ABSTRACT
Phosphorus (P) in animal manure compost (AMC) is one of the most important resources of P for crop production. The P characteristics in AMC are affected by animal type, feed, additive, and composting method, among others. Clarifying the P characteristics in AMC is necessary to utilize P effectively and eco-sustainably. Several approaches exist for characterizing P in AMC. The chemical method (extraction) is widely used and can evaluate P according to solubility. Other methods include non-destructive methods like X-ray diffraction (XRD), 31P nuclear magnetic resonance (NMR) spectroscopy, and X-ray absorption spectroscopy (XAS), which can determine P compounds or speciation in AMC. In this paper, we review recent studies relating to the P characteristics in AMC. The chemical P composition or solubility was differed by animal types. The ratio of labile P (water and 0.5M NaHCO3 soluble P) to total P (T-P) was highest in cattle manure compost, followed by swine and poultry. This difference in P solubility was explained by the kind of P compounds in each AMC determined by non-destructive analysis. Struvite (MgNH4PO4·6H2O) was contained in all types of AMC and considered to be the main source of labile P. The relative efficiency of struvite P was higher than commercial chemical P fertilizer, increasing the P recovery rate of AMC. Poultry manure compost had a higher ratio of organic P (Po), mainly phytate P, than others. However, there were few studies which demonstrated the accumulation of Po in AMC-applied soils, indicating the mineralization of Po in soils. Finally, we suggested the best management of P derived from AMC.
1. Introduction
Japan has been importing the most phosphorus (P) for both agricultural and industrial usage, either in the form of phosphate fertilizer (mainly ammonium phosphate) or rock phosphate. In Japan, the quantity of P in annually discharged animal manure was estimated to reach 1.2 × 105 Mg (Mishima et al. Citation2009). This quantity is equal to about 75% of P fertilizer consumption in the same year (1.6 × 105 Mg; FAOSTAT). Using animal manure P for agriculture is expected to reduce dependency on P imports and to save P fertilizer costs. Most animal manure in Japan is composted in order to decompose phytotoxic substances, kill weed seeds and pathogens, and improve the handling of the manure.
Relative efficiency has been used to indicate the available P in animal manure compost (AMC). Relative efficiency is the ratio of P use efficiency of AMC to that of inorganic P fertilizer. The relative efficiency of P in AMC, as evaluated by cultivation experiments, widely fluctuates [Arakawa Citation2012 (66–68% with pelletized cattle and broiler manure compost for komatsuna (Brassica campestris L.) and buckwheat (Fagopyrum esculentum Moench)); Komiyama et al. Citation2014b (50–109% with cattle or layer manure compost for silage coon (Zea mays L.)); Kouno et al. Citation1992 (115–176% with cattle or poultry manure compost for African millet (Eleusine coracana Gaertn)); National Grassland Research Institute (NGRI) Citation1983 (60–70% with all types of manure compost for several crops); Otabe et al. Citation2014 (61–125% with all types of manure compost for komatsuna (Brassica campestris L.)); Oyanagi et al. Citation2005 (100%< with all types of manure compost for komatsuna (Brassica campestris L.)); Ushio et al. Citation2004 (80% with all types of manure compost for several crops)]. Prasad (Citation2009) reviewed the results of pot and field trials on availability of P from composts including sewage sludge and kitchen waste, and concluded that there was a large variation in the relative efficiency among the kind of composts. The relative efficiency of P in AMC is also affected by soil type (P fixing capacity), available P content in the soil, crop species, and length of cultivation, among others.
Because it is difficult to comprehensively evaluate the relative efficiency of P in AMC and to properly adopt it, excessive P has been introduced to agricultural land from AMC or from chemical P fertilizer that is applied at the same time as AMC application. As a result, many studies have revealed soil P buildup by AMC application. [Ito et al. Citation1982 (cattle manure compost, Andosol); Kaji et al. Citation1990 (several manures, Andosol); Kasuya et al. Citation2011 (swine manure compost, Cambisol); Komiyama et al. Citation2014b (cattle and poultry manure compost, Andosol); Murakami and Kuroyanagi Citation2009 (several animal manure composts, Andosol); Ohashi and Okamoto Citation1985 (cattle manure compost mixed with sawdust, Gleysols); Taki et al. Citation2006 (cattle manure compost, Fluvisol)].
This P accumulation in agricultural soils can increase the potential risk of P runoff into lakes and streams, potentially accelerating their eutrophication (Carpenter et al. Citation1998; Kleinman et al. Citation2005). Soil P runs off in the form of particle P (P absorbed into the soil) or dissolved P, via surface or subsurface flow. Several studies have examined particle P runoff (Cully et al. Citation1983; Kasuya et al. Citation2010); Sharpley et al. (Citation1992) revealed that 9–69% of particle P was bioavailable P. Although soil P in the plow layer rarely transfers to subsurface flow (especially Andosols, which cover more than half of Japanese upland soil showing a high P fixing capacity), some studies have revealed that applying excessive P in AMC to sandy soils could contribute to the increase of P concentration in subsurface water (Ito et al. Citation2009; Tani et al. Citation2010; Tsunekawa et al. Citation2013).
Clarifying the P characteristics in AMC is critically important from both an agricultural and an environmental point of view. There are some factors affecting the P characteristics or composition in AMC (Toor et al. Citation2006). The feeds are one of the largest factors. The addition of phytase or using low phytate grain can result in reducing P and change P composition in raw manure of swine and poultry which lacks the inherent phytase enzyme in the digestive tract (Maguire et al. Citation2004; Angel et al. Citation2005; Toor et al. Citation2005). The other factor is handling including physical, chemical and biological treatments, storage and composting. During composting, it is considered that phytate decomposition and stabilization of inorganic P occurs as He et al. (Citation2016) have reviewed. Although Ito et al. (Citation2010) showed the effects of handling (co-composting materials, composting facilities, fermentation period) on P composition in AMC, the largest factor was the type of animals. The purposes of this paper are: 1) to review existing researches on chemical P composition, P solubility, and the species of P compounds in different types of AMC; 2) to associate those characteristics; and 3) to suggest appropriate P fertilization considering the P characteristics in AMC.
2. The chemical P composition in AMC
P in AMC is roughly divided into inorganic P (Pi) and organic P (Po). The determination of Pi and Po can be conducted by wet digestion method (Barnett Citation1994) or dry combustion method (Yokota et al. Citation2003b). In the dry combustion method, AMC and combusted (ashed) AMC is extracted by hot HCl and the amount of P in solution is determined. The difference in P content between before and after combustion was proved with Po. In terms of characterizing the chemical composition of P in animal manure or AMC, the most-used analytical method is the sequential extraction method developed by Hedley et al. (Citation1982) and modified by Frossard et al. (Citation1994) [Ito et al. Citation2010; Sharpley and Moyer Citation2000; Turner and Leytem Citation2004; Waldrip et al. Citation2011; Yamamoto et al. Citation2016; Yokota et al. Citation2003a; Zuvomuya et al. Citation2006]. In this method, air-dried AMC is sequentially extracted by deionized water, 0.5 M NaHCO3 (pH = 8.5), 0.1 M NaOH, and 1 M HCl. Each extraction is conducted with continuous shaking for 16 h at 25°C at the extraction ratio of 1:200. In each fraction, the Pi concentration of the supernatant is determined colorimetrically (Murphy and Riley Citation1962). The chemical compositions of P in AMCs are shown in . Residual P (Resid-P) is the difference of total P (T-P) and the sum of four P fractions, nearly equal to Po (Frossard et al. Citation1994; Ito et al. Citation2010).
Table 1. The chemical P composition in AMC by sequential extraction (Ito et al. Citation2010)
Yokota et al. (2003) showed that the ratios of Pi in cattle manure compost (CMC), swine manure compost (SMC), and poultry manure compost (PMC) were 90%, 93%, and 83%, respectively. The ratios of Pi in AMCs determined by the sequential extraction method (67 ~ 93%, ) were consistent with those determined by the dry combustion method by Yokota et al. (2003), except for broiler litter compost (BLC), which had the lowest Pi ratio. The ratios of Po (Resid-P) in dairy manure compost (DMC; 12%) and beef manure compost (BMC; 12%) were smaller than those in raw manure (21 ~ 37%, Sharpley and Moyer Citation2000; Turner and Leytem Citation2004; Zuvomuya et al. Citation2006). The reason for this was thought to be the decomposition of Po during composting. On the other hand, the ratio of Po in SMC (7%) did not significantly differ from that in raw manure (9%, Sharpley and Moyer Citation2000; Turner and Leytem Citation2004). Layer manure compost (LMC) and broiler litter compost (BLC) had larger ratios of Po (22 ~ 34%) compared with other animal types.
Ito et al. (Citation2010) defined the sum of H2O-soluble P (H2O-P) and NaHCO3-soluble P (NaHCO3-P) as ‘labile P’. The ratio of labile P to T-P was highest in BMC (72%), followed by DMC (65%), SMC (49%), BLC (31%), and LMC (30%). The ratio was negatively correlated with total calcium (T-Ca) content in AMC (r= −0.706, p< 0.01). The high T-Ca content, or T-Ca/T-P ratio, resulted in an increase of HCl-soluble P (HCl-P), particularly in LMC. The ratio of NaOH-soluble P (NaOH-P), which evaluates aluminum (Al) and iron (Fe) bound P, was less than 10% in all types of AMCs. Komiyama et al. (Citation2014a) determined the maximum amount of H2O-extractable P (WEPmax) by continuous water extraction and showed that the WEPmax was consistent with the sum of H2O-P and NaHCO3-P (labile P). The WEPmax was strongly correlated with the amount of H2O-soluble magnesium (Mg), indicating that most labile P was bonded with Mg.
The P composition of phosphate fertilizer has been evaluated by the official testing method for fertilizers (Food and Agricultural Materials Inspection Center (FAMIC) Citation2016). In this method, the sample is extracted by water (1:100, 30 min.) or 2% citric acid solution (1:200, 1 hr.). The P composition of AMC was evaluated with this method in some studies (; Oyanagi et al. Citation2005; Otabe et al. Citation2014). The ratio of H2O-soluble P by the official method was less than that by Hedley’s method. This result is thought to be caused by the difference in AMC/H2O ratios (1:100 vs. 1:200) and extraction periods (1 hr. vs. 16hr). In both extraction methods ( and ), CMC seems to have a higher ratio of more soluble P compounds than SMC or PMC. On the other hand, the ratio of residual P by the official method was about the same as that by Hedley’s method. The ratio of citric acid-soluble P was almost comparable to the sum of each P fraction by Hedley’s method.
Table 2. The chemical P composition in AMC by the official fertilizer analysis method
3. P compounds in AMC
3.1. The analytical methods to identify P compounds in AMC
The species of P compounds in AMC can be estimated, but cannot be identified, from the chemical P compositions. The P compounds may be changed by repeating acid/alkaline extraction, and they are estimated from assumed compounds (except for unexpected ones). Non-destructive methods and structure analyses have been applied to identify P compounds in AMC.
The X-ray diffraction (XRD) method is typically used to evaluate chemical (inorganic) fertilizers; it is effective when the AMC contains crystal P compounds. Some studies apply the XRD method to identify P compounds in AMC, although those may be masked by the existence of many organic substances or calcium compounds (Güngör et al. (Citation2006) (dairy manure); Hunger et al. (Citation2008) (poultry litter); Komiyama et al. (Citation2013) (swine manure compost)).
The 31P nuclear magnetic resonance (NMR) spectroscopy has been applied to P structure elucidation in AMC or raw manure. The solution-state NMR spectroscopy is typically used to characterize organic P compounds in extracts like NaOH (Hashimoto et al. (Citation2014) (poultry litter compost); He et al. (Citation2007a) (poultry litter); He et al. (Citation2007b) (dairy and poultry manures); Leytem et al. (Citation2004) (swine manure). In contrast, the solid-state NMR spectroscopy is used to analyze both organic and inorganic P species in non-destructive samples or residues of extracts (Hunger et al. (Citation2005) (aluminum amended poultry litter); Hunger et al. (Citation2008) (poultry litter)).
The X-ray absorption spectroscopy (XAS) is widely used to determine the chemical speciation or structures of targeted elements in samples. The P K-edge X-ray absorption near-edge structure (XANES) spectroscopy has been employed to analyze P compounds in soils, manures, and AMCs because it has a higher analytical sensitivity and can provide P-specific information, thus avoiding the interferences of other elements (Güngör et al. (Citation2006) (dairy manure); Hashimoto et al. (Citation2014) (poultry litter compost); Peaks et al. (Citation2002) (poultry litter); Sato et al. (Citation2005) (layer manure); Shober et al. (Citation2006) (dairy manure and poultry litter); Toor et al. (Citation2005) (broiler litter and turkey manure); Ymamoto and Hashimoto (Citation2017) (swine manure compost)).
3.2. P compounds in cattle manure compost (CMC)
Güngör et al. (Citation2006) analyzed sieved raw dairy manure and anaerobically digested manure using the XRD and the P K-edge XANES spectroscopy methods. In raw manure, they detected struvite (MgNH4PO4·6H2O) and dicalcium phosphate anhydrous (CaHPO4, DCPA); in anaerobically digested manure, 78% of P was present as struvite and 22% of P was associated with hydroxylapatite (HAp, Ca5(PO4)3OH). Although CMC is usually digested aerobically in Japan, it is possible that part of P is transformed into HAp during composting.
Tanahashi et al. (Citation2010) also showed the existence of struvite in CMC by simultaneous dissolution patterns of P, Mg, and ammonium nitrogen (NH4-N) to HCl or EDTA solution. Horta (Citation2017) fractionated struvite crystal by sequential extraction (Headley’s method) and showed that the solubilities of struvite P to H2O and to 0.5 M NaHCO3 solution were 25 and 170 mg L−1, respectively. Even if all of P in AMC was struvite P, it would dissolve in H2O and NaHCO3 steps judging from the solubility and extraction ratio, while HAp P dissolves in an HCl solution (Wang et al. Citation1995).
He et al. (Citation2007b) showed that the ratio of phytate P in raw dairy manure (5 ~ 6%) was smaller than in poultry manure (24 ~ 33%) with an NaOH-EDTA extract (NaOH-EDTA could extract 99% of P in dairy manure). However, the ratio of other monoester P (14 ~ 22%) in raw dairy manure was higher than in poultry manure (7 ~ 12%). Most Po (27 ~ 35%; He et al. Citation2007b) in raw manure was considered to be digested during composting, resulting in low Po ratio (12%) in CMC ().
3.3. P compounds in swine manure compost (SMC)
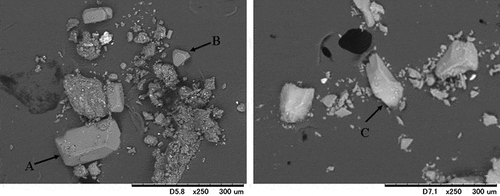
Tanahashi et al. (Citation2010) showed the existence of magnesium ammonium phosphate (MgNH4PO4) in SMC by measuring NH4-N in extracts with various pH levels. The existence of struvite (MgNH4PO4·6H2O) in SMC was confirmed by the XRD method (Komiyama et al. Citation2013; Nanzyo and Kanno Citation2018) and by the P K-edge XANES spectroscopy (Yamamoto and Hashimoto Citation2017). The crystal of struvite was observed by a scanning electron microscope (SEM) image (). If all of NH4-N in SMC (5.7 mg N kg−1; Livestock Industry’s Environmental Improvement Organization (LEIO) Citation2007) originated from struvite, the amount of P originating from struvite is calculated as 12.6 mg P kg−1, equal to 52% of T-P in SMC. This ratio is consistent with that of labile (H2O and NaHCO3 extractable) P (49%; ) as evaluated by the sequential extraction method. Yamanoto and Hashimoto (Citation2017) also found tricalcium phosphate (TCA, Ca3(PO4)2) as a secondary P compound in SMC. This compound is thought to be extracted by HCl.
Figure 1. Scanning electron microscope (SEM) images of struvite (A and B) collected from a swine manure compost (SMC) and struvite crystal removed from animal wastewater (C) (Komiyama et al. Citation2013)
The Po ratio of SMC was lowest in all types of AMC (). Turner and Leytem (Citation2004) showed that the percentage of phosphate monoester (including phytate P) to T-P was 9% in grain-fed swine manure, lower than that in cattle manure (14%) or broiler litter (58%). This result corresponds with the lowest content of Po in SMC ().
3.4. P compounds in poultry manure compost (PMC)
PMC has also been confirmed as containing struvite (Tanahahi Citation2011; Hunger et al. Citation2008). Hunger et al. (Citation2008) detected struvite in poultry litter by the XRD method and by solid-state NMR spectroscopy. However, the proportion of struvite-P to T-P was 0 ~ 23% (Hunger et al. Citation2008), indicating that struvite was not a major P compound of PMC.
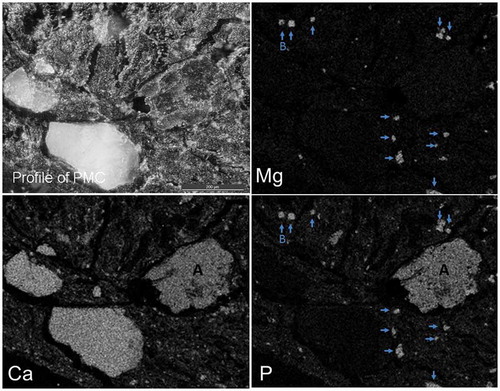
shows the profile of PMC and the two-dimensional distribution images of Ca, Mg, and P by electron probe micro-analyzer (EPMA), referring to Nanzyo and Kanno (Citation2018). The distribution of P overlapped with those of Ca and Mg (). The compound composed of P and Mg was thought to be struvite as a result of combining the XRD result (data not shown).
Figure 2. The profile of a poultry manure compost (PMC) and the two-dimensional distribution images of calcium (Ca), magnesium (Mg), and phosphorus (P) by electron probe micro-analyzer (EPMA). A: P compound bound with Ca; B: P compound bound with Mg
Several studies (Peaks et al. Citation2002; Sato et al. Citation2005; Toor et al. Citation2005) have shown that poultry manure or litter contains DCPA (CaHPO4). Hashimoto et al. (Citation2014) found an increase of hydroxyapatite (HAp) in poultry manure during composting by the P K-edge XANES spectroscopy. Toor et al. (Citation2005) showed that a high Ca/P ratio (>2) resulted in transforming more-soluble P compounds like DCPA to less-soluble P compounds like HAp. In sequential extraction, struvite and DCPA are understood to be dissolved in H2O or NaHCO3 solutions, and HAp is dissolved in HCl (Horta Citation2017; McDowell et al. Citation2002; Wang et al. Citation1995).
Poultry manure is known to have higher contents of phytate P than other types of animal manures. Maguire et al. (Citation2004) showed that 26 ~ 56% of T-P in broiler and turkey manures was phytate P, and Leytem et al. (Citation2006) showed that the ratio was 35 ~ 80% in layer manure. He et al. (Citation2007a) analyzed poultry litter P speciation in the extracts of each step with the solution-state NMR spectroscopy and showed that 6%, 63%, and 80% of P was phytate P in H2O, NaOH, and HCl extracts, respectively. From these results, the proportion of phytate P to T-P was calculated to be 54%. Although a certain portion of phytate P decomposes during composting, it remains in PMC, resulting in a higher ratio of Po (21 ~ 33%, ).
3.5. The differences of p compounds by the type of AMC
The conceptual diagram of P compounds in each AMC, drawn from the results of the chemical and non-destructive analysis above, is shown in . The major factors of the P compound formation are the concentrations of elements and their ratios (). The higher Ca and Ca/P are thought to contribute to the formation of HAp in LMC, BLC, and DMC. SMC had a lower Ca/P ratio where HAp was difficult to form (Toor et al. Citation2005). As a result, TCP became the P compound bound with Ca in SMC.
Table 3. The chemical properties relating to the formation of P compounds
Figure 3. The conceptual diagram of the relationship between P solubility and P compounds. CMC–cattle manure compost; SMC–swine manure compost; PMC-poultry manure compost. †NaOH-extractable P, which is considered to be Al or Fe bound P, was excluded in this diagram
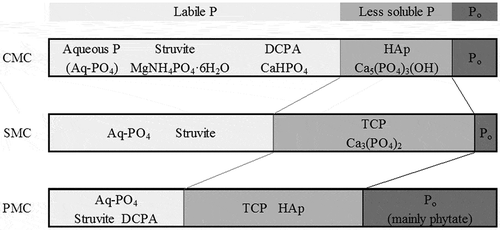
Struvite was found in all types of AMCs, and it is presumed to be dissolved in water or an NaHCO3 solution in the sequential method (Horta Citation2017; Komiyama et al. Citation2013, Citation2014a). SMC had the highest Mg and NH4-N contents, indicating that it was easy to form more struvite in it. However, it is possible that the formation of struvite was suppressed by higher Ca and uric acid, which contributed to the growth of HAp and ammonium acid urate (Tanahashi Citation2011) in PMC (LMC and BLC). The size of struvite in SMC is thought to grow to more than 100 µm (Nanzyo and Kanno Citation2018; Komiyama et al. Citation2013), although struvite size in PMC is around 10 µm (), supporting our hypothesis that the struvite crystal is difficult to grow in PMC.
The ratio of phytate P is highest in PMC (LMC and BLC), followed by CMC and SMC, corresponding to the phytate P contents in raw manures.
4. P fertilization based on the chemical P composition and P compounds in AMC
The information concerning the chemical P composition and the species of P compounds in AMC should be reflected in the best P fertilization that enables maintenance of crop yield and minimizes the P buildup in the agricultural soil.
Struvite is the main labile P compound in all types of AMC and shows higher P recovery rate for crops than chemical fertilizers like superphosphate (SP) or double superphosphate (DSP) (Katanda et al. Citation2015; Komiyama et al. Citation2013). Although the solubility of struvite P to H2O is low, its solubility to NaHCO3 solution is about 7 times as high as H2O (Horta Citation2017). This moderate solubility of struvite P is thought to increase the P recovery rate, because struvite P can readily avoid absorption into Al and Fe oxides in soil and can be released into soil solution with crop growth.
Although the availabilities of the other labile P compound (DCPA) or aqueous P (HPO42-, H2PO4−) are the same as SP or DSP, organic substances like the humic acids in AMC are known to increase P use efficiency by chemically and physically suppressing P fixation to soil (Kato et al. Citation2010; Sharif et al. Citation1974).
As for HAp, which is categorized as a less-soluble P compound, Ando et al. (Citation1988) showed that the relative efficiency of P in synthetic-precipitated HAp for rice and Chinese cabbage was the same as in calcined defluorinated phosphate fertilizer. This result means that the HAp in AMC shows the same P availability for crops as a commercial P fertilizer. Hashimoto et al. (Citation2014) observed the formation of HAp in poultry manure during the composting period and found that the availability of HAp precipitated in poultry manure was the same as that in the synthetic-precipitated HAp by Ando et al. (Citation1988). The official fertilizer analysis () showed that citric acid solution extracted most of the less-soluble P, including HAp, in PMC. This result supports the availability of HAp in PMC because citric acid soluble P was demonstrated as plant-available P (Otabe et al. Citation2014; Oyanagi et al. Citation2005).
PMC contained more Po, most of which is considered to be phytate P. Dou et al. (Citation2009) surveyed the P speciation of several soils that had received manure application for 8 ~ 10 years, and they showed that the concentrations of inositol hexakisphosphate (phytate) were similar in manure-applied soils (52 ~ 116 mg P kg−1, 2 ~ 8% of T-P) and unapplied soils (43 ~ 137 mg P kg−1, 6 ~ 22%). Annaheim et al. (Citation2015) also surveyed the Po speciation of the soil which had a 62-year application of dairy manure and compost by 31P NMR spectroscopy or enzyme addition, and showed no difference in organic P speciation compared with mineral P fertilizer or no fertilization treatments. Schmieder et al. (Citation2018) reported that long-term (more than 40 years) cattle manure amendment did not lead to any enrichment with organic P in the soil. Leytem et al. (Citation2006) demonstrated the phytate decomposition and increase of Olsen P in calcareous soil during a 12-week incubation. He et al. (Citation2007a) investigated the effects of poultry litter application on Po in soil and showed that poultry litter did not lead to a significant accumulation of hydrolyzable Po. Gerke (Citation2015) indicated that phytate bound with soil (Al or Fe oxides) was immobilized and hard to react with phytase. These results indicate that manure-derived Po, mainly phytates, may not be stable and mineralized to an inorganic form, which may contribute to the increase of available P in the soil.
Another factor relating to P use efficiency of AMC is the proliferation of roots that are enhanced biologically and physically by soil improvement (Matsuguchi and Nitta Citation1988).
These characteristics of P in AMC (higher recovery rate of soluble P and struvite, possible HAp dissolution or phytate decomposition) are considered to contribute to the higher relative effectiveness of P in AMC. In some cases, it is possible that the relative efficiency of P in AMC exceeds 100%, for the reasons mentioned in this paper. A P-based AMC management that considers the chemical P characteristics or the species of P compounds are required both for increasing crop productivity and for decreasing the risk of P loss (Eghball and Power Citation1999; Komiyama et al. Citation2014b; Toth et al. Citation2006).
5. Conclusion
This review associated the existing studies on the chemical P composition in AMC and P speciation determined by 31P NMR spectroscopy, XRD and XANES. Although the sequential extraction method can determine P composition easily, the information on P speciation or compounds was limited. The species of P compounds in AMC and their solubility can be evaluated comprehensively by combining chemical and non-destructive methods in response to both agricultural and environmental needs. It is needed to clarify the relationships between P characteristics in AMC and soil reactions or crop responses to establish eco-sustainably P management system.
References
- Ando J, Nagano Y, Iguchi N 1988: Recovery and fertilizer use of phosphorus in wastewaters (Part 1) Basic studies on recovery and fertilizer use of phosphorus in sewage. Jpn. J. Soil Sci. Plant Nutr., 59, 33–40. (In Japanese with English summary).
- Angel CR, Powers WJ, Applegate TJ, Tamim NM, Christman MC 2005: Influence of phytase on water-soluble phosphorus in poultry and swine manure. J. Environ. Qual., 34, 563–571.
- Annaheim KE, Doolette AL, Smernik RJ, Mayer J, Oberson A, Frossard E, Bunemann EK 2015: Long-term addition of organic fertilizers has little effect on soil organic phosphorus as characterized by 31P NMR spectroscopy and enzyme additions. Geoderma, 257-258, 67–77. doi:10.1016/j.geoderma.2015.01.014
- Arakawa Y 2012: Pelletization of composted manure improves the phytoavailability of contained phosphorus. Jpn. J. Soil Sci. Plant Nutr., 83, 249–255. (In Japanese with English summary).
- Barnett GM 1994: Phosphorus forms in animal manure. Bioresour. Technol., 49, 139–147. doi:10.1016/0960-8524(94)90077-9
- Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH 1998: Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Applic., 8, 559–568. doi:10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2
- Cully JEB, Bolton EF, Bernyk V 1983: Suspended-solids and phosphorus loads from a clay soil. I. Pilot Studies. J. Environ. Qual., 12, 493–498. doi:10.2134/jeq1983.00472425001200040011x
- Dou Z, Ramberg CF, Toth JD, Wang Y, Sharpley AN, Boyd SE, Chen CR, Williams D, Xu ZH 2009: Phosphorus speciation and sorption-desorption characteristics in heavily manured soils. Soil Sci. Soc. Am. J., 73, 93–101. doi:10.2136/sssaj2007.0416
- Eghball B, Power JF 1999: Phosphorus- and nitrogen-based manure and compost applications: corn production and soil phosphorus. Soil Sci. Soc. Am. J., 63, 895–901. doi:10.2136/sssaj1999.634895x
- Food and Agricultural Materials Inspection Center (FAMIC) 2016: Testing methods for fertilizers. http://www.famic.go.jp/ffis/fert/obj/TestingMethodsForFertilizers2016.pdf
- Frossard E, Tekely P, Grimal JY 1994: Characterization of phosphate species in urban sewage sludges by high-resolution solid-state 31P-NMR. Eur. J. Soil Sci., 45, 403–408. doi:10.1111/j.1365-2389.1994.tb00525.x
- Gerke J 2015: Phytate (inositol hexakisphosphate) in soil and phosphate acquisition from inositol phosphates by higher plants. A Review. Plants, 4, 253–266. doi:10.3390/plants4020253
- Güngör K, Jürgensen A, Karthikeyan KG 2006: Determination of phosphorus speciation in dairy manure using XRD and XANES spectroscopy. J. Environ. Qual., 36, 1856–1863. doi:10.2134/jeq2006.0563
- Hashimoto Y, Takamoto A, Kikkawa R, Murakami K, Yamaguchi N 2014: Formation of hydroxyapatite and inositol hexakisphosphate in poultry litter during the composting period: sequential fractionation, P K-edge XANES and solution 31P NMR investigations. Environ. Sci. Technol., 48, 5486–5492. doi:10.1021/es404875j
- He Z, Cade-Menum BJ, Senwo ZN, Tazisong IA 2007a: Phosphorus in poultry litter and soil: enzymatic and nuclear magnetic resonance characterization. Soil Sci. Soc. Am. J., 72, 1425–1433. doi:10.2136/sssaj2007.0407
- He Z, Cade-Menum BJ, Toor GS, Fortuna AM, Honeycutt CW, Sims JT 2007b: Comparison of phosphorus forms in wet and dried animal manures by solution phosphorus-31 nuclear magnetic resonance spectroscopy and enzymatic hydrolysis. J. Environ. Qual., 36, 1086–1095. doi:10.2134/jeq2006.0549
- He Z, Pagliari PH, Waldrip HM 2016: Applied and environmental chemistry of animal manure: A review. Pedsphere, 26, 779–816. doi:10.1016/S1002-0160(15)60087-X
- Hedley MJ, Stewart JWB, Chauhan BS 1982: Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J., 46, 970–976. doi:10.2136/sssaj1982.03615995004600050017x
- Horta C 2017: Bioavailability of phosphorus from composts and struvite in acid soils. Rev. Bras. Eng. Agric. Ambient., 21, 459–464. doi:10.1590/1807-1929/agriambi.v21n7p459-464
- Hunger S, Sims JT, Sparks DL 2005: How accurate is the assessment of phosphorus pools in poultry litter by sequential extraction? J. Environ. Qual., 34, 382–389.
- Hunger S, Sims JT, Sparks DL 2008: Evidence for struvite in poultry litter: effect of storage and drying. J. Environ. Qual., 37, 1617–1625. doi:10.2134/jeq2007.0331
- Ito T, Komiyama T, Saigusa M, Morioka M 2010: Phosphate composition of swine and poultry manure composts. Jpn. J. Soil Sci. Plant Nutr., 81, 215–223. (In Japanese with English summary).
- Ito T, Komiyama T, Yonai K, Sano O, Suzuki K, Uno T 2009: The effect of phosphorus composition in manure composts on phosphorus leaching in the soil with those applications. The Proceedings of Jpn. Soc. Soil Sci. Plant Nutr., 55, 161. (In Japanese).
- Ito Y, Shiozaki H, Hashimoto H 1982: Effects of continuous heavy-application of farmyard manure on the fertility of a humus-rich volcanic ash soil. Bull. Kyusyu Agric. Exp. Sta., 22, 259–320. (In Japanese with English summary).
- Kaji T, Ikeda K, Kusamizu T 1990: Effect of successive application on various animal manures on the yield of crop plants and chemical property of soil. Bull. Kagoshima Agric. Exp. Stn., 18, 33–50. (In Japanese with English summary).
- Kasuya M, Banzai K, Itahashi S, Ogino K, Hiroto S 2010: Nitrogen and phosphorus runoffs into a stream from an upland basin with high stocking density in a red-yellow soil area. Jpn. J. Soil Sci. Plant Nutr., 81, 481–488. (In Japanese with English summary).
- Kasuya M, Ogino K, Hiroto S, Ishikawa H, Suzuki R 2011: Dynamics of nutrients in a yellow soil vegetable field using cattle or pig feces compost for five years. Res. Bull. Aichi Agric. Res. Ctr., 43, 137–149. (In Japanese with English summary).
- Katanda Y, Zvomuya F, Flaten D, Cicek N 2015: Hog-manure-recovered struvite: effects on canola and wheat biomass yield and phosphorus use efficiencies. Soil Sci. Soc. Am. J., 80, 135–146. doi:10.2136/sssaj2015.07.0280
- Kato M, Komiyama T, Fujisawa E, Morikuni H 2010: Repressions of adsorptions of available phosphate derived from fertilizer by combined applications with cow or poultry manure compost in upland soil conditions. Jpn. J. Soil Sci. Plant Nutr., 81, 367–371. (In Japanese).
- Kleinman PJA, Wolf AM, Sharpley AN, Beegle DB, Saporito LS 2005: Survey of water-extractable phosphorus in livestock manures. Soil Sci. Soc. Am. J., 69, 701–708. doi:10.2136/sssaj2004.0099
- Komiyama T, Ito T, Saigusa M 2014a: Measurement of the maximum amount of water-extractable phosphorus in animal manure compost by continuous and sequential water extraction. Soil Sci. Plant Nutr., 60, 196–207. doi:10.1080/00380768.2013.879836
- Komiyama T, Ito T, Saigusa M 2014b: Effects of phosphorus-based application of animal manure compost on the yield of silage corn and soil phosphorus accumulation in an upland Andosol in Japan. Soil Sci. Plant Nutr., 60, 863–873. doi:10.1080/00380768.2014.955449
- Komiyama T, Niizuma S, Fujisawa E, Morikuni H 2013: Phosphorus compounds and their solubility in swine manure compost. Soil Sci. Plant Nutri., 59, 419–426. doi:10.1080/00380768.2013.789397
- Kouno K, Ogata S, Marumoto K 1992: Comparison of the recovery rates of N, S and P in added organic matter and chemical fertilizer in Regosols and Andosols. Jpn. J. Soil Sci. Plant Nutr., 63, 146–153. (In Japanese with English summary).
- Leytem AB, Smith DR, Applegate TJ, Thacker PA 2006: The influence of manure phytic acid on phosphorus solubility in calcareous soils. Soil Sci. Soc. Am. J., 70, 1629–1638. doi:10.2136/sssaj2006.0003
- Leytem AB, Turner BL, Thacker PA 2004: Phosphorus composition of manure from swine fed low-phytate grains: evidence for hydrolysis in animal. J. Environ. Qual., 33, 2380–2383.
- Livestock Industry’s Environmental Improvement Organization (LIEO) 2007: The Nutrient List and the Usage Method that Adopted Fertilizer Efficiency of Animal Manure Compost. Livestock Industry’s Environmental Organization, Tokyo.
- Maguire RO, Sims JT, Saylor WW, Turner BL, Angel R, Applegate TJ 2004: Influence of phytase addition to poultry diets on phosphorus forms and solubility in litters and amended soils. J. Environ. Qual., 33, 2306–2316.
- Matsuguchi T, Nitta T 1988: Effects of organic amendment on root development and the rhizosphere microflora of monocropped upland crops. Jpn. J. Soil Sci. Plant Nutr, 59, 1–11. (In Japanese with English summary).
- McDowell RW, Condron LM, Mahieu N, Brookes PC, Poulton PR, Sharpley AN 2002: Analysis of potentially mobile phosphorus in arable soils using solid state nuclear magnetic resonance. J. Environ. Qual., 31, 450–456.
- Mishima S, Endo A, Shirato Y, Kimura DS 2009: Quantity of organic waste resources in Japan and capacity of local farmyard to receive composted wastes. Jpn. J. Soil Sci. Plant Nutr., 80, 226–232. (In Japanese with English summary).
- Murakami H, Kuroyanagi Y 2009: Effects of animal manure application on soil environment in cabbage field. Bull. Natl. Inst. Veg. & Tea Sci., 8, 139–156. (In Japanese with English summary).
- Murphy J, Riley JP 1962: A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta., 27, 31–36. doi:10.1016/S0003-2670(00)88444-5
- Nanzyo M, Kanno H 2018: Role of inorganic soil constituents in selected topics. In “Inorganic Constituents in Soil”, pp. 133–176. Springer Singapore, Singapore.
- National Grassland Research Institute (NGRI) 1983: The Report of the Study on the Processing and Use of Animal Feces and Urine, pp. 60–61. National Grassland Research Institute, Tokyo. (In Japanese).
- Ohashi K, Okamoto M 1985: Effects of continuous application of farmyard manure mixed with sawdust on the nutrient uptake by vegetables and on the chemical properties of soil. Jpn. J. Soil Sci. Plant Nutr., 56, 378–383. (In Japanese).
- Otabe H, Fujita Y, Ueta T, Orimoto Y 2014: Estimation of phosphoric acid and potassium fertilizer effect on the poultry manure compost by citric acid-soluble content, examination by pot cultivation of Brassica campestris L. var. Komatsuna. Jpn. J. Soil Sci. Plant Nutr., 85, 461–465. (In Japanese).
- Oyanagi W, Wada T, Ando Y 2005: Property and fertilizer effect of phosphorus included animal waste compost. Bull. Niigata Animal Husbandry Exp. Sta., 15, 6–9. (In Japanese).
- Peaks D, Sims JT, Sparks DL 2002: Solid-state speciation of natural and alum-amended poultry litter using XANES spectroscopy. Environ. Sci. Technol., 36, 4253–4261.
- Prasad M 2009: A literature review on the availability of phosphorus from compost in relation to the nitrate regulations SI 378 of 2006. Environmental Protection Agency Ireland. http://www.cre.ie/docs/Phosphorus%20Review.pdf
- Sato S, Solomon D, Hyland C, Ketterings QM, Lehamann J 2005: Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ. Sci. Technol., 39, 7485–7491.
- Schmieder F, Bergstrom L, Riddle M, Gustafsson JP, Klysubun W, Zehetner F, Condron L, Kirchmann H 2018: Phosphorus speciation in a long-term manure-amended soil profile – evidence from wet chemical extraction, 31P NMR and K-edge XANES spectroscopy. Geoderma, 322, 19–27. doi:10.1016/j.geoderma.2018.01.026
- Sharif M, Chaudhry FM, Latif A 1974: Suppression of superphosphate-phosphorus fixation by farmyard manure Ⅰ. High phosphorus uptake from superphosphate. Soil Sci. Plant Nutr., 20, 387–393. doi:10.1080/00380768.1974.10432610
- Sharpley AN, Moyer B 2000: Phosphorus forms in manure and compost and their release during simulated rainfall. J. Environ. Qual., 29, 1462–1469. doi:10.2134/jeq2000.00472425002900050012x
- Sharpley AN, Smith SJ, Jones OR, Berg WA, Coleman GA 1992: The transport of bioavailable phosphorus in agricultural runoff. J. Environ. Qual., 21, 30–35. doi:10.2134/jeq1992.00472425002100010003x
- Shober AL, Hesterberg DL, Sims JT, Gardner S 2006: Characterization of phosphorus species in biosolids and manures using XANES spectroscopy. J. Environ. Qual., 35, 1983–1993. doi:10.2134/jeq2006.0100
- Taki N, Kumagai C, Hatanaka A 2006: Phosphorus accumulation after continuous application of animal manure compost to grey lowland soil of upland field. Bull. Miyagi Pref. Furukawa Agric. Exp. Sta., 6, 35–41. (In Japanese).
- Tanahashi T 2011: Development of the practical nitrogen fertilizer efficiency appraisal method of animal waste compost based on the form analysis of the nitrogen. Jpn. J. Soil Sci. Plant Nutr., 82, 357–359. (In Japanese).
- Tanahashi T, Yano H, Itou H, Oyanagi W 2010: Magnesium ammonium phosphate in cattle and swine manure composts and an extraction method for its evaluation. Jpn. J. Soil Sci. Plant Nutr., 84, 329–335. (In Japanese with English summary).
- Tani M, Mizota C, Yagi T, Kato T, Koike M 2010: Vertical distribution and accumulation of phosphate in virgin soils and arable soils of Tokachi district, Hokkaido. J. Soil Sci. Plant Nutr., 81, 350–359. (In Japanese with English summary).
- Toor GS, Hunger S, Peak JD, Sims JT, Sparks DL 2006: Advances in the characterization of phosphorus in organic wastes: environmental and agronomic applications. Adv. Agron., 89, 1–72.
- Toor GS, Peak JD, Sims JT 2005: Phosphorus speciation in broiler litter and turkey manure produced from modified diets. J. Environ. Qual., 34, 687–697.
- Toth JD, Dou Z, Ferguson JD, Galligan DT, Ramberg CF Jr. 2006: Nitrogen- vs. phosphorus-based dairy manure application to field crops: nitrate and phosphorus leaching and soil phosphorus accumulation. J. Environ. Qual., 35, 2302–2312. doi:10.2134/jeq2005.0479
- Tsunekawa A, Ikeda A, Tsuji M, Taki K 2013: Nutrient dynamics in sandy field soil amended using composted livestock manure during an eight-year period. Res. Bull. Aichi Agric. Res. Ctr., 45, 1–9. (In Japanese with English summary).
- Turner BL, Leytem AB 2004: Phosphorus compounds in sequential extracts of animal manures: chemical speciation and a novel fractionation procedure. Environ. Sci. Technol., 38, 6101–6108.
- Ushio S, Yoshimura N, Saitou K, Anzai T 2004: The spreadsheets that show the characteristic of ingredient contents of animal waste compost and the proper rate of animal waste compost application. Jpn. J. Soil Sci. Plant Nutr., 75, 99–102. (In Japanese).
- Waldrip HM, He Z, Erich MS 2011: Effects of poultry manure amendment on phosphorus uptake by ryegrass, soil phosphorus fractions and phosphate activity. Biol. Fertil. Soils, 47, 407–418. doi:10.1007/s00374-011-0546-4
- Wang HD, Harris WG, Reddy KR, Flaig EG 1995: Stability of phosphorus forms in dairy-impacted soils under simulated leaching. Ecol. Eng., 5, 209–227. doi:10.1016/0925-8574(95)00047-X
- Yamamoto K, Hashimoto Y 2017: Chemical species of phosphorus and zinc in water-dispersible colloids from swine manure compost. J. Environ. Qual., 46, 461–465. doi:10.2134/jeq2016.11.0433
- Yamamoto T, Tsuji M, Suzuki R, Kasuya M, Takeuchi M 2016: Changes of phosphorus species in cabbage field using cattle manure compost. Res. Bull. Aichi Agric. Res. Ctr., 48, 101–107. (In Japanese with English summary).
- Yokota T, Ito T, Ono T, Takahashi M, Saigusa M 2003a: Composition of inorganic phosphate in cattle manure compost with different production conditions. Jpn. J. Soil. Sci. Plant. Nutr., 74, 133–140. (In Japanese with English summary).
- Yokota T, Ito T, Saigusa M 2003b: Measurement of total phosphorus and organic phosphorus contents of animal manure composts by the dry combustion method. Soil Sci. Plant Nutr., 49, 267–272. doi:10.1080/00380768.2003.10410006
- Zuvomuya F, Helgason BL, Larney FJ, Janzen HH, Akinremi OO, Olsen BM 2006: Predicting phosphorus availability from soil-applied composted and non-composted cattle feedlot manure. J. Environ. Qual., 35, 928–937. doi:10.2134/jeq2005.0409
