ABSTRACT
At present, there is no comprehensive soil quality assessment practice for soil stockpiles in the South African coal mining industry. Soil microorganisms and enzymes are suitable indicators for soil quality monitoring. Therefore, this study investigated the microbial community and enzyme (beta-glucosidase and urease) activities in soil stockpiles of opencast coal mines in the coal-rich region of South Africa. Soil stockpiles of three opencast coal mines were sampled at depths of 0–20 cm (‘topsoil’) and >20 cm (‘subsoil’) across three seasons. Beta-glucosidase and urease activities were mostly higher in soil stockpiles than in unmined soils and were significantly influenced (P < 0.05) by the interaction of site and seasonal factors. However, analyses of PCR-denaturing gradient gel electrophoresis (PCR-DGGE) profiles of partial 16S rRNA gene and internally transcribed spacer 2 (ITS2) sequences revealed higher microbial diversity in unmined (reference) soils compared to soil stockpiles across all seasons. Redundancy analysis further revealed that microbial communities of topsoil were not significantly (P > 0.05) influenced by soil properties, whereas microbial communities of subsoils were significantly (P < 0.05) influenced by pH, organic carbon, total nitrogen and phosphorus contents. Furthermore, operational taxonomic units (OTUs) belonging to genera of known phytobeneficial species such as Azomonas, Aureobasidium, Phialocephala, Phoma and Sordariomycetes were detected in these soils. Overall, results suggest that the microbial community structure and diversity observed in stockpiles is impaired (compared to the unmined site), although variations in the microbial community structure of soil stockpiles across seasons are site-specific. The impaired microbial community of stockpiles may have negative implications on soil biological processes driven by microbes; especially those that are critical for nutrient cycling and ecosystem sustainability. More importantly, such alteration in soil biodiversity may impair post-mining land use capability of stockpile soils.
1. Introduction
The soil is a non-renewable resource which plays crucial roles that are paramount to human existence and sustainability of other ecosystems (Faber et al. Citation2013). Often, this non-renewable resource is disturbed through several anthropogenic activities, including agriculture and mining. The coal deposits (largely bituminous) in South Africa are mostly below arable soils, especially in the Mpumalanga Highveld portion of the grassland biome area. This area provides important ecosystem services such as provisioning services (e.g., arable use) and cultural services (recreational). Unfortunately, surface (opencast) mining of these coal deposits contributes to the loss of such important ecosystem services. Therefore, the rehabilitation of mined lands towards restoring pre-disturbance ecosystem services or achieving an acceptable post-mining land use capability is paramount.
For the post-mining restoration of the soil profile and ecosystem services, mining regulations stipulate that the original topsoil and reusable horizons – the nutrient-rich and plant-growth supporting horizons – must be carefully excavated and stockpiled (stored) separately from other overburden materials until post-mining rehabilitation (Strohmayer Citation1999). In most cases, the soil is stored for several years, during which changes in the quality of the soil may occur (Strohmayer Citation1999). Unfortunately, these changes may contribute to the impairment of important soil health components, including physico-chemical properties and diversity of the soil biota (Harris et al. Citation1989; Cardoso et al. Citation2013; Ezeokoli et al. Citation2019). Previous studies have established that the stockpiling operations contribute to the hard-setting of soil, increased soil compaction levels, poor soil structure, reduction in soil water-holding capacity, decreased soil biomass, loss of nutrients and poor overall quality of topsoil (Abdul-Kareem and McRae Citation1984; Ghose Citation2004; Coetzee Citation2016; Paterson et al. Citation2019). Logically, the quality of stockpiled soil is linked to the success of post-mining rehabilitation because the soil is reapplied during rehabilitation and prior to revegetation (Sheoran et al. Citation2010). Hence, an assessment of the quality of soil stockpiles in coal mines will provide an early forecast of the likelihood of post-mining land rehabilitation success or otherwise.
For soil health assessments, a combination of physical, chemical, and biological components of the soil are important parameters. Such an assessment provides a comprehensive insight into the condition of the soil (Doran and Parkin Citation1994; Claassens et al. Citation2008, Citation2012). With regard to soil biological components, microbes and enzymes play several important roles in the soil ecosystem. Such roles include the mobilization of essential nutrients in the soil, mediation of plant nutrient uptake, plant-pathogen resistance, secretion of plant growth-promoting hormones and decomposition of soil organic matter (Tinker Citation1984; van Veen and Kuikman Citation1990; van der Heijden et al. Citation2008; Adeleke et al. Citation2012, Citation2017). Hence, enzyme activities and diversity of microbes have been used as soil quality indices for ascertaining nutrient cycling and availability in the soil (Tabatabai and Dick Citation2002). Such indices are also used for assessing changes in soil organic matter (Six et al. Citation2004) and the impacts of farming practices (García-Ruiz et al. Citation2009), soil management (Bending et al. Citation2004), tillage (Fließbach et al. Citation2007), plant genotype (van Wyk et al. Citation2017) and fertilizer application (Mandal et al. Citation2007). Generally, higher soil microbial diversity and activity suggest the functional plasticity or stability of the soil ecosystem to external constraints.
At present, there is no comprehensive soil health assessment practice for soil stockpiles in the South African coal mining industry. It has been previously established that soil microorganisms and enzymes are sensitivity to anthropogenic disturbances (Dose et al. Citation2015; Nkuekam et al. Citation2018). However, there is still a knowledge gap especially as this relates to the extent at which microbial communities and enzyme activities in soil stockpiles are influenced across climatic seasons. In the present study, we tested two hypotheses viz (1.) that enzyme activities and microbial diversity of topsoil stockpiles (disturbed) are impaired compared to adjacent unmined (undisturbed) soils, and (2.) that microbial communities and enzyme activities in soil stockpiles vary across seasons. To achieve this, the present study explored the microbial community structure and dynamics, as well as activities of selected enzymes of soil stockpiles in three South African coal mines and an unmined reference site across three seasons. By making comparisons of these parameters between reference soils and soil stockpiles, we aimed to provide some indication of the health status of soil stockpiles as well as appropriateness of current soil stockpiling processes in the South African coal industry.
2. Materials and methods
2.1. Study sites and soil sampling
Three opencast coalmines (designated A, B, and C) located in the coal-rich eMalahleni area (24°0′–27°30′ S, 28°15′–32°5′ E) of the Mpumalanga Province of South Africa, were selected for this study (exact locations of mines and names are withheld due to a confidentiality agreement). These mines are actively being mined for coal (bituminous thermal grade coal). Mine B is approximately 48 km from mine C, while mine A was 160 km from mine B and C. The climate of the eMalahleni area is usually warm and moist in the summer, while the winter season is usually cold and dry with frost. This area receives about 750 mm of rainfall annually, 85% of which occurs during the growing season (October to March) (ARC, Citation2016). The vegetation across all sites was predominantly grass species (Digitaria eriantha, Cynodon dactylon and Eragrostis curvula) with an estimated basal cover of 10% – 35% across soil stockpiles. The soil stockpiles at the time of sampling were sparsely vegetated (~10% grass and ~2% forbs cover) and have been stored for at least 5 years. The age of stockpiles was not considered in this study due to possible differences in mining practices as previously reported in a parallel study (Ezeokoli et al. Citation2019). Unmined lands adjacent to mine A (approx. 200–500 m distant) served as ‘reference’. The plant cover (grass species) of the reference site was approximately 60% and predominantly comprised Digitaria eriantha and Cynodon dactylon. At the time of sampling, the reference site was not being utilized for any anthropogenic activities (especially mining). However, due to the proximity of the reference site to infrastructures such as roads and settlements, total absence of anthropogenic influences cannot be excluded.
Soil sampling was conducted during the summer (February), winter (July), and spring (September) seasons of 2015. At each study site, a minimum of five soil stockpiles was randomly sampled per season at depths of 0–20 cm (hereafter referred to as ‘topsoil’) and ˃20 cm (hereafter referred to as ‘subsoil’), respectively, by using a sterile auger. Samples were collected in sterile bags and immediately placed on ice. Samples were appropriately stored prior to the enzyme and microbial analyses detailed below.
2.2. Determination of physical and chemical properties of soil
Physico chemical properties of soils, including texture, bulk density, pH (H2O), cation exchange capacity (CEC), total nitrogen (N), organic carbon (OC), extractable cations (calcium, magnesium, sodium, and potassium) and available phosphorus (Bray 1) were analyzed using standard methods of the Non-Affiliated Soil Analysis Work Committee (Citation1990). Soils were ground and passed through a 2 mm sieve before analyses. Briefly, pH was determined from a 1:2.5 soil-water suspension using a pre-calibrated pH meter (pH 700, Eutech Instruments Pte Ltd, Singapore). The particle size distribution was determined by the Bouyoucos method. Cations and exchangeable cations were determined from soil ammonium acetate (1 M, pH 7) extracts by using Inductively coupled plasma – optical emission spectrometry (ICP-OES). Bulk density (BD) was determined by using a bulk density sampler core of known volume after overnight drying at 105°C.
2.3. Determination of beta-glucosidase and urease activities in soil
Determination of beta-glucosidase (β-D-glucoside glucohydrolase, EC 3.2.1.21) and urease (urea amidohydrolase, EC 3.5.1.5) activities were performed by the methods of Dick et al. (Citation1996) and Kandeler and Gerber (Citation1988). The analyses were performed on topsoil samples only because previous studies have indicated that soil enzymes activities are mostly concentrated in the topsoil (0–15 cm) region (Das and Varma Citation2010). Soil samples were passed through a 2-mm sieve and oven dried at 40°C prior to beta-glucosidase and urease activity assays of beta-glucosidase and urease activities.
2.4. Microbial community analyses
2.4.1. Extraction of total community DNA
Total community DNA was extracted from soil by using ZR Soil Microbe DNA extraction kit (Zymo Research, Irvine, CA, USA) according to the instruction of the manufacturer. DNA integrity and concentration were determined by agarose gel electrophoresis and fluorometric quantification (Qubit 2.0 fluorimeter, Invitrogen, California, USA), respectively, prior to downstream analyses.
2.4.2. PCR-Denaturing gradient gel electrophoresis (PCR-DGGE) analyses of soil microbial communities
PCR-DGGE analysis was performed to obtain a snapshot of the microbial (bacterial and fungal) community in soils. Universal primers 341F (5′-CCTACGGAGGCAGCAG-3′) and 907R (5′ CCGTCAATTCCTTTGAGTTT-3′) were used for amplification of the bacterial 16S rRNA gene, while primers ITS3 (5′-GCATCGATGAAGAACGCAGC-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al. Citation1990) were used for amplification of fungal internally transcribed spacer-2 (ITS2) region. A 40 bp GC clamp was attached to the 5′-end of all forward primers (Muyzer et al. Citation1993). PCR was performed in a C100TM thermal cycler (Bio-Rad Laboratories, CA, USA). Each PCR reaction contained 12.5 µl OneTaq 2X master mix (New England Biolabs, MA, USA), 0.2 µM of each primer, 15 ng DNA template and PCR-grade water to a final reaction volume of 25 µl. PCR conditions for both bacterial 16S rRNA and fungal ITS2 amplifications were exactly as described by Ezeokoli et al. (Citation2016b). Thereafter, PCR products of all samples collected at similar depths from each site during a given season were pooled in equal proportion (per volume basis).
For DGGE, 30 µl of pooled PCR amplicons that included 6x loading dye at 3:1 mixture ratio, were loaded onto a 1 mm thick 6% (for bacteria) or 8% (for fungi) (w/v) polyacrylamide gel of denaturing gradient 40%-60% urea and formamide (100% denaturant is defined as 7 M urea and 40% (v/v) formamide). DGGE was performed on the Dcode™ Universal Mutation Detector System (Bio-Rad, Hercules, CA, USA) as described by Roopnarain et al. (Citation2017). Following DGGE runs, gels were stained in 0.1% (v/v) ethidium bromide solution (in 1 x TAE buffer) and subsequently photographed using the ChemiDocTM MP imaging system (Bio-Rad Laboratories, USA).
2.4.3. Diversity analyses of PCR-DGGE fingerprints
DGGE gel images were subjected to densitometric analyses by using the Gene Tools software version 4.03.1.0 (Syngene, Cambridge, UK) as previously described by Mashiane et al. (Citation2017). An unweighted (absence or presence) and weighted (presence and relative intensity of individual peaks calculated separately for each gel image) similarity matrix generated from band positions was subjected to hierarchical clustering using the average linkage method (UPGMA) in R software version 3.4.0 (R Core Team Citation2013). Furthermore, diversity indices, including Shannon-Weiner diversity index (H´) and species evenness (J´), were estimated based on the assumption that different species (sequence) migrate to different positions in the DGGE gel (Muyzer and Smalla Citation1998). H´ and J´ were computed in the vegan package of R software.
2.4.4. Sequencing, operational taxonomic units (OTUs) clustering and taxonomic assignments of dominant PCR-DGGE bands
Dominant bands on the DGGE gels were excised with a sterile scalpel and eluted of DNA as described previously by Ezeokoli et al. (Citation2016b). Precisely two microlitres of the eluted DNA were used as the template for a PCR ‘re-amplification’ involving the same set of primers stated earlier, but without the 40 bp GC-rich clamp attached to the forward primers. PCR amplicons were further purified and sequenced (Sanger sequencing) at the Central Analytical Facilities of Stellenbosch University, Cape Town, South Africa. Sequence electropherograms were manually inspected and edited for ambiguous nucleotides by using Chromas Lite (v.2.1, Technelysium Pty Ltd, South Brisbane, Australia). Edited sequences were clustered into OTUs at 97% 16S rRNA gene (for bacteria) and ITS2 sequence (for fungi) similarities as described by Ezeokoli et al. (Citation2016a). OTU representatives were assigned into taxonomic ranks through the Ezbiocloud (https://www.ezbiocloud.net/) and UNITE (https://unite.ut.ee/analysis.php) databases for 16S rRNA gene and ITS2 sequences, respectively.
Sequence data have been deposited in the GenBank under the accession numbers KY985473-KY985518 (for bacterial) and MF001318-MF001351 (for fungi).
2.5. Statistical analyses
Soil physico-chemical data for each soil horizon were analyzed separately by the aligned rank transform of non-parametric factorial (site x season) analysis of variance (ANOVA) by using the ARTool package in R software version 3.4.0 (R Core Team Citation2013). Beta-glucosidase and urease activities data were subjected to a two-way (site x season) ANOVA with weighted means by using R software. Prior to ANOVA, enzyme activity data were square root-transformed to near-normality and to meet homoscedastic assumption for parametric tests. After ANOVA, the Tukey honest significant difference (HSD) post hoc test was used to separate significant means at P < 0.05. Pearson correlation was used to test the relationship between enzyme activities (normalized by square root transformation) and soil physico-chemical properties by using the R software. Based on the outcome (i.e., significant differences, P < 0.05) of ANOVA, correlations were conducted across sites, as well as on a site-by-site basis. Furthermore, to test the relationship of the microbial communities with the physico-chemical properties at each sampling depth, a redundancy analysis (RDA) was performed in the vegan package (Oksanen et al. Citation2015) of R software by using the unweighted similarity data matrix generated above (Section 2.3.4) and soil physicochemical properties. Thereafter, the environmental factors (vectors) were then fitted into the RDA model and their significance tested by permutations using the ‘envfit’ function of the vegan package.
3. Results
3.1. Physico-chemical properties of soils
Soils (topsoil and subsoil) were characterized as predominantly sandy loam. However, soils from mine B contained higher clay contents compared to other mines and reference soils (Supplementary Table S1 and Table S2). pH was generally acidic (pH 4.3–5.8) (Table S1 and Table S2). BD was highest in mine C (1.76 ± 0.22 g cm−3) and ranged between 1.34 and 1.62 g cm−3 in the reference site and between 1.22 and 1.80 g cm−3 in soil stockpiles across seasons (Table S1 and Table S2). CEC was between 3.19 and 8.52 cmol kg−1 100 g−1 for reference soils and between 2.39 and 10.94 cmol kg−1 in soil stockpiles. OC ranged from 0.46 to 1.71% in reference soils, and from 0.50 to 4.40% in soil stockpiles. In both topsoils and subsoils, total N of the reference soil was lower than in soil stockpiles of mine B and mine C (Table S1 and Table S2). Consequently, the C: N ratio was lower in most stockpiles (particularly mine B and mine C) compared to the reference site.
3.2. Beta-glucosidase and urease activities in soils
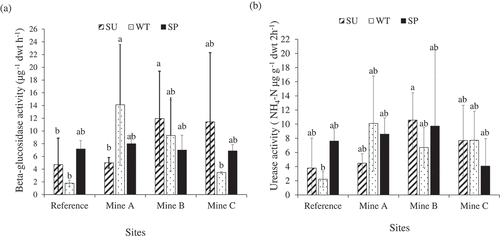
Two-way ANOVA revealed that site and the interaction between factors (site and season) had significant effects (P < 0.05) on beta-glucosidase and urease activities (). In winter, the differences observed in the beta-glucosidase between mine C and mine A and between reference site and mine A are significant (factors interaction effects, Tukey HSD P < 0.05). By contrast, only the differences in urease activity between the winter values of the reference site and summer values of mine B was significant (interaction effects, Tukey HSD P < 0.05). There were more significant differences (based on factors interaction) in beta-glucosidase activity amongst treatments () than in urease activity (), suggesting that beta-glucosidases may be more sensitive to environmental factors compared to ureases. Overall, with the exception of the spring season for all sites, beta-glucosidase activity in stockpile soils was mostly higher than those of the reference soils (). This suggests a higher biological activity in the stockpiled soils in response to availability of higher (compared to unmined soils) organic carbon in most of the soil stockpiles (See Table S1). Similarly, among sites, the urease activity was mostly higher (except in the spring season) in stockpile soils than in unmined soils (). Across sites, beta-glucosidase activity was only positively correlated with C: N in mine A (Pearson’s correlation coefficient, r = 0.91, P = 0.013) (data not shown), while urease activity was significantly correlated with the sum of exchangeable cations (S-V) only in the reference site (Pearson’s correlation coefficient, r = 0.57, P = 0.021) (data not shown).
Figure 1. Mean enzymatic activities in soils (a) Beta-glucosidase activity (b) Urease activity. Sample size (N) ≥ 5. Bars with different letters are significantly different based on the effect of interactions between sites and season (Tukey HSD, P < 0.05). Error bars are standard deviations from means. SU, summer; WT, winter; SP, spring
3.3. PCR-DGGE profile of microbial communities in soil across seasons
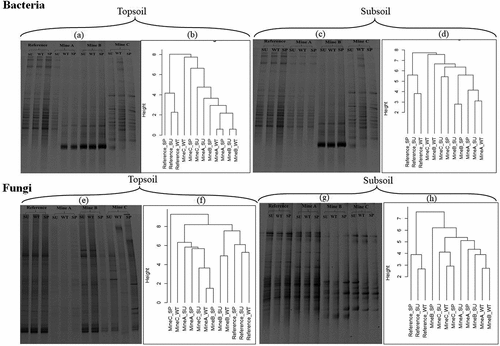
The PCR-DGGE profiles shown in suggest that bacterial and fungal diversities of unmined soil are higher than those of stockpiles in the topsoil horizon (see also Supplementary Table S3 for some alpha diversity indices). In the topsoil, the least microbial diversity was observed in Mine A: with bacterial diversity lowest in the spring while fungal diversity was lowest in winter. Across seasons, the soil microbial diversity did not vary greatly within the unmined reference soil ( and Table S3), whereas varying levels of differences were observed in bacterial and fungal diversities amongst seasons in each of the coal mining sites (Table S3). Based on the associations depicted by the weighted hierarchical cluster dendrograms in , microbial community structure of the unmined reference soil is dissimilar to those of stockpiles in all three seasons. Within the reference site, summer and winter microbial community structures were more similar compared to the spring season in both topsoil and subsoil (, , and ). While microbial communities were mostly clustered based on site, close associations were observed in microbial community structure between some sites, such as the subsoil fungal community structures of mine A and mine B in winter ().
Figure 2. PCR-DGGE gel image and weighted hierarchical cluster dendrogram of microbial communities in soils. (a-b) Bacterial 16S rRNA gene diversity in topsoils (c-d) Bacterial 16S rRNA gene diversity in subsoils. (e-f) Fungal ITS2 gene diversity in topsoils. (g-h) Fungal ITS2 gene diversity in subsoils. SU, summer; WT, winter; SP, spring See also Supplementary Fig. S3 for weighted hierarchical cluster dendrogram
3.4. Taxonomic affiliations of OTUs obtained from excised PCR-DGGE bands
A total of 44 bacterial and 33 fungal sequences were successfully (without ambiguous nucleotide base positions) obtained from the excised bands. These sequences yielded seven bacterial and 19 fungal OTUs (). Taxonomically, the bacterial OTUs spanned 2 phyla [Firmicutes (4 OTUs) and Proteobacteria (3 OTUs)] and four genera viz Bacillus (3 OTUs), Pseudomonas (2 OTUs), Azomonas (1 OTU) and Lysinibacillus (1 OTU) ( & Supplementary Fig. S1). All 19 fungal OTUs belonged to the Ascomycota phylum and included species of Alternaria, Aureobasidium, Austroplaca, Cenangium, Claviceps, Curvularia, Dendroclathra, Diaporthe, Fusarium, Helotiales, Macrohilum, Neoscytalidium, Phialocephala, Phoma, Pyrrhospora, Thyronectra and Valdensinia ( & Fig. S2).
Table 1. Taxonomic affiliations (closest relative) and economic importance of operational taxonomic units obtained from sequenced excised DGGE bands
3.6. Relationship between soil physico-chemical properties and microbial communities
The RDA model for the species-environmental relationship for topsoil as depicted in the biplot of is not significant (P = 0.725), suggesting that physicochemical properties in the topsoil did not influence the microbial community of the topsoil in general (Table S4). In contrast, the RDA model for the species-environmental constraints in the subsoil is significant (P = 0.011). Specifically, in the subsoil, pH, organic carbon, total nitrogen and phosphorus contents of the subsoil significantly (P < 0.05) influenced the microbial community of the subsoil (Table S4). In the fitted model (correlations of environmental variables with both axis of the RDA model), the correlation of the first two axes with the silt content is significant (R2 = 0.566, P = 0.027) (Table S5), suggesting that the silt content is a predictor of topsoil microbial community. In the subsoil, total nitrogen (R2 = 0.544, P = 0.039) and phosphorus (R2 = 0.559; P = 0.030) were the predictors of the soil microbial community structure (Table S5).
Figure 3. Redundancy analysis (RDA) biplot showing the relationship between soil physico-chemical properties and unweighted PCR-DGGE profile of microbial communities in soils. (a) Topsoil. (b) Subsoil. In both RDA plots, black dots are indicative of ‘microbial species’ in the context of combined (per soil horizon) number of different bands in the bacterial and fungal PCR-DGGE image of . Wherever present in site names, ‘SU’, ‘SP’ and ‘WT’ denotes summer, spring and winter samples. SV, sum of the exchangeable cations (Ca, Mg, Na, and K). See also Table S4 and S5 for the significance of the environmental factors in the RDA model
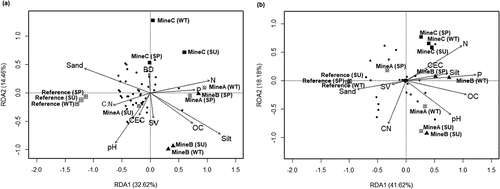
Based on the RDA biplot of , the microbial community of the topsoil and subsoil in the reference and mine C sites are least influenced by physicochemical parameters. However, in mine A and mine B, selected physico-chemical properties influence seasonal variations in the soil microbial communities. For example, pH, and cation exchange capacity of the topsoil strongly influenced the microbial community of mine A in summer (), while in winter and spring, phosphorus and total nitrogen content of soil influenced microbial communities of mine A. The biggest predictor of the microbial communities in mine B subsoils during winter and spring is phosphorus ().
4. Discussion
With respect to the hypothesis of this study, the pattern of differences observed in the physico-chemical properties (except for the total exchangeable cations in the topsoil horizon) of soils between unmined/reference site and stockpiles did not clearly suggest that stockpiled soils were in less adequate physico-chemical conditions. However, the differences appeared to be site-specific and variable across seasons (Table S1 and Table S2). Ghose (Citation2004) reported drastic alterations in quality of topsoil dumps generated during opencast coal mining and that such alteration in soil quality increased with duration of storage. The high bulk density in some soil stockpiles soils may indicate high soil compaction attributable to heavy machineries, such as those used during the stripping and stockpiling process (Shrestha et al. Citation2005). Within a post-mining revegetation context, such high bulk density soils may pose restrictions on the growth of deep-rooted plants (Ghose Citation2004; Shrestha et al. Citation2005; Mensah et al. Citation2015). Higher organic carbon in coal mine stockpiles compared to the reference site may be due to the deposition of fossil (coal) dusts on the soil stockpile surfaces (Čížková et al. Citation2018). The observed acidic pH in all soils may be due to leaching of basic cations, as well as from the oxidation of sulfide minerals and acid mine drainage (Katzur and Haubold-Rosar Citation1996; Pietrzykowski Citation2014).
The observed higher beta-glucosidase activity in stockpile compared to unmined soils is similar to the findings of Claassens et al. (Citation2012) who observed that the beta-glucosidase activity in coal discard and asbestos sites had a higher maximum value compared to reference sites. Similar observations of higher urease activities in stockpile soils may be attributed to the relatively higher total nitrogen present in most stockpile soils (Table S1). Generally, urease activity correlates with soil nitrogen, especially when a source of urea is available (Meyer et al. Citation2015).
According to Das and Varma (Citation2010), enzyme activity in soil ecosystems is mostly dependent on organic carbon levels, soil type, soil composition and microbial community diversity and function. For example, numerous authors have reported that beta-glucosidase activity is sensitive to soil pH and management practices (Tabatabai and Dick Citation2002; Makoi and Ndakidemi Citation2008; Meyer et al. Citation2015). In contrast, Meyer et al. (Citation2015) observed that beta-glucosidase and urease activities correlated significantly with soil carbon, NO3− and pH but that the correlations between beta-glucosidase and soil carbon, NO3− and pH were weaker compared to correlations with urease. Furthermore, standard deviations from the mean beta-glucosidase and urease activities were higher in soil stockpiles than in reference soils, suggesting higher intra-site variations within coal mines. Similarly, Claassens et al. (Citation2012) observed higher variations in enzyme activities on disturbed sites (coal discard sites) compared to undisturbed reference sites. The higher variation may be attributable to the mixtures of soil horizons (physically observed on-site) and intra-site variations in ages of soil stockpiles which was not considered in the design of this study (due to of lack of comprehensive information on stockpile ages).
The observed highest number of different bands (‘species richness’) in the topsoil and subsoil of reference soils compared to stockpile soils at all three sampling seasons (Table S3), suggests soil disturbances during stockpiling impair microbial diversity. Similar observations in the microbial diversity of disturbed and undisturbed sites have been previously reported (Fresquez and Aldon Citation1984; Harris et al. Citation1989, Citation1993). The variation of microbial communities in response to environmental conditions is well documented (Habekost et al. Citation2008; Baldrian et al. Citation2010; López-Mondéjar et al. Citation2015). For example, López-Mondéjar et al. (Citation2015) reported that the spring and summer bacterial community of temperate deciduous soils significantly differed from those of the autumn and winter seasons. The changes in microbial diversity across seasons may be due to different supply of nutrients (resource availability) as a consequence of certain seasonal (environmental) factors such as temperature and moisture (Rasche et al. Citation2011; Fekete et al. Citation2012). In the soil ecosystem, these factors influence the allocation of photosynthates to the soil by roots of primary producers, the inputs of fresh litter, and above- or below-ground biomass production (López-Mondéjar et al. Citation2015). Invariably, the alteration in plant-derived exudates and biomass influences microbial community structure and composition of the soil and may give rise to niche partitioning of microorganisms.
The biodiversity of soil microbial communities is vital to the sustainability of soil ecosystem functioning. The detection of specific phylotypes at only certain depths suggests a vertical niche differentiation of species in the soil stockpiles (Hansel et al. Citation2008). Generally, vertical and seasonal variations in nutrient and physical parameters predispose the selection (selective pressure) of species with different adaptability (e.g., adaptation to energy and nutrient limiting conditions) and capabilities (e.g., nutrient mineralization) along the soil physico-chemical differentiation profile (Hansel et al. Citation2008; Rasche et al. Citation2011; López-Mondéjar et al. Citation2015; Stone et al. Citation2015). Niche differentiation of species in the soil ecosystem is important for the sustainability of soil health through biological processes that regulate, amongst others, nutrient cycling (Lennon et al. Citation2012).
Although the partial 16S rRNA gene sequences used for taxonomic identification of bacterial OTUs may not be sufficient to delineate sequences into the species taxonomic rank, reference to the closest relative at the species level is used herein to facilitate discussions towards potential roles of these species in agroecosystems and for comparison with the scientific literature. The observation of dominant phylotypes of the Firmicutes and Proteobacteria phyla agrees with observations made in previous soil microbial ecology studies (Lennon et al. Citation2012; López-Mondéjar et al. Citation2015). The dominance of Bacillus spp. in these soils suggest that these species are well-adapted to the prevailing nutrient-limiting conditions of the soils and are likely contributors to ecosystem processes in these soils. Phylogenetically similar species of Bacillus detected in this study (taxonomic clade) have been associated with mining soils (Jamal et al. Citation2016; Oladipo et al. Citation2018). Bacillus spp. are functionally diverse and play roles in the mineralization of plant-derived materials, plant-growth promotion, humus formation, biocontrol of plant pests and the degradation of hydrocarbons in the soil (Garbeva et al. Citation2003; Mandic-Mulec and Prosser Citation2011). For example, strains of Bacillus amyloliquefaciens have been shown to promote the yield of tomato (Gül et al. Citation2008) and improve the tolerance of wheat to heat stress (El-Daim et al. Citation2014). Other phylotypes whose strains are known to have ecological relevance in the agroecosystem include Lysinibacillus (e.g., L. macroides) and Azomonas (e.g., A. macrocytogenes) (). Similarly, some Pseudomonas spp. are able to demineralize coal (Singh et al. Citation2016), degrade polychlorinated biphenyl and polycyclic aromatic hydrocarbons (Nam et al. Citation2014; Bello-Akinosho et al. Citation2016), perform soil ecological functions (Naseby and Lynch Citation1999), promote plant-growth (Wicaksono et al. Citation2017) and participate in the remediation of metal-contaminated soils (Wasi et al. Citation2013; Oladipo et al. Citation2018).
Interestingly, most of the fungal species detected in the present study are known pathogens of plants (). The presence of these pathogens in the soil suggests that these soils may also serve as reservoirs for economically important plant pathogens. Hence, within the context of post-mining land use, especially for agriculture, the use of these soils may have implications on the productivity of certain crops if adequate disease management practices are not implemented. In contrast, other fungal strains such as Aureobasidium pullulans, Phialocephala humicola, Phoma herbarum and Sordariomycetes sp. detected in this study have been reported to have beneficial importance to industry and agriculture (Neumann and Boland Citation2002; Ferreira-Pinto et al. Citation2006; Mahmoud and Narisawa Citation2013) ().
5. Conclusion
While there were no clear differences between the physico-chemical properties of soil stockpiles and unmined sites, the differences in enzyme activities (mostly beta-glucosidase) and microbial diversity between soil stockpiles and unmined sites suggests that biological components of the soil are quite sensitive to soil management practice and/or disturbance. The impaired (compared to an unmined site) microbial community structure and diversity observed in stockpiles may have negative implications for soil biological processes driven by microbes, especially those that are critical for nutrient cycling and ecosystem sustainability. More importantly, the alteration observed in microbial communities of soil stockpiles in comparison to the reference site may impair post-mining land use capability. In addition, several microbial species detected in both reference and stockpile soils are closely related to characterized phytobeneficial species. Thus, these soils may serve as sources for the bioprospection of microbes of both agricultural and industrial applications. Further studies are required to investigate the influence of storage durations on the properties and biological parameters of stockpile soils.
figure_S2_new.pdf
Download PDF (242.6 KB)figure_S1_new.pdf
Download PDF (151.9 KB)Supplementary_Materials.docx
Download MS Word (42.1 KB)Acknowledgments
We sincerely thank Nicky Mushia and Siseko Mkula for assisting with soil sampling. The statistical and technical contributions of Deidre van Wyk and members of the Microbiology and Environmental Biotechnology Research Group of the Agricultural Research Council are hereby acknowledged.
Disclosure statement
The authors have no conflict of interests to disclose.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Abdul-Kareem AW, McRae SG 1984: The effects on topsoil of long-term storage in stockpiles. Plant Soil, 76, 357–363. doi:10.1007/BF02205593
- Adeleke R, Cloete T, Khasa D 2012: Culturable microorganisms associated with Sishen iron ore and their potential roles in biobeneficiation. World J. Microbiol. Biotechnol., 28, 1057–1070. doi:10.1007/s11274-011-0904-2
- Adeleke R, Nwangburuka C, Oboirien B 2017: Origins, roles and fate of organic acids in soils: A review. S. Afr. J. Bot., 108, 393–406. doi:10.1016/j.sajb.2016.09.002
- Annesi T, Luongo L, Vitale S, Galli M, Belisario A 2016: Characterization and pathogenicity of Phomopsis theicola anamorph of Diaporthe foeniculina causing stem and shoot cankers on sweet chestnut in Italy. J. Phytopathol., 164, 412–416. doi:10.1111/jph.2016.164.issue-6
- ARC (Agricultural Research Council) 2016: Geo-Informatics Database, Agricultural Research Council, Pretoria, South Africa.
- Baldrian P, Merhautová V, Cajthaml T, Petránková M, Šnajdr J 2010: Small-scale distribution of extracellular enzymes, fungal, and bacterial biomass in Quercus petraea forest topsoil. Biol. Fert. Soil, 46, 717–726. doi:10.1007/s00374-010-0478-4
- Bassimba D, Mira J, Baixauli C, Vicent A 2012: First report of Alternaria petroselini causing leaf blight of fennel in Spain. Plant Dis., 96, 907. doi:10.1094/PDIS-01-12-0059-PDN
- Bello-Akinosho M, Makofane R, Adeleke RA, Thantsha M, Pillay M, Chirima GJ 2016: Potential of polycyclic aromatic hydrocarbon-degrading bacterial isolates to contribute to soil fertility. BioMed. Res. Int., 2016, 10. doi:10.1155/2016/5798593
- Bending GD, Turner MK, Rayns F, Marx M-C, Wood M 2004: Microbial and biochemical soil quality indicators and their potential for differentiating areas under contrasting agricultural management regimes. Soil Biol. Biochem., 36, 1785–1792. doi:10.1016/j.soilbio.2004.04.035
- Cardoso EJBN, Vasconcellos RLF, Bini D, Miyauchi MYH, Santos C, Alves PRL, Paula A, Nakatani AS, Pereira J, Nogueria MA 2013: Soil health: looking for suitable indicators. What should be considered to assess the effects of use and management on soil health? Sci. Agric., 70, 274–289. doi:10.1590/S0103-90162013000400009
- Carter L, Leslie J, Webster R 2008: Population structure of Fusarium fujikuroi from California rice and water grass. Phytopathology, 98, 992–998. doi:10.1094/PHYTO-98-9-0992
- Choi I, Cho S, Park J, Shin H-D 2014: First report of leaf spot caused by a Phoma sp. on Schisandra chinensis in Korea. Plant Dis., 98, 157. doi:10.1094/PDIS-05-13-0489-PDN
- Čížková B, Woś B, Pietrzykowski M, Frouz J 2018: Development of soil chemical and microbial properties in reclaimed and unreclaimed grasslands in heaps after opencast lignite mining. Ecol. Eng., 123, 103–111. doi:10.1016/j.ecoleng.2018.09.004
- Claassens S, van Rensburg PJ, Liebenberg D, van Rensburg L 2012: A comparison of microbial community function and structure in rehabilitated asbestos and coal discard sites. Water Air Soil Pollut., 223, 1091–1100. doi:10.1007/s11270-011-0927-1
- Claassens S, van Rensburg PJ, Maboeta M, van Rensburg L 2008: Soil microbial community function and structure in a post-mining chronosequence. Water Air Soil Pollut., 194, 315–329. doi:10.1007/s11270-008-9719-7
- Coetzee M 2016: Organic matter contribution of ameliorants to reclaimed surface-mined soil and carbon storage. Doctoral dissertation, pp 1–121. University of Pretoria, Pretoria.
- Das SK, Varma A 2010: Role of enzymes in maintaining soil health. In Soil Enzymology, Ed. Shukla G, Varma GA, pp. 25–42. Springer, Berlin.
- Dick RP, Breakwell DP, Turco RF 1996: Soil enzyme activities and biodiversity measurements as integrative microbiological indicators. In Methods of Assessing Soil Quality, Ed. Doran JW, Jones AJ, pp. 247–271. Soil Science Society of America, Madison, Wisconsin.
- Doran JW, Parkin TB 1994: Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment, Ed. Doran JW, Coleman DC, Bezdicek DF, Stewart BA, pp. 3–21. American Society of Agrnomy Special Publication, Madison, Wisconsin.
- Dose HL, Fortuna A-M, Cihacek LJ, Norland J, DeSutter TM, Clay DE, Bell J 2015: Biological indicators provide short term soil health assessment during sodic soil reclamation. Ecol. Indic., 58, 244–253. doi:10.1016/j.ecolind.2015.05.059
- El-Daim IAA, Bejai S, Meijer J 2014: Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil, 379, 337–350. doi:10.1007/s11104-014-2063-3
- Ezeokoli O, Gupta A, Popoola T, Bezuidenhout C 2016a: Molecular analysis of bacterial community dynamics during the fermentation of soy-daddawa condiment. Food Sci. Biotech., 25, 1081–1086. doi:10.1007/s10068-016-0174-8
- Ezeokoli OT, Gupta AK, Mienie C, Popoola TO, Bezuidenhout CC 2016b: PCR-denaturing gradient gel electrophoresis analysis of microbial community in soy-daddawa, a Nigerian fermented soybean (Glycine max (L.) Merr.) condiment. Int. J. Food Microbiol., 220, 58–62. doi:10.1016/j.ijfoodmicro.2016.01.003
- Ezeokoli OT, Nwangburuka CC, Adeleke RA, Roopnarain A, Paterson DG, Maboeta MS, Bezuidenhout CC 2019: Assessment of arbuscular mycorrhizal fungal spore density and viability in soil stockpiles of South African opencast coal mines. S. Afr. J. Plant Soil, 36, 91–99. doi:10.1080/02571862.2018.1537011
- Faber JH, Creamer RE, Mulder C, Römbke J, Rutgers M, Sousa JP, Stone D, Griffiths BS 2013: The practicalities and pitfalls of establishing a policy‐relevant and cost‐effective soil biological monitoring scheme. Integr. Environ. Assess. Manag., 9, 276–284. doi:10.1002/ieam.1398
- Fekete I, Kotroczó Z, Varga C, Hargitai R, Townsend K, Csányi G, Várbiró G 2012: Variability of organic matter inputs affects soil moisture and soil biological parameters in a European detritus manipulation experiment. Ecosystems, 15, 792–803. doi:10.1007/s10021-012-9546-y
- Ferreira-Pinto M, Moura-Guedes M, Barreiro M, Pais I, Santos M, Silva M 2006: Aureobasidium pullulans as a biocontrol agent of blue mold in “Rocha” pear. Commun. Agric. App. Biol. Sci., 71, 973–978.
- Fließbach A, Oberholzer H-R, Gunst L, Mäder P 2007: Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ., 118, 273–284. doi:10.1016/j.agee.2006.05.022
- Fresquez PR, Aldon EF 1984: Distribution of Fungal Genera in Stockpiled Topsoil and Coal Mine Spoil Overburden, Vol. 447. USDA Forest Service, Rocky Mountain Forest and Range Experiment Station, Fort Collins, Colorado.
- Garbeva P, Van Veen J, Van Elsas J 2003: Predominant Bacillus spp. in agricultural soil under different management regimes detected via PCR-DGGE. Microb. Ecol., 45, 302–316. doi:10.1007/s00248-002-2034-8
- García-Ruiz R, Ochoa V, Vinegla B, Hinojosa MB, Pena-Santiago R, Liébanas G, Linares JC, Carreira JA 2009: Soil enzymes, nematode community and selected physico-chemical properties as soil quality indicators in organic and conventional olive oil farming: influence of seasonality and site features. App. Soil Ecol., 41, 305–314. doi:10.1016/j.apsoil.2008.12.004
- Ghose MK 2004: Effect of opencast mining on soil fertility. J. Sci. Ind. Res., 63, 1006–1009.
- Gül A, Kidoglu F, Tüzel Y 2008: Effects of nutrition and Bacillus amyloliquefaciens on tomato (Solanum lycopersicum, L.) growing in perlite. Span. J. Agric. Res., 6, 422–429. doi:10.5424/sjar/2008063-335
- Habekost M, Eisenhauer N, Scheu S, Steinbeiss S, Weigelt A, Gleixner G 2008: Seasonal changes in the soil microbial community in a grassland plant diversity gradient four years after establishment. Soil Biol. Biochem., 40, 2588–2595. doi:10.1016/j.soilbio.2008.06.019
- Hansel CM, Fendorf S, Jardine PM, Francis CA 2008: Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. App. Environ. Microbiol., 74, 1620–1633. doi:10.1128/AEM.01787-07
- Harris J, Birch P, Short K 1989: Changes in the microbial community and physico‐chemical characteristics of topsoils stockpiled during opencast mining. Soil Use Manage., 5, 161–168. doi:10.1111/j.1475-2743.1989.tb00778.x
- Harris J, Birch P, Short K 1993: The impact of storage of soils during opencast mining on the microbial community: a strategist theory interpretation. Restor. Ecol., 1, 88–100. doi:10.1111/j.1526-100X.1993.tb00014.x
- Jamal Q, Ahmed I, Rehman SU, Abbas S, Kim KY, Anees M 2016: Isolation and characterization of bacteria from coal mines of Dara Adam Khel, Pakistan. Geomicrobiol. J., 33, 1–9. doi:10.1080/01490451.2014.964886
- Kandeler E, Gerber H 1988: Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fert. Soils, 6, 68–72. doi:10.1007/BF00257924
- Katzur J, Haubold-Rosar M 1996: Amelioration and forestation of sulfurous mine soils in Lusatia (Eastern Germany). Water Air Soil Pollut., 91, 17–32. doi:10.1007/BF00280920
- Khadka R 2016: First Report of Curvularia trifolii causing leaf spot on Trifolium alexandrinum (Berseem clover) in Nepal. Plant Dis., 100, 1246. doi:10.1094/PDIS-07-13-0720-PDN
- Khan AL, Shahzad R, Al-Harrasi A, Lee I-J 2017: Endophytic microbes: a resource for producing extracellular enzymes. In Endophytes: crop Productivity and Protection, Ed. Maheshwari DK, Annapurna K, Vol. 2 pp. 95–110. Springer International Publishing, Cham, Switzerland.
- Lennon JT, Aanderud ZT, Lehmkuhl B, Schoolmaster DR 2012: Mapping the niche space of soil microorganisms using taxonomy and traits. Ecology, 93, 1867–1879.
- López-Mondéjar R, Voříšková J, Větrovský T, Baldrian P 2015: The bacterial community inhabiting temperate deciduous forests is vertically stratified and undergoes seasonal dynamics. Soil Biol. Biochem., 87, 43–50. doi:10.1016/j.soilbio.2015.04.008
- Mahmoud RS, Narisawa K 2013: A new fungal endophyte, Scolecobasidium humicola, promotes tomato growth under organic nitrogen conditions. PLoS ONE, 8, e78746. doi:10.1371/journal.pone.0078746
- Makoi JH, Ndakidemi PA 2008: Selected soil enzymes: examples of their potential roles in the ecosystem. Afr. J. Biotechnol., 7, 181–191.
- Mandal A, Patra AK, Singh D, Swarup A, Masto RE 2007: Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresour. Technol., 98, 3585–3592. doi:10.1016/j.biortech.2006.11.027
- Mandic-Mulec I, Prosser JI 2011: Diversity of endospore-forming bacteria in soil: characterization and driving mechanisms. In Endospore-Forming Soil Bacteria, Ed. Logan N, Vos P, pp. 31–59. Springer, Heidelberg, Berlin.
- Mashiane RA, Ezeokoli OT, Adeleke RA, Bezuidenhout CC 2017: Metagenomic analyses of bacterial endophytes associated with the phyllosphere of a Bt maize cultivar and its isogenic parental line from South Africa. World J. Microbiol. Biotechnol., 33, 80. doi:10.1007/s11274-017-2249-y
- Mensah AK, Mahiri IO, Owusu O, Mireka OD, Wireko I, Kissi EA 2015: Environmental impacts of mining: A study of mining communities in Ghana. App. Ecol. Environ. Sci., 3, 81–94.
- Meyer AH, Wooldridge J, Dames JF 2015: Variation in urease and β-glucosidase activities with soil depth and root density in a ‘Cripp’s Pink’/M7 apple orchard under conventional and organic management. S. Afr. J. Plant Soil, 32, 227–234. doi:10.1080/02571862.2015.1053155
- Millar C 1974: Decomposition of coniferous leaf litter. In Biology of Plant Litter Deomposition, Ed. Dickinson CH, Pugh GJF, Vol. 1 pp. 105–128. Academic Press Inc., London.
- Muyzer G, De Waal EC, Uitterlinden AG 1993: Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. App. Environ. Microbiol., 59, 695–700.
- Muyzer G, Smalla K 1998: Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Van Leeuwenhoek, 73, 127–141.
- Nam I-H, Chon C-M, Jung K-Y, Kim J-G 2014: Biodegradation of biphenyl and 2-chlorobiphenyl by a Pseudomonas sp. KM-04 isolated from PCBs-contaminated coal mine soil. Bull. Environ. Contam. Toxicol., 93, 89–94. doi:10.1007/s00128-014-1286-6
- Naseby D, Lynch J 1999: Effects of Pseudomonas fluorescens F113 on ecological functions in the pea rhizosphere are dependent on pH. Microb. Ecol., 37, 248–256.
- Nekoduka S, Kanematsu S, Tanaka K, Harada Y, Sano T 2012: Valdensia leaf blight of highbush blueberry caused by Valdensinia heterodoxa, a new fungal disease in Japan. J. Gen. Plant Pathol., 78, 151–159. doi:10.1007/s10327-012-0373-y
- Neumann S, Boland GJ 2002: Influence of host and pathogen variables on the efficacy of Phoma herbarum, a potential biological control agent of Taraxacum officinale. Can. J. Bot., 80, 425–429. doi:10.1139/b02-024
- Nkuekam GK, Cowan DA, Valverde A 2018: Arable agriculture changes soil microbial communities in the South African grassland biome. S. Afr. J. Sci., 114, 1–7.
- Non-Affiliated Soil Analysis Work Committee 1990: Handbook of Standard Soil Testing Methods for Advisory Purposes, Soil Science Society of South Africa, Pretoria.
- Nouri MT, Lawrence DP, Yaghmour M, Michailides T, Trouillas F 2018: Neoscytalidium dimidiatum causing canker, shoot blight and fruit rot of almond in California. Plant Dis., 102, 1638–1647. doi:10.1094/PDIS-12-17-1967-RE
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H 2015: Package ‘Vegan’. Community Ecology Package, Version, 2. https://CRAN.R-project.org/package=vegan.
- Oladipo OG, Ezeokoli OT, Maboeta MS, Bezuidenhout JJ, Tiedt LR, Jordaan A, Bezuidenhout CC 2018: Tolerance and growth kinetics of bacteria isolated from gold and gemstone mining sites in response to heavy metal concentrations. J. Environ. Manage., 212, 357–366. doi:10.1016/j.jenvman.2018.01.038
- Page WJ, Collinson SK 1987: Characterization of Azomonas macrocytogenes strains isolated from Alberta soils. Can. J. Microbiol., 33, 830–833. doi:10.1139/m87-145
- Paterson DG, Mushia MN, Mkula SD 2019: Effects of stockpiling on selected properties of opencast coal mine soils. S. Afr. J. Plant Soil, 36, 101–106. doi:10.1080/02571862.2018.1493161
- Pietrzykowski M 2014: Soil quality index as a tool for Scots pine (Pinus sylvestris) monoculture conversion planning on afforested, reclaimed mine land. J. Forest. Res., 25, 63–74. doi:10.1007/s11676-013-0418-x
- R Core Team 2013: R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria.
- Rasche F, Knapp D, Kaiser C, Koranda M, Kitzler B, Zechmeister-Boltenstern S, Richter A, Sessitsch A 2011: Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. Isme J., 5, 389–402. doi:10.1038/ismej.2010.138
- Roopnarain A, Mukuba M, Adeleke RA, Moeletsi M 2017: Biases during DNA extraction affect bacterial and archaeal community profile of anaerobic digestion samples 3. Biotech., 7, 375.
- Sheoran V, Sheoran A, Poonia P 2010: Soil reclamation of abandoned mine land by revegetation: a review. Int. L J. Soil Sed. Water, 3, 13.
- Shrestha G, Stahl PD, Ingram L 2005: Influence of reclamation management practices on soil bulk density and infiltration rates on surface coal mine lands in Wyoming. Paper presented at the National Meeting of the American Society of Mining and Reclamation, June 19–23, 2005, Breckenridge, Colorado.
- Singh PK, Singh AL, Singh MP, Naik A, Singh D, Rai S, Kumar A 2016: Demineralization of Gondwana coal with Pseudomonas mendocina strain B6-1: a case study of coal from Gopinathpur top and bottom seams of Mugma mine, Dhanbad, Jharkhand (India). Int. J. Coal Sci. Technol., 3, 235–245. doi:10.1007/s40789-016-0128-z
- Six J, Bossuyt H, Degryze S, Denef K 2004: A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till. Res., 79, 7–31. doi:10.1016/j.still.2004.03.008
- Stone MM, Kan J, Plante AF 2015: Parent material and vegetation influence bacterial community structure and nitrogen functional genes along deep tropical soil profiles at the Luquillo Critical Zone Observatory. Soil Biol. Biochem., 80, 273–282. doi:10.1016/j.soilbio.2014.10.019
- Strobel G, Singh SK, Riyaz-Ul-Hassan S, Mitchell AM, Geary B, Sears J 2011: An endophytic/pathogenic Phoma sp. from creosote bush producing biologically active volatile compounds having fuel potential. FEMS Microbiol. Lett., 320, 87–94. doi:10.1111/j.1574-6968.2011.02297.x
- Strohmayer P 1999: Soil stockpiling for reclamation and restoration activities after mining and construction. Restor. Reclam. Rev., 4, 1–6.
- Swart H 1988: Australian leaf-inhabiting fungi. XXVI. Some noteworthy coelomycetes on Eucalyptus. Trans. Br. Mycol. Soc., 90, 279–291. doi:10.1016/S0007-1536(88)80100-1
- Tabatabai MA, Dick WA 2002: Enzymes in soil. In Enzymes in the Environment: activity, Ecology and Applications, Ed. Burns RG, Dick RP, pp. 567–596. Marcel Dekker, New York.
- Tinker P 1984: The role of microorganisms in mediating and facilitating the uptake of plant nutrients from soil. Plant Soil, 76, 77–91. doi:10.1007/BF02205569
- Tudzynski P, Scheffer J 2004: Claviceps purpurea: molecular aspects of a unique pathogenic lifestyle. Mol. Plant Pathol., 5, 377–388. doi:10.1111/j.1364-3703.2004.00237.x
- Vakalounakis D, Kavroulakis N, Lamprou K 2016: Occurrence of leaf spot caused by Alternaria tenuissima on Aloe barbadensis in Greece. Plant Dis., 100, 1015. doi:10.1094/PDIS-07-15-0803-PDN
- van der Heijden MG, Bardgett RD, van Straalen NM 2008: The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett., 11, 296–310. doi:10.1111/j.1461-0248.2007.01139.x
- van Veen J, Kuikman P 1990: Soil structural aspects of decomposition of organic matter by micro-organisms. Biogeochem., 11, 213–233. doi:10.1007/BF00004497
- van Wyk DA, Adeleke R, Rhode OH, Bezuidenhout CC, Mienie C 2017: Ecological guild and enzyme activities of rhizosphere soil microbial communities associated with Bt‐maize cultivation under field conditions in North West Province of South Africa. J. Basic Microbiol., 57, 781–792. doi:10.1002/jobm.201700043
- Vikrant P, Verma K, Rajak R, Pandey A 2006: Characterization of a phytotoxin from Phoma herbarum for management of Parthenium hysterophorus L. J. Phytopathol., 154, 461–468. doi:10.1111/jph.2006.154.issue-7-8
- Wasi S, Tabrez S, Ahmad M 2013: Use of Pseudomonas spp. for the bioremediation of environmental pollutants: a review. Environ. Monit. Assess., 185, 8147–8155. doi:10.1007/s10661-013-3163-x
- White TJ, Bruns T, Lee S, Taylor J 1990: Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: a Guide to Methods and Applications, Ed. Innis MA, Gelfand DH, Sninsky JJ, White TJ, pp. 315–322. Academic Press Inc., New York.
- Wicaksono WA, Jones EE, Sansom CE, Perry NB, Monk J, Black A, Ridgway HJ 2017: Indigenous bacteria enhance growth and modify essential oil content in Leptospermum scoparium (mānuka). N. Zeal. J. Bot., 55, 306–317. doi:10.1080/0028825X.2017.1330272
- Xiang N, Lawrence KS, Kloepper JW, Donald PA, McInroy JA, Lawrence GW 2017: Biological control of Meloidogyne incognita by spore-forming plant growth-promoting rhizobacteria on cotton. Plant Dis., 101, 774–784. doi:10.1094/PDIS-09-16-1369-RE
- Zijlstra JD, Van’t Hof P, Baar J, Verkley GJ, Summerbell RC, Paradi I, Braakhekke WG, Berendse F 2005: Diversity of symbiotic root endophytes of the Helotiales in ericaceous plants and the grass, Deschampsia flexuosa. Stud. Mycol., 53, 147–162. doi:10.3114/sim.53.1.147
