved in P and N cycling (AcPase, AlPase, β-acetylglucosaminidase, Leu-petidase, and Tyr-peptidase) were higher in rhizosphere soil than in bulk soil ( and ). AcPase activity increased from young to senescent cluster roots in P-deficient treatment. It was highest in senescent cluster roots of −P treatment (). In contrast, AlPase showed highest enzyme activities in mature cluster root samples and was stimulated mainly by P deficiency in young, mature, and senescent cluster roots (). β-Acetylglucosaminidase activity was high in the rhizosphere of cluster roots formed under P-deficient conditions (). Both peptidase activities showed similar levels of activity among all treatments (). The results of discriminant analyses including enzyme data revealed separation between mature cluster roots at 27 and 41 DAT and other cluster roots along axis 1 which explained 46.4% of the variation within the dataset (Fig. S1 and Table S1). Bulk samples and rhizosphere samples separated along axis 2, which explained an additional 41.8% of the variance within the dataset. Control and −N treatments were not separated along axis 1 or 2.
Figure 1. Acid phosphatase (A) and alkaline phosphatase (B) activities in rhizosphere soils.
White and gray bars, respectively, show data for 27 and 41 DAT. B, N, Y, M, and S, respectively, stand for bulk soil, and rhizosphere soils for normal roots, young, mature, and senescent cluster roots. Values represent means of three replicates ± SE. Different letters denote significant differences (P ≤ 0.05).
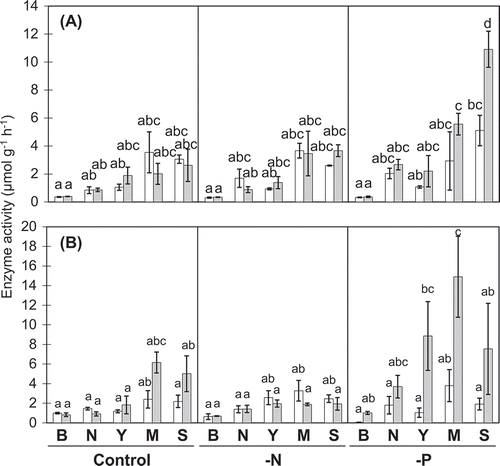
Figure 2. β-Acetylglucosaminidase (A), Leu-peptidase (B), and Tyr-peptidase (C) activities in rhizosphere soils.
White and gray bars, respectively, show data for 27 and 41 DAT. B, N, Y, M, and S, respectively, stand for bulk soil, and rhizosphere soils for normal roots, young, mature, and senescent cluster roots. Values represent means of three replicates ± SE. Different letters denote significant differences (P ≤ 0.05).
3.4. Microbial community structure
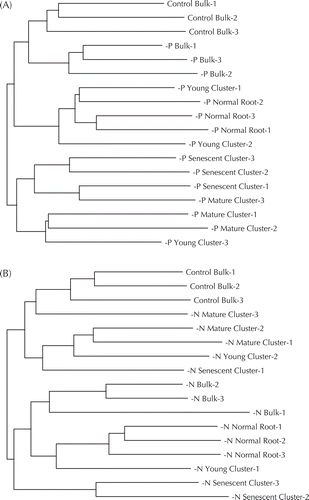
Cluster analyses based on results from DGGE pattern were conducted for all samples at 41 DAT, revealing a separation between bulk soil and rhizosphere samples (). In addition, the DGGE pattern of normal and young cluster roots showed higher similarity than the mature and senescent cluster roots of −P treatments did. Nevertheless, no clear trend was found for −N treatments ().
Figure 3. Cluster analyses for rhizosphere bacterial fingerprintings of white lupin at 41 DAT. (A) and (B), respectively, denote results for comparing with P or N deficiency to control treatment.
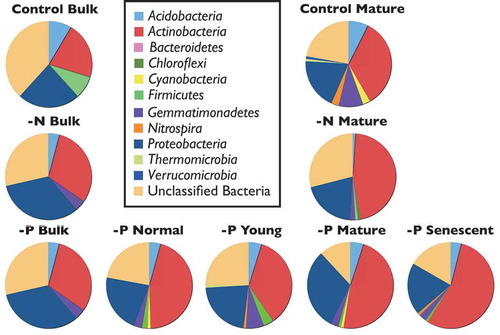
Microbial community structures were compared according to the 16S rDNA sequences, as determined by clone libraries ( and Table S2). The sequencing of 16S rDNA was mainly intended to clarify the alteration of the microbial communities during development of cluster roots under P-deficient conditions. Therefore, rhizosphere of all types for −P samples and mature cluster root samples for control and −N treatments were compared. At the phyla level, Actinobacteria, Proteobacteria as well as Acidobacteria were detected in bulk soil of all treatments ( and Table S2). Around 25 percent of the sequences could not be assigned for all samples. Independent of treatment, we found a higher relative contribution of Actinobacteria of mature cluster roots in comparison to the bulk soil. Rhizosphere samples showed a higher diversity of phyla than in the bulk soil (). The following phyla were detected: Acidobacteria, Actinobacteria, Bacteroidetes, Chlamydiae, Chloroflexi, Cyanobacteria, Firmicutes, Gemmatimonadetes, Nitrospira, Planctomycetes, Proteobacteria, Thermomicrobia, and Verrucomicrobia. The relative abundance of Actinobacteria increased from young to senescent cluster roots, whereas Gemmatimonadetes, Firmicutes and unclassified bacteria decreased their relative abundance simultaneously. Micrococcineae and Streptomycineae showed dominant representative suborders within the phylum Actinobacteria in senescent cluster roots (). Burkholderiales were an important order representing Proteobacteria mainly in the rhizosphere, whereas Rhizobiales had high relative abundance under N deficiency as well as in young and mature cluster roots under −P treatment ().
Table 5. Composition of dominant rhizobacteria at (sub)order level (%).
Figure 4. Relative abundance of different bacterial phyla of rhizosphere soil of white lupin at 41 DAT.
To evaluate the alteration of microbial diversities, Shannon’s diversity indices were calculated from a dataset of 16S rDNA sequencing as phyla level (). No difference was found in bulk soil samples among treatments. The diversities of control treatment were similar between bulk soil and rhizosphere soil of the matured cluster. It is particularly interesting that indices were decreased in elder cluster roots formed under P- or N-deficiency.
Table 6. Shannon index calculated by phyla level of 16S rDNA sequences. Values represent mean of three replicates ± SE (standard error). Different letters in a given column denote significant differences (P ≤ 0.05). n.d.; not determined.
4. Discussion
4.1. Activities of phosphomonoesterase during cluster root development
Higher activities of AcPase and concentrations of citrate in elder cluster roots than in other roots were found ( and ). These results corresponded with those of previous studies indicating notable mobilization of the sparingly available P in the rhizosphere of cluster roots formed under low-P conditions. It was demonstrated that exudative bursts such as AcPase and carboxylates contributed to a great degree to P mobilization (Gardner et al. Citation1983; Neumann and Martinoia Citation2002; Wasaki et al. Citation2003). An increase of cluster roots in the mature stage found in −P at 41 DAT () and similar growth level among treatments () provided evidence that P mobilization by root exudates contributed strongly to P uptake from sparingly forms under P-deficient conditions. The highest activity of AcPase was found in senescent cluster roots at 41 DAT, although the highest exudative burst is usually found in the mature stage (Neumann and Martinoia Citation2002). A similar result was also found in a previous study, which also investigated white lupin (Wasaki et al. Citation2005). A high stability of LASAP2, which was the sole isoform of root-secreted AcPase (Tadano et al. Citation1993; Wasaki et al. Citation2000), is regarded as a reason for maintaining higher activity after the maturation of cluster roots.
Not only cluster roots mobilize P from of organic P compounds, but also soil microorganisms could contribute to the P nutrition of white lupin. Our results showed that the absolute AlPase activities were higher than the activities of AcPase (). Whereas AcPase is produced mainly by plants, the high activity of AlPase might derive from microbial phosphatase production as well as plant-derived AcPase, which show a broader pH optimum than microbial AlPase (Sakurai et al. Citation2008). However, the temporal patterns between both phosphatases differed. The AlPase showed the highest activity in mature cluster roots at 41 DAT (), whereas AcPase, mainly consisted with LASAP2 secreted from cluster roots (Ozawa et al. Citation1995), was highest in the senescent stage (). Based on results of a specific microbial community in elder cluster roots, we suggest that these microorganisms are responsible for additional excretion of AlPases (). Therefore, alteration of the microbial community in the rhizosphere of mature cluster roots under P-deficient conditions might induce higher activity of AlPase. In contrast, Wasaki et al. (Citation2005) found similar patterns of AcPase and AlPase during cluster root development. This inconsistency with the results of the present study might be explained by the differences in richness or diversity of microbes of the two experiments.
4.2. Activities of N-cycling enzymes during cluster root development
Although root nodule development is a well-known phenomenon under N deficiency, the contribution of cluster root formation for N cycling under this condition is not well understood. Results showed that low availability of nitrate measured at 27 DAT () induced nodule formation. This increase in nodule formation was detected at 41 DAT in N-deficient lupin plants in comparison to control and −P plants (). In addition, the number of cluster roots (especially in senescent cluster roots) tended to be high under N-deficiency conditions (). An increase of the number of cluster roots had been found in white lupin and other plant species under N-deficiency (Schmidt et al. Citation2003; Paungfoo-Lonhienne et al. Citation2009). The following mechanisms are currently under discussion: organic N mobilization by hydrolytic enzymes might be the physiological role of cluster roots under N deficiency. Measurements of peptidase and β-acetylglucosaminidase revealed differences in activities between the rhizosphere and bulk soil, but no difference was found between N-deficient and P-deficient and control plants ().
Therefore, N-cycling enzymes derived from cluster roots did not contribute to N mobilization in the rhizosphere of N-deficient plants. Because DGGE fingerprints showed no difference between N-deficient plants and control plants, we infer that the alteration of microbial community structure of cluster roots formed under N deficiency was not a mechanism of N-deficient plants to acquire nitrogen. Nevertheless, plants might have directly taken up N through endocytosis of high-molecular-weight compounds. This process was proven for Hakea actities (Paungfoo-Lonhienne et al. Citation2008), but it remains unknown whether endocytosis is important also for lupin N nutrition. Therefore, the increase of symbiotic nitrogen fixation is the most important pathway of N assimilation in our study to compensate for limited N in the soil.
β-Acetylglucosaminidase activity was not increased in N-deficient conditions, but it was increased under P-deficient conditions (). Induction of β-acetylglucosaminidase is known to cleave the cell walls of microorganisms that are consuming carboxylates. Consequently, it reduces compounds acting as metal-chelators (Wasaki et al. Citation2005; Weisskopf et al. Citation2006). Therefore, the role of β-acetylglucosaminidase in the rhizosphere of cluster roots of P-deficient plants might be protection of carboxylates from degradation, without a significant contribution to N mineralization.
4.3. Interactions between nitrogen and phosphorus nutrition for white lupin
Several publications described the N responses of white lupin under P deficient conditions. For instance, ammonium supply stimulated the cluster root formation and proton extrusion by roots of P deficient white lupin (Sas et al. Citation2002). The high cluster root formation under −P conditions at early stage of this study may be supported by the ammonium supplement into the soil. Le Roux et al. (Citation2009) suggested that white lupin may be better adapted to maintaining biological nitrogen fixation during short-term P starvation. In our study, N concentration of the shoots and nodule numbers at 41 DAT tended to be higher than control, regardless of similar growth. The enhancement of P uptake by cluster root formation may support the biological nitrogen fixation by nodules.
In contrast, the involvement of P nutrition under N-deficient condition has not been well discussed. In our study, P concentration under N deficient conditions was significantly higher than under sufficient N supply (). Therefore, cluster roots under N deficiency might also contribute to P nutrition of plants. Since the activities of AcPase and AlPase in the rhizosphere soils of −N treatment had similar levels than other root types and control treatment (), microbial degradation of organic P was not stimulated under N deficient conditions. In contrast, citrate concentration in the rhizosphere soil of mature cluster roots had similar levels under −P and −N treatments (), and results were in agreement with previous reports of different authors (20–120 µmol root−1 h−1 by Wasaki et al. Citation2005; 150–400 µM in soil solution of cluster roots by Dessureault-Rompré et al. Citation2006; less than 60 µg g−1 by Le Bayon et al. Citation2006). We suggest that cluster roots formed under −N condition had also capacity to solubilize sparingly soluble inorganic P. The number of cluster roots at senescent stage was highest under −N condition at 41 DAT probably due to a faster cluster root development than under other conditions. This hypothesis is supported by the high citrate release from mature cluster roots mobilizing inorganic P under −N conditions. This study showed that nitrogen fixation by nodules of white lupin under N deficient conditions was important for N-acquisition. It has been also shown that the P concentration of nodules was greater than that in other organs regardless of the P supply (Schulze et al. Citation2006). In addition, P homeostasis in nodules is important for symbiotic nitrogen fixation (Sulieman and Tran Citation2015; Esfahani et al. Citation2016). Therefore, our results gave evidence that cluster roots have an important role in proving P for the functioning of symbiotic N fixation under −N conditions. Nevertheless, further studies have to clarify P transport from cluster roots to nodules as well as the molecular mechanisms of P uptake from soils into cluster roots.
4.4. Changes of microbial community structures in the rhizosphere during development of cluster roots and their function
Rhizosphere microorganisms and microbial communities of bulk soils differed mainly because of a higher proportion of Actinobacteria and Proteobacteria in the vicinity of roots and cluster roots (). Both groups are well-known key players in the rhizosphere feeding on root exudates. Amongst Actinobacteria, suborder Streptomycineae was highly increased in all rhizosphere samples (Table S2b). Members of Streptomycineae are known to produce antifungal compounds, indole-3-acetic acid and siderophores (Khamna et al. Citation2009). The higher relative proportion of Acidobacteria in the bulk soil in comparison to the rhizosphere suggested that these oligotrophic microorganisms feed preferentially from high-molecular-weight substrates of organic matter in soils (Naether et al. Citation2012).
The microbial community structure changed during root cluster development ( and ). Either P or N deficiency modified the microbial succession during cluster root development ( and ). Although neither P nor N nutrient limitation changed the Shannon’s index in the bulk soil, results show that nutrient limitation reduced microbial diversity in the rhizosphere of mature cluster roots ().
We found a trend for lower microbial diversity during development of cluster roots under P deficiency. Therefore, microbial community composition changed during cluster root formation toward a more specialized community. This result is supported by cluster analyses of DGGE patterns derived from all samples (). Cloning and sequencing analyses revealed that mainly unclassified microorganisms were lost and that Proteobacteria were enhanced during cluster root development under P deficiency (). Among Proteobacteria, an increase of Burkholderiales was detected in the rhizosphere under control and −P treatments (Table S2c). Weisskopf et al. (Citation2011) reported that Burkholderia species are major inhabitants of white lupin cluster roots. A specific increase of Burkholderiales in cluster roots might contribute to the mobilization of organic P including phytate (myo-inositol hexakisphosphate). The high activity of microbial derived phosphomonoesterase in elder cluster roots supports the view that Burkholderia species might be producers of this enzyme (). Unno et al. (Citation2005) found more than 300 isolates harboring phytate degradation activity from the rhizosphere of white lupin grown in the same soil. In our study, Burkholderia species might be reasonable candidates contributing to organic P mobilization.
The proportion of order Rhizobiales was generally higher in P-deficient soil than in controls (Table S2). This order includes genus Rhizobium, which has been reported frequently as a group of plant growth promoting rhizobacteria (PGPR) (Sessitsch et al. Citation2002; Dessaux et al. Citation2016; Verbon and Liberman Citation2016). Under N-deficiency, we found an increase in the relative proportion of Rhizobiales as well as numbers of nodules (Table S2). It has been shown that inoculation of a PGPR belonging to Bradirhizobium stimulated cluster root formation of V juncea (Fabaceae; Lamont et al. Citation2014). The increase of order Rhizobiales may be involved in the emergence of cluster roots of white lupin. The order Rhodospirillales was found for the first time in the rhizosphere of cluster roots under N-deficient conditions. Because this order contains genus Azospirillum, a group harboring PGPR activities, Rhodospirillales might contribute to the N uptake of plants. The specific microbial community structure in the rhizosphere of N-deficient lupin gave evidence that symbiotic as well as associative N fixation occurred in our experiment and contributed to N nutrition of lupin. In contrast, the formation of cluster roots was unable to contribute directly to N uptake by plants because N cycling enzymes were not stimulated under these conditions ().
5. Conclusion
This study investigated the microbe–plant interactions of white lupin (L. albus L.), which form a unique root structure, so-called cluster roots, under low P and N conditions. We expected that nutrient limitations change rhizosphere microbial community to a more specialized community characterized by modified enzyme production. Results showed that P-deficiency induced the release of carboxylates and AcPase from roots and stimulated the production of microbe-derived AlPase in the rhizosphere. An increased relative abundance of Burkholderiales in the rhizosphere might contribute to microbial degradation of different organic P fractions such as phytates. N deficiency also stimulated cluster root development, but microorganisms might have only an indirect contribution to the N nutrition of lupin. Higher production of nodules, and higher abundance of members of Rhizobiales and Rhodospirillales gave evidence that symbiotic and associative N fixation occurred in the rhizosphere of N-deficient plants. P demand required for N fixation might be supported by citrate release of enhanced numbers of cluster roots under N-deficiency mobilizing sparingly soluble P of soils. In contrast, rhizosphere microorganisms mineralizing organic nitrogen compounds by extracellular enzymes might play only a negligible role under N deficiency.
Supplemental Material
Download Zip (319.1 KB)Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Adams MA, Pate JS 1992: Availability of organic and inorganic forms of phosphorus to lupins (Lupinus spp.). Plant Soil, 145, 107–113. doi:10.1007/BF00009546
- Dessaux Y, Grandclément C, Faure D 2016: Engineering the rhizosphere. Trends Plant Sci., 21, 266–278. doi:10.1016/j.tplants.2016.01.002
- Dessureault-Rompré J, Nowack B, Schulin R, Luster J 2006: Modified micro suction cup/rhizobox approach for the in-situ detection of organic acids in rhizosphere soil solution. Plant Soil, 286, 99–107. doi:10.1007/s11104-006-9029-z
- Dinkelaker B, Marschner H 1992: In vivo demonstration of acid phosphatase activity in the rhizosphere of soil-grown plants. Plant Soil, 144, 199–205. doi:10.1007/BF00012876
- Dissanayaka DMSB, Maruyama H, Masuda G, Wasaki J 2015: Interspecific facilitation of P acquisition in the intercropping of maize with white lupin in two contrasting soils as influenced by different rates and forms of P supply. Plant Soil, 390, 223–236. doi:10.1007/s11104-015-2392-x
- Dissanayaka DMSB, Wickramasinghe WMKR, Marambe B, Wasaki J 2017: Phosphorus-mobilization strategy based on carboxylate exudation in lupins (Lupinus, Fabaceae): a mechanism facilitating the growth and phosphorus acquisition of neighboring plants under phosphorus-limited conditions. Exp. Agr., 53, 308–319. doi:10.1017/S0014479716000351
- Esfahani MN, Kusano M, Nguyen KH, Watanabe Y, Ha CV, Saito K, Sulieman S, Herrera-Estrella L, Tran LSP 2016: Adaptation of the symbiotic Mesorhizobium–chickpea relationship to phosphate deficiency relies on reprogramming of whole-plant metabolism. Proc. Natl. Acad. Sci. USA, 113, E4610–E4619. doi:10.1073/pnas.1609440113
- Gardner WK, Barber DA, Parbery DG 1983: The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant Soil, 70, 107–124. doi:10.1007/BF02374754
- Hagström J, James WM, Skene KR 2001: A comparison of structure, development and function in cluster roots of Lupinus albus L. under phosphate and iron stress. Plant Soil, 232, 81–90. doi:10.1023/A:1010334003073
- Hawkins H-J, Wolf G, Stock WD 2005: Cluster roots of Leucadendron laureolum (Proteaceae) and Lupinus albus (Fabaceae) take up glycine intact: an adaptive strategy to low mineral nitrogen in soils? Ann. Bot., 96, 1275–1282. doi:10.1093/aob/mci279
- Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH 1997: Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol., 63, 3233–3241.
- Hue NV 2009: Iron and phosphorus fertilizations and the development of proteoid roots in macadamia (Macadamia integrifolia). Plant Soil, 318, 93–100. doi:10.1007/s11104-008-9820-0
- Khamna S, Yokota A, Lumyong S 2009: Actinomycetes isolated from medicinal plant rhizosphere soils: diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World J. Mirobiol. Biotechnol., 25, 649–655. doi:10.1007/s11274-008-9933-x
- Lambers H, Clements JC, Nelson MN 2013: How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot., 100, 263–288. doi:10.3732/ajb.1200474
- Lamont B 1972a: ‘Proteoid’ roots in the legume Viminaria juncea. Search, 3, 90–91.
- Lamont B 1972b: The morphology and anatomy of proteoid roots in the genus Hakea. Aust. J. Bot., 20, 155–174. doi:10.1071/BT9720155
- Lamont BB, Pérez-Fernández M, Rodríguez-Sánchez J 2014: Soil bacteria hold the key to root cluster formation. New Phytol., 206, 1156–1162. doi:10.1111/nph.13228
- Le Bayon CC, Weisskopf L, Martinoia E, Jansa J, Frossard E, Keller F, Föllmi KB, Gobat JM 2006: Soil phosphorus uptake by continuously cropped Lupinus albus: a new microcosm design. Plant Soil, 283, 309–321. doi:10.1007/s11104-006-0021-4
- Le Roux MR, Khan SZ, Valentine AJ 2009: Nitrogen and carbon costs of soybean and lupin root systems during phosphate starvation. Symbiosis, 48, 102–109. doi:10.1007/BF03179989
- Li MG, Tadano T 1996: Comparison of characteristics of acid phosphatases secreted from roots of lupin and tomato. Soil Sci. Plant Nutr., 42, 753–763. doi:10.1080/00380768.1996.10416623
- Marschner P, Neumann G, Kania A, Weisskopf L, Lieberei R 2002: Spatial and temporal dynamics of the microbial community structure in the rhizosphere of cluster roots of white lupin (Lupinus albus L.). Plant Soil, 246, 167–174. doi:10.1023/A:1020663909890
- Miller SS, Liu J, Allan DL, Menzhuber CJ, Fedorova M, Vance CP 2001: Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin. Plant Physiol., 127, 594–606. doi:10.1104/pp.010097
- Naether A, Foesel BU, Naegele V et al. 2012: Environmental factors affect acidobacterial communities below the subgroup level in grassland and forest soils. Appl. Environ. Microbiol., 78, 7398–7406. doi:10.1128/AEM.01325-12
- Neumann G, Martinoia E 2002: Cluster roots – an underground adaptation for survival in extreme environments. Trends Plant Sci., 7, 162–167. doi:10.1016/S1360-1385(02)02241-0
- Ozawa K, Osaki M, Matsui H, Honma M, Tadano T 1995: Purification and properties of acid phosphatase secreted from lupin roots under phosphorus-deficiency conditions. Soil Sci. Plant Nutr., 41, 461–469. doi:10.1080/00380768.1995.10419608
- Paungfoo-Lonhienne C, Lonhienne TGA, Rentsch D, Robinson N, Christie M, Webb RI, Gamage HK, Carroll BJ, Schenk PM, Schmidt S 2008: Plants can use protein as a nitrogen source without assistance from other organisms. PNAS, 105, 4524–4529. doi:10.1073/pnas.0712078105
- Paungfoo-Lonhienne C, Schenk PM, Lonhienne TGA, Brackin R, Meier S, Rentsch D, Schmidt S 2009: Nitrogen affects cluster root formation and expression of putative peptide transporters. J. Exp. Bot., 60, 2665–2676. doi:10.1093/jxb/erp111
- Rath M, Salas J, Parhy B et al. 2010: Identification of genes induced in proteoid roots of white lupin under nitrogen and phosphorus deprivation, with functional characterization of a formamidase. Plant Soil, 334, 137–150. doi:10.1007/s11104-010-0373-7
- Sakurai M, Wasaki J, Tomizawa Y, Shinano T, Osaki M 2008: Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci. Plant Nutr., 54, 62–71. doi:10.1111/j.1747-0765.2007.00210.x
- Sas L, Rengel Z, Tang C 2002: The effect of nitrogen nutrition on cluster root formation and proton extrusion by Lupinus albus. Ann. Bot., 89, 435–442. doi:10.1093/aob/mcf066
- Schmidt S, Mason M, Sangtiean T, Stewart GR 2003: Do cluster roots of Hakea actities (Proteaceae) acquire complex organic nitrogen? Plant Soil, 248, 157–165. doi:10.1023/A:1022352415728
- Schulze J, Temple G, Temple SJ, Beschow H, Vance CP 2006: Nitrogen fixation by white lupin under phosphorus deficiency. Ann. Bot., 98, 731–740. doi:10.1093/aob/mcl154
- Sessitsch A, Howieson JG, Perret X, Antoun H, Martínez-Romero E 2002: Advances in Rhizobium research. Crit. Rev. Plant Sci., 21, 323–378. doi:10.1080/0735-260291044278
- Shane MW, Lambers H 2005: Cluster roots: a curiosity in context. Plant Soil, 274, 101–125. doi:10.007/s11104-004-2725-7
- Sulieman S, Tran LSP 2015: Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci., 239, 36–43. doi:10.1016/j.plantsci.2015.06.018
- Tadano T, Ozawa K, Sakai H, Osaki M, Matsui H 1993: Secretion of acid phosphatase by the roots of crop plants under phosphorus-deficient conditions and some properties of the enzyme secreted by lupin roots. Plant Soil, 155–156, 95–98. doi:10.1007/BF00024992
- Trinick MJ 1977: Vesicular-arbuscular infection and soil phosphorus utilization in Lupinus spp. New Phytol., 78, 297–304. doi:10.1111/j.1469-8137.1977.tb04833.x
- Truog E 1930: The determination of the readily available phosphorus in soils. J. Am. Soc. Agron., 22, 874–882. doi:10.2134/agronj1930.00021962002200100008x
- Unno Y, Okubo K, Wasaki J, Shinano T, Osaki M 2005: Plant growth promotion abilities and micro-scale bacterial dynamics in the rhizosphere of lupin analyzed by phytate utilization ability. Environ. Microbiol., 7, 396–404. doi:10.1111/j.1462-2920.2004.00701.x
- Verbon EH, Liberman LM 2016: Beneficial microbes affect endogenous mechanisms controlling root development. Trends Plant Sci., 21, 218–229. doi:10.1016/j.tplants.2016.01.013
- Wang Q, Garrity GM, Tiedje JM, Cole JR 2007: Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol., 73, 5261–5267. doi:10.1128/AEM.00062-07
- Wasaki J, Omura M, Ando M, Dateki H, Shinano T, Osaki M, Ito H, Matsui H, Tadano T 2000: Molecular cloning and root specific expression of secretory acid phosphatase from phosphate deficient lupin (Lupinus albus L.). Soil Sci. Plant Nutr., 46, 427–437. doi:10.1080/00380768.2000.10408796
- Wasaki J, Rothe A, Kania A, Neumann G, Römheld V, Shinano T, Osaki M, Kandeler E 2005: Root exudation, P acquisition and microbial diversity in the rhizosphere of Lupinus albus as affected by P supply and atmospheric CO2 concentration. J. Environ. Qual., 34, 2157–2166. doi:10.2134/jeq2004.0423
- Wasaki J, Yamamura T, Shinano T, Osaki M 2003: Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant Soil, 248, 129–136. doi:10.1023/A:1022332320384
- Watanabe T, Osaki M, Tadano T 1998: Effects of nitrogen source and aluminum on growth of tropical tree seedlings adapted to low pH soils. Soil Sci. Plant Nutr., 44, 655–666. doi:10.1080/00380768.1998.10414489
- Weisskopf L, Abou-Mansour E, Fromin N, Tomasi N, Santelia D, Edelkott I, Neumann G, Aragno M, Tabacchi R, Martinoia E 2006: White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ., 29, 919–927. doi:10.1111/j.1365-3040.2005.01473.x
- Weisskopf L, Heller S, Eberl L 2011: Burkholderia species are major inhabitants of white lupin cluster roots. Appl. Environ. Microbiol., 77, 7715–7720. doi:10.1128/AEM.05845-11
