ABSTRACT
Pineapple (Ananas comosus [L.] Merrill) is a commercial crop commonly planted in strongly acid soils in Taiwan. However, aluminum (Al) in strongly acid soils may be toxic and inhibits the root growth of various pineapples. This study evaluated the cell morphology, 1,3-β-glucan, and malondialdehyde (MDA) of the root apex cells of Al-resistant Cayenne and nonresistant Tainung No. 17 pineapples under two Al concentrations (0 and 300 μM AlCl3). An observation using transmission electron microscopy indicated no particular variation in the cells of the root apices of the Cayenne pineapple after a treatment with 300 μM AlCl3. However, apparent changes in the cells of the root apices of the Tainung No. 17 pineapple were observed, for example, separation of the cell wall and cell membrane, enlargement of vacuoles, shrinking and gathering of mitochondria beside the inner cell membrane). The 1,3-β-glucan (callose) and malondialdehyde (MDA) contents in the Cayenne pineapple were not affected by the Al treatment. However, in the Tainung No. 17 pineapple, those contents showed substantial increases under the treatment with 300 μM AlCl3, indicating definite damage on the root apices of Tainung No. 17. The results further revealed that the non-Al-resistance pineapple cultivars should be cultivated on the soil that contains low toxic aluminum.
1. Introduction
In strongly acid soils (pH < 5.5), Al exists mainly in the Al3+, Al(OH)2+, and Al(OH)2+ biotoxic forms (Marion et al. Citation1976). Thus, Al toxicity is commonly recognized as one of the main causes of damage to plant growth in strongly acidic soils. In general, damage to the root apex cells of plants can be observed using an electron microscope. Goodwin and Sutter (Citation2009) employed scanning electron microscopy to observe the root apices of Arabidopsis species treated with 50 μM AlCl3, and discovered that the cell wall of the root apices became thicker to defend against Al toxicity. Le Van et al. (Citation1994) studied Al toxicity in the root apices of pumpkins, and Tabuchi and Matsumoto (Citation2001) studied that in wheat. These studies yielded similar results. Because root apex cells are subject to Al toxicity, the hemicellulose content increases gradually, making the cell wall thicker and more tenacious. This phenomenon plays a critical role in the mechanism of resistance to Al toxicity. Minochaa et al. (Citation2001) used transmission electron microscopy (TEM) to observe the internal cells of Picea rubens root apices after a treatment with 0.5 mM Al concentration for 24 h. They observed that the vacuoles in the cells were enlarged, the cell walls were thickened and separated from the cell membrane, and the number of mitochondrian had increased. These signs manifested cell damage when compared with those treated with low Al concentration. When plant roots are subject to Al toxicity, Al blocks the formation of fiber on the cell wall and induces callose synthase on the cell membrane to form callose (Kaneko et al. Citation1999; Teraoka et al. Citation2002). As Al concentration increases, the amount of callose on the root also increases (Bhuja et al. Citation2004). In the case of Al toxicity, the root cellulose synthase stops within 5 min and induces the formation of large quantities of callose (Nakashima et al. Citation2003). Thus, the formation of callose is a protective mechanism for damaged plants (Jaffe and Leopold Citation1984). The same phenomenon was found in the studies of other Al-resistant crops, such as oats (Schreiner et al. Citation1994; Bhuja et al. Citation2004), corn (Horst et al. Citation1997; Eticha et al. Citation2005), tobacco (Chang et al. Citation1999), barley (Kaneko et al. Citation1999), and bean (Massot et al. Citation1999). Al causes lipid peroxidation on the cell membrane of root apex cells (Basu et al. Citation2001; Yamamoto et al. Citation2001). This phenomenon leads to the inhibition of root growth in soybeans (Cackmak and Horst Citation1991), peas (Yamamoto et al. Citation2001; Kobayashi et al. Citation2004), rape (Basu et al. Citation2001), rice (Meriga et al. Citation2004; Achary et al. Citation2008), wheat (Darko et al. Citation2004; Hossain et al. Citation2005), onions (Achary et al. Citation2008), and corn (Boscolo et al. Citation2003). Under normal conditions, the production and removal of free radicals in plants are in a dynamic equilibrium. However, after being intoxicated by Al, this equilibrium is destroyed and the free radicals begin to accumulate, leading to phospholipid peroxidation on the cell membrane and the production of phosphatidylcholine hydroxide. These effects are followed by the conversion to a secondary metabolic product, MDA (Yamamoto et al. Citation2002). MDA is the final product of the reaction between unsaturated fatty acid and reactive oxygen species, and it is a highly active compound containing aldehyde. The higher the concentration of MDA in a plant, the worse is the damage to its cells or tissue. Therefore, the concentration of MDA can reflect the degree of damage in plants under environmental stress.
Some researches about the examining experiments of Al-resistance for different pineapple cultivars were published. For example, the root elongation, the dry weight and the concentration of calcium and magnesium in Cayenne were increased with increasing Al concentration after treating with AlCl3 for 4 weeks, however, that of Tainung No.17 were decreased (Lin Citation2010; Lin and Chen Citation2011). On the other hand, the Al concentration in Tainung No.17 in whole plant was higher than Cayenne in the treatments of different Al concentrations. Based on these researches, we know that Cayenne was Al-resistance and Tainung No.17 was non-Al-resistance in high Al concentration (300 μM AlCl3). This study was conducted to evaluate the cell morphology of traditional (Cayenne) and new (e.g., Tainung No.17) pineapple cultivars by non-Al and high-Al environment. The results will provide the knowledge for the farmers to appropriately choose soil properties when cultivating the new pineapple cultivars.
2. Material and methods
2.1. Cultivation of pineapples
Approximately 81 ± 8 g in fresh weight of Cayenne and Tainung No. 17 pineapple was cleaned with deionized water before being planted in a circular plastic container (inner diameter: 25 cm, height: 30 cm) with a hydroponic solution in triplicate. The composition of the hydroponic solution was that of Hoagland and Arnon (Citation1938) with some modifications. The pH was adjusted to 4.5 by using 0.1 N HCl and 0.1 N NaOH. The container with the pineapple cultivar was aerated uniformly by using a pump; this was then moved into a growth chamber (Hipoint, FH-302, Taiwan) regulated at 27°C during daytime (14 h, RH 65%) and 23°C during night-time (10 h, RH 85%). The hydroponic solution was replaced once weekly. The cultivars with different Al concentrations (0 and 300 μM AlCl3) were cultivated for 4 weeks.
2.2. Effects of Al concentration on the cell morphology of pineapple root apices under TEM observation
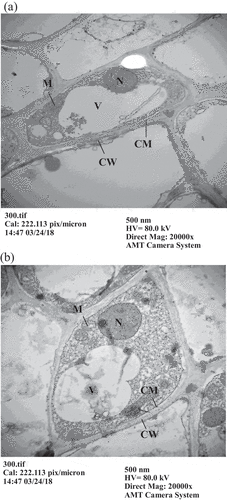
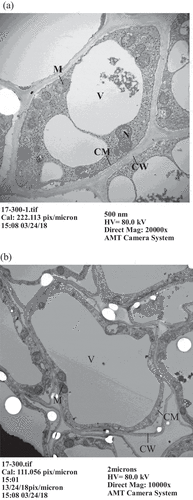
The root apices (1 cm) of Cayenne and Tainung No. 17 grown in 0 and 300 μM AlCl3 solution for 4 weeks were excised using alcohol-cleaned stainless scissors and observed through TEM. The root apices of the pineapples were fixed with 2.5% glutaraldehyde-Na phosphate at a pH of 7 for 2 h and were rinsed with 0.1 M Na phosphate buffer (pH 7) and 5% sucrose solution for 10 min each. The root apices were then fixed with 1% osmium tetroxide and 0.1M Na phosphate at a pH of 7 for 1 h. After rinsing with water three times, alcohol at concentrations of 50%, 70%, 80%, 90%, 95%, and 100% and 100% acetone were used to dehydrate the sample. The dehydrated sample was then enclosed in Spurr’s resin, after which it was cut with an Ultramicrotome (Reichert–Jung Ultracut E), and finally dyed with uranyl acetate and lead citrate. Then the samples were prepared for examination of the root apex cells through TEM (Hitachi, H7100).
2.3. Effects of Al concentration on callose and MDA in the root apices of pineapples with different al resistance
2.3.1. Determination of callose
After treatment with 0 and 300 μM AlCl3, the root apices (1 cm) of Cayenne and Tainung No. 17 pineapples were cut using alcohol-cleaned stainless scissors to measure the callose. The spectrophotometric method described by Zhang et al. (Citation1994) was used. Root apices were fixed with alcohol (boiling at 70°C for 10 min). After removing the alcohol, the sample was stored at −40°C until the analysis. The samples were immersed in 1 mL NaOH, and then sonicated for 2 min. After being placed in a water bath at 80°C for 30 min, each sample was centrifuged for 15 min at 10,000g. After filtration, the callose was measured using a fluorescence spectrophotometer (Shimadzu, RF-1501, Japan). The excitation wavelength was set at 350 nm and the emission wavelength at 500 nm. Laminarin was employed as a standard, and the callose content was expressed in mg laminarin.
2.3.2. Determination of MDA
The MDA content in the root apices (1 cm) of the Cayenne and Tainung No. 17 pineapples was determined following the method of Heath and Packer (Citation1968). Sample of root apex (0.1 g) were rinsed with 4 mL of trichloroacetic acid (TCA; 5% w/v) and ground. The mixture was centrifuged at 10,000g for 5 min. The filtrate (1 mL) was added to 4 mL of thiobarbituric acid (0.5% w/v, in 20% w/v TCA) and immersed in a water bath at 95°C for 30 min. The sample was quickly frozen and sonicated to remove bubbles before centrifugation at 5,000g for 10 min. The absorption at a wavelength of 532 nm was compared with the standard curve to obtain the MDA content.
3. Results and discussion
3.1. Effects of Al concentration on the morphology of pineapples root apices
Through TEM observation, we found no notable variation in cell nucleus, mitochondria or vacuoles for Cayenne pineapples treated either with 0 or 300 μM AlCl3 (). However, the vacuole in the cell was enlarged, the cell nucleus became smaller, and mitochondria were sprayed around the cell membrane in Tainung No. 17 pineapples (). Goodwin and Sutter (Citation2009) investigated the change in Arabidopsis species cells due to Al toxicity and found that the cell walls of root apices became thicker and that Al accumulated in the vacuoles when the root apices were treated with 50 μM AlCl3. This did not occur without Al treatment. This study demonstrates that the cell membrane and cell wall in Tainung No. 17 root apices were separated when treated with 300 μM AlCl3. Miyasaka obtained similar results regarding Al toxicity with the root apices of Picea rubens after a treatment with 0.5 μM Al for 24 h. They discovered that vacuoles in the cells played a role in the storing of toxic substances and detoxification. Because Tainung No. 17 is not Al-resistant, its vacuoles were spread widely in the cell to mitigate Al toxification when treated with Al solution. To dispel Al from the cell, mitochondria were widely distributed around the cell membrane. These changes in the root apex cells were quite similar to those of rapeseed subject to Al toxicity, as observed by Clune and Copeland (Citation2001).
3.2. Effects of Al concentration on callose and MDA in the root apices of pineapples
Under normal conditions, the fiber synthase on the cell membrane of most plants continues to enhance the cell to form fiber. However, the function of fiber synthase is inhibited in response to environmental stress. By contrast, the function of callose synthase is initiated to defend against environmental stress (Delrner, Citation1987). Bouazizi et al. (Citation2009) treated soybean root apices with 75 μM CuSO4 and discovered that callose was produced in abundance. They deduced that the increased thickness of the cell wall in root apices was related to the production of callose. Volgger et al. (Citation2009) reported that the root of wheat produces a large quantity of callose to defend against environmental stress due to high permeability. Vicedo et al. (Citation2009) suggested that hexanoic acid can induce the production of callose to stave off powdery mildew. Therefore, the production of callose plays a major role in the defense against environmental stress for plants. indicates that no callose was measured in the root apices of Cayenne and Tainung No. 17 pineapples when they were treated with hydroponic solutions without Al. However, when they were treated with 300 μM AlCl3, the callose content in the root apices of Cayenne and Tainung No. 17 pineapples was 1.1 and 4.9 mg µmole/g, respectively. The MDA content in the root apices of Cayenne and Tainung No. 17 pineapples was approximately equivalent (3.2 and 3.4 μmole/g FW, respectively) when they were not treated with Al. However, when they were treated with Al, the MDA content in the root apices of Cayenne pineapples increased to 8.5 μmol/g FW, whereas that of Tainung No. 17 pineapples increased to 28 μmol/g FW. These results reveal that the damage on the root apices is more severe in Tainung No. 17 pineapples than in Cayenne pineapples. When plants are subject to damage, callose is produced to protect the cell. Therefore, its content can serve as an index for plant damage (Jaffe and Leopold Citation1984). This study demonstrates that the root apices of Tainung No. 17 pineapples are more severely damaged than are those of Cayenne pineapples when they are treated with 300 μM AlCl3. This phenomenon is due to the formation of fiber being inhibited on the cell wall of the root apices and to callose synthase being induced on the cell membrane to form callose (Kaneko et al. Citation1999; Teraoka et al. Citation2002). MDA can serve as an index for oxidization damage to plant cell or tissue. The higher the MDA content in plants, the more the cell or tissue is damaged (Yamamoto et al. Citation2001). This study also reveals that the MDA content in the root apices of Tainung No. 17 pineapples is considerably higher under a treatment of 300 μM AlCl3 than without Al treatment. However, the MDA content in the root apices of Cayenne pineapples is only slightly increased by the treatment of 300 μM AlCl3 compared with no Al treatment. Therefore, when treated with 300 μM AlCl3, the root apices of Tainung No. 17 pineapples appear to be more oxidized compared with those of the Cayenne pineapples.
Table 1. Callose and malondialdehyde content in the root apices of Cayenne and Tainung No. 17 pineapples after treatment with 0 and 300 µM AlCl3.
4. Conclusion
After observation through TEM, there were no significant changes on the cell morphology of the Cayenne pineapple under a no Al or high Al treatment. However, the cell morphology of the Tainung No. 17 pineapple displayed great changes. Based on the analysis of callose and MDA, the cell in the root apices of Tainung No. 17 was actually damaged under high Al concentration. This study can serve as a reference for Al resistance in pineapples.
Acknowledgments
The authors would like to thank Professor Yu-Chia Chung at the National Sun Yat-sen University, in Kaohsiung, Taiwan, for the critical reading of the manuscript. The English of this article was critically corrected by Wallace Academic Editing.
References
- Achary VM, Jena MS, Panda KK 2008: Aluminium-induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotox Environ. Safe., 70, 300–310. doi:10.1016/j.ecoenv.2007.10.022
- Basu U, Good AG, Taylor GJ 2001: Transgenic Brassica mapus plants overexpressing aluminum-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminum. Plant Cell Environ., 24, 1269–1278. doi:10.1046/j.0016-8025.2001.00783.x
- Bhuja P, McLachian K, Stephens J, Taylor G 2004: Accumulation of 1,3-B-D-glucans, in response to aluminum and cytosolic calcium in triticum aestivum. Plant Cell Physiol., 45, 543–549. doi:10.1093/pcp/pch068
- Boscolo PRS, Menossi M, Jorge RA 2003: Aluminum induced oxidative stress in maize. Phytochemistry., 62, 181–189.
- Bouazizi H, Jouili H, Geitmann A, Ferjani E 2009: Structural changes of cell wall and lignifying enzymes modulations in bean roots in response to copper stress. Biol Trace Elem Res., 136, 232–240.
- Cackmak L, Horst WJ 1991: Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant., 83, 463–468. doi:10.1111/j.1399-3054.1991.tb00121.x
- Chang YC, Yamamoto Y, Matsumoto H 1999: Enhancernent of callose production by a combination of aluminum and iron in suspension-cultured tobacco cells. Soil Sci. Plant Nutr., 45, 337–347. doi:10.1080/00380768.1999.10409348
- Clune TS, Copeland L 2001: Uptake of aluminum by intact seedlings. Commun. Soil Sci. Plant Anal., 32, 2819–2829. doi:10.1081/CSS-120000964
- Darko E, Ambrusa H, Stefanovits-Bonyai E 2004: Aluminium toxicity, Al tolerance and oxidative stress in an Al-sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Sci., 166, 583–591. doi:10.1016/j.plantsci.2003.10.023
- Delmer DP 1987: Cellulose biosynthesis. Annu. Rev. Plant Physiol., 38, 259–290. doi:10.1146/annurev.pp.38.060187.001355
- Eticha D, The C, Welcker C, Narro L, Stab A, Horst WJ 2005: Aluminum-induced callose formation in root apices: inheritance and selection trait for adaptation of tropical maize to acid soils. Field Crops Res., 93, 252–263. doi:10.1016/j.fcr.2004.10.004
- Goodwin SB, Sutter TR 2009: Microarray analysisss of Arabidopsis genome response to aluminum stress. Biol. Plant., 53, 85–99. doi:10.1007/s10535-009-0012-4
- Heath RL, Packer L 1968: Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125, 189–198.
- Hoagland DR, Arnon DL 1938: The water culture method for growing plants without soil. Cali. Agr. Expt. Sta. Circ., 347, 1–24.
- Horst WJ, Puschel K, Schmohl N 1997: Induction of callose formation is a sensitive marker for genotypic aluminum sensitivity in maize. Plant Soil., 192, 23–30. doi:10.1023/A:1004204120863
- Hossain MA, Hossain AK, Kihara T 2005: Aluminium-induced lipid peroxidation and lignin deposition are associated with an increase in H2O2 generation in wheat seedlings. Soil Sci. Plant Nutr., 51, 223–230. doi:10.1111/j.1747-0765.2005.tb00026.x
- Jaffe M, Leopold AC 1984: Callose deposition during gravitropism of Zea mays and Pisum sativum and its inhibition by 2-deoxy-D-glucose. Planta, 161, 20–26.
- Kaneko M, Yoshimura E, Nishizawa NK, Mori S 1999: Time course study of aluminum-induced callose formation in barley roots as observed by digital microscopy and low-vacuum scanning electron microcopy. Soil Sci. Plant Nutr., 45, 701–712. doi:10.1080/00380768.1999.10415833
- Kobayashi Y, Yamamoto Y, Matsumoto H 2004: Studies on the mechanism of aluminum tolerance in pea (Pisum sativwn L.) using aluminum-tolerant cultivar ‘Alaska’ and aluminum-sensitive cultivar ‘Hyogo’. Soil Sci. Plant Nutr., 50, 197–204. doi:10.1080/00380768.2004.10408468
- Le Van H, Kuraishi S, Sakurai N 1994: Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiol., 106, 971–976. doi:10.1104/pp.106.3.971
- Lin YH 2010: Effects of aluminum on root growth and absorption of nutrients by two pineapple cultivars [Ananas comosus (L.) Merr.]. Afr. J. Biotech., 9, 4034–4041.
- Lin YH, Chen JH 2011: Effects of aluminum on nutrient uptake in different parts of four pineapple cultivars. Afr. J. Biotech., 6, 1438–1446.
- Marion G, Gessner W, Beherens HJ 1976: Multinuclear studies of aluminum compounds. Soil Sci., 121, 76–82. doi:10.1097/00010694-197602000-00003
- Massot N, Llugany M, Poschenrieder C, Barcelo J 1999: Callose production as indicator of aluminum toxicity in bean cultivara. J. Plant Nutr., 22, 1–10. doi:10.1080/01904169909365601
- Meriga B, Reddy BK, Rao KR 2004: Aluminium-induced production of oxygen radicals, lipid peroxidation and DNA damage in seedlings of rice (Oryza sativa). J. Plant Physiol., 161, 63–68.
- Minochaa R, McQuattieb C, Fagerbergc W, Longa S, Nohd EW 2001: Effects of aluminum in red spruce (Picea rubens) cell cultures: cell growth and viability, mitochondrial activity, ultrastructure and potential sites of intracellular aluminum accumulation. Physiol. Plant., 113, 486–498.
- Nakashima J, Laosinchal W, Cui XJ, Brown RM 2003: New insight into the mechanism of cellulose and callose biosynthesis: proteasesmay regulate callose biosynthesis upon wounding. Cellulose, 10, 369–389. doi:10.1023/A:1027336605479
- Schreiner KA, Hoddinott J, Taylor GJ 1994: Aluminum-induced deposition of (1,3) B-glucans(callose) in Triticum aestivum L. Plant Soil., 162, 273–280. doi:10.1007/BF01347714
- Tabuchi A, Matsumoto H 2001: Changes in cell-wall properties of wheat (Triticum aestivum) roots during aluminium-induced growth inhibition. Physiol. Plant, 112, 353–358. doi:10.1034/j.1399-3054.2001.1120308.x
- Teraoka T, Kaneko M, Mori S, Yoshimura E 2002: Aluminum rapidly inhiblts cellulose synthesis in roots of barley and wheat seedlings. J. Plant Physiol., 159, 17–23. doi:10.1078/0176-1617-00678
- Vicedo B, Flors V, Leyva M, Finiti I, Kravchuk Z, Real MD, García-Agustín P, González-Bosch C 2009: Hexanoic acid-induced resistance against Botrytis cinerea in tomato plants. Mol. Plant-Microbe Interact., 22, 1455–1465. doi:10.1094/MPMI-22-11-1455
- Volgger M, Lang I, Ovečka M, Lichtscheid I 2009: Plasmolysis and cell wall deposition in wheat root hairs under osmotic stress, 17, Jun.
- Yamamoto Y, Kobayashi Y, Devi SR 2002: Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol., 128, 63–72.
- Yamamoto Y, Kolayashi Y, Matsumoto H 2001: Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol., 125, 199–208. doi:10.1104/pp.125.1.199
- Zhang G, Hoddinott J, Taylor GJ 1994: Characterization of 1,3-β-Dglucan (callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. J. Plant Physiol., 144, 229–234. doi:10.1016/S0176-1617(11)80548-1