ABSTRACT
The response of soil organic matter (SOM) to global warming is a crucial subject. However, the temperature sensitivity of SOM turnover remains largely uncertain. Changes in the mineralization of native SOM, i.e., priming effect (PE) may strongly affect the temperature sensitivity of SOM turnover in the presence of global warming. We investigated the direction and magnitude of the PE in a Japanese volcanic ash soil at different temperatures (15°C, 25°C, and 35°C) using a natural 13C tracer (C4-plant, maize leaf) in a short-term (25 days) incubation study. In addition, we evaluated the temperature sensitivity expressed as Q10 value with and without the addition of maize to the soil and their relations to PE. We found that positive PE occurred at each temperature condition and tended to increase with decreased temperature, and these PE results were consistent with the microbial biomass at the end of the incubation period. CO2 emission from control soil (without maize) increased with increasing temperature (Q10 = 2.6), but CO2 emission from the soil with added maize did not significantly change with increasing temperature (Q10 = 1.0). This was caused by the suppression of CO2 emission from the soil with increasing temperature (Q10 = 0.9). On the other hand, soil-originated CO2 emission clearly increased with increasing temperature (Q10 = 3.4) when Q10 values were calculated on the assumption that the temperature and substrate supply increase at the same time (from 25°C). These results suggest that not only the temperature increase but also the labile carbon supply may be important for the temperature sensitivity of Japanese volcanic ash soil.
1. Introduction
The amount of carbon (C) stored in the upper 1 m of mineral soils is estimated to be 1500 Pg (Jobbágy and Jackson Citation2010), which is approximately twice the amount in the atmosphere and triple the amount held in vegetation (Batjes Citation1996; Fröberg et al. Citation2003). Any change in the rate of soil CO2 efflux would have major impacts on atmospheric CO2 concentration and the Earth’s climate (Davidson and Janssens Citation2006). Many studies have reported the effects of warming on soil organic matter (SOM) dynamics (i.e., the temperature sensitivity of SOM turnover, often expressed as Q10: the proportional increase in CO2 released by soil for a 10°C increase in temperature), yet our understanding of SOM dynamics, including its temperature sensitivity, remains highly uncertain (Davidson and Janssens Citation2006; Han and Jin Citation2018).
SOM is a heterogeneous mixture of organic substances with different forms and degradability. These characteristics of SOM make the temperature sensitivity of SOM turnover difficult to understand. General explanations of SOM stability include chemical recalcitrance (Watanabe et al. Citation2006; Ikeya et al. Citation2011) and physical protection resulting in the unavailability of C to soil microbes (Sollins et al. Citation2009; Heater et al. Citation2015). However, these factors cannot explain the priming effect (PE) observed in recent studies.
The PE, a change in the mineralization of native recalcitrant SOM due to the addition of new labile C substrates, has been observed in many laboratory and field studies (e.g., Kuzyakov et al. Citation2000; Fontaine et al. Citation2004, Citation2007, Citation2011; Wang et al. Citation2014). Fontaine et al. (Citation2007) found that the input of labile C (cellulose) primed microbial activity, leading to faster decomposition of older recalcitrant SOM in deep soil layers. The theory has recently been proposed that the stability of SOM is attributable to a lack of labile C for soil microbes. Carbon allocation by plants thus plays an important part in SOM dynamics (Schumidt et al. Citation2011). It is generally considered that global warming increases labile C input to the soil through increased photosynthesis and C allocation below ground (Karhu et al. Citation2017). Therefore, the PE may strongly affect the temperature sensitivity of SOM turnover with global warming.
Volcanic ash soil is a major type of soil in Japan. It has very thick, dark-colored A horizons (Kumada Citation1987; Shindo and Honma Citation2001), with a large amount of organomineral complexes of dark-colored organic matter (Kuwatsuka, Tsutsuki, and Kumada Citation1978; Yonebayash and Hattori Citation1988) with short-range-order minerals (particularly allophane and imogolite) and/or monomeric Al and Fe ions (i.e., active Al and Fe) (Wada and Higashi Citation1976; Inoue Citation1990; Asano and Wagai Citation2014). Although it has been hypothesized that this dark-colored SOM is very stable, yet remains highly uncertain temperature sensitivity of SOM turnover and PE in this Japanese black soil.
We need a better understanding of the temperature sensitivity of SOM turnover, including the PE phenomenon, particularly for volcanic ash soil in Japan. We conducted a short-term (3 weeks) laboratory incubation study to investigate the temperature sensitivity of SOM turnover, expressed as Q10 values, at 15°C, 25°C, and 35°C in a volcanic ash soil with and without labile C input. We also investigated the temperature sensitivity of the PE by quantifying the direction and magnitude of the PE at each temperature.
2. Materials and methods
2.1. Study site and soil sampling
The study site was situated on the campus of the Takayama Experimental Forest, River Basin Research Center, Gifu University, Japan (36° 08′ N, 137° 25′ E, 1425 m above sea level). According to the records of the Takayama Experimental Field Station (1342 m above sea level), the mean annual temperature is 7.3°C and the mean annual precipitation is about 2400 mm (2014 − 2015). The ground is covered by snow from December to April, with a depth of usually 1–2 m. According to Chen et al. (Citation2017), the dominant tree species are Quercus crispula (26.9% of the basal area) and Betula platyphylla var. japonica (14.6% of the basal area). A few evergreen conifer species (2.8% of the basal area) are also present. The height of the dominant forest canopy ranges from 13 to 20 m. The forest floor is 100% covered (ca. 40 stems/m2) by very dense dwarf bamboo (Sasa senanensis) with a height of 1−1.5 m. A permanent plot of 1 ha (100 m × 100 m) was established in this area. A detailed description of this site (Takayama site) is provided in other studies (Ohtsuka et al. Citation2005).
Soil samples were taken from the Takayama site in May 2018. We focused on the surface mineral horizon (0–20 cm), which is most likely to be affected by warming. After the soil was collected, the fresh soil samples were passed through a 2-mm mesh sieve after all visible plant debris and roots had been thoroughly removed with tweezers. The fresh soil samples were immediately stored in a refrigerator at 5°C for about 2 weeks until they were used for the incubation experiment (see Section 2.3). Subsamples for soil characterization were air-dried and subjected to the same process as described above.
2.2. Soil analysis
Soil pH was measured using an air-dried soil to solution (H2O or KCl) ratio of 1:5 after shaking for 1 h. To measure the pH of NaF, 1 g of air-dried soil was mixed with 50 ml of 1 M NaF (pH 7.0), the suspension was stirred continuously for 2 min, and the pH of the suspension was measured. Soil C and nitrogen (N) contents were measured with an NC analyzer (Sumigraph NC-22F, Sumika Analysis Service, Japan). The CEC was determined by the ammonium acetate (1 M and pH 7.0) method. Microbial biomass carbon was determined by the ATP method (Aoyama 2005). The humification degree of humic acids (A600/C) was measured by the methods of Han et al. (Citation2019) and Ikeya and Watanabe (Citation2003). The δ13C value of the soil sample was measured by an elemental analysis/stable isotopic ratio mass spectrometer (EA/IRMS) continuous flow system (Thermo Scientific, USA). The δ13C measurements were repeated until the standard deviation reached <0.2‰.
2.3. Incubation experiment
The experimental units consisted of fresh sieved soil (10-g oven-dried samples) placed in 500-ml jars and incubated at 15°C, 25°C, and 35°C. The incubation experiment was continued for 25 days until CO2 emission from the soil slowed down. After 10 days of preincubation, dried and milled maize leaf (<2 mm) was added to yield a total organic C content of 2.5 g kg−1 dry soil (ca. 1 g C-maize leaf per kg soil was prepared according to Fontaine et al. Citation2007, Citation2011) and mixed with the soil. Total N, C:N ratio, and δ13C of maize leaf were 1.7%, 24.4, and −14.8‰, respectively. Soil without maize leaf was prepared as a control and was mixed to provide the same physical disturbance. The incubation experiment included six treatments (three replicates): soil only at 15°C, 25°C, and 30°C (15S, 25S 35S, respectively) and soil with maize leaf at 15, 25 and 30°C (15S+M, 25S+M 35S+M, respectively). The moisture content of the soil was adjusted to 60% of water-holding capacity with distilled water during incubation. The released CO2 was trapped in a NaOH solution at 3, 7, 14, and 25 days of incubation until CO2 emission from the soil slowed down. A glass vial containing 20 ml of 0.2 M NaOH solution was placed in each jar to trap the CO2 emitted from the soil, and the jars were sealed. The jars were flushed with CO2-free air each time the NaOH solution was replaced. The C content of the NaOH solution was measured by a total inorganic C analyzer. δ13C of CO2 (composite sample for 25 days) was analyzed by an EA/IRMS continuous flow system after precipitation of the carbonates with excess BaCl2 and filtration (Fontaine et al. Citation2011). The δ13C measurements were repeated until the standard deviation reached <0.2‰.
2.4. Calculation of PE
The substrates (maize leaf) added to the soil allowed the separation of the respiration rates (mg CO2-C kg−1 soil) of native soil C (Rsoil) and of substrates (Rsub) using mass balance equations (Fontaine et al. Citation2011):
Rsoil + Rsub = Rtotal
Rsoil × δ13C soil + Rsub × δ13C sub = Rtotal × δ13C total
where δ13C soil is the δ13C of soil C, δ13C sub is the δ13C of maize leaf C, Rtotal is the total CO2 emitted by soil with maize leaf, and δ13C total is its δ13C.
The PE (mg C-CO2 kg−1 soil) induced by the addition of substrates was calculated as:
PE = (Rsoil soil with substrates) – (Rsoil control soil)
where (Rsoil control soil) is the CO2 emitted by control soil.
The cumulative CO2 emission was used to calculate the Q10 value for the temperature range (15°C–35°C). According to Yashiro et al. (Citation2012), the temperature dependence of CO2 efflux and Q10 value was calculated as:
R = R0 exp (kT)
Q10 = exp10k
where R is the respiration rate, R0 and k are fitting parameters, and T is temperature.
3. Results
3.1. Soil characteristics
shows some characteristics of the soil used. The soil is weakly acidic, with a pH (NaF) of 9.5 or more, and has a very high C content; it is characterized as andosol (FAO et al. Citation2014). The humification degree of humic acids (A600/C) is 8.2, and they are classified as type A humic acids, which are commonly observed in black volcanic ash soil in Japan (Kuroboku). These results are consistent with the findings of a previous study (Chen et al. Citation2017) that the soil in Takayama site can be classified as an andic Japanese volcanic ash soil (Kuroboku). We confirmed that a very thick, dark-colored A horizon with high C content was widely distributed at the study site.
Table 1. Some characteristics of the soil used in this study
3.2. CO2 emission and PE
Cumulative total CO2 emission, soil-derived CO2 emission, and maize-derived CO2 emission are shown in . Cumulative total CO2 emission during 25 days of incubation increased with increasing temperature regardless of whether or not the soil was mixed with maize leaf, but overall, it was extremely high in soils with added maize leaf. In particular, the cumulative CO2 emission at 35°C was significantly higher than that at 15°C, and 25°C. Maize-derived CO2 was a major component of total CO2 emission at all temperatures, accounting for 86–90% of total CO2 emission. The amount of maize-derived CO2 emission decreased in the following order: 35 S + M > 25 S + M > 15 S + M.
Table 2. Cumulative CO2 emission from control soil and soil with added maize (total, soil-derived, and maize-derived), and Q10 value for each temperature condition
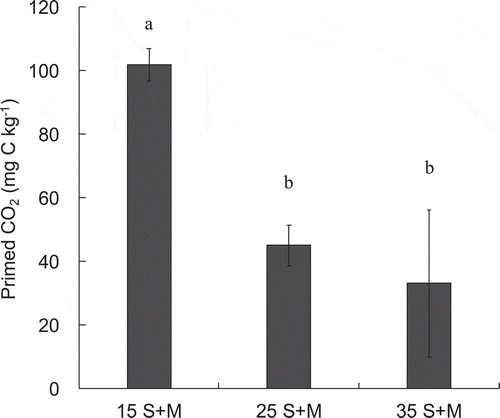
The primed CO2 emission in each treatment (15S+M, 25S+M 35S+M) is shown in . Addition of maize to soil significantly increased cumulative soil-derived CO2 emission during incubation at 15°C (P < 0.005) and 25°C (P < 0.05) for comparison with control (t test), causing a positive PE of 101.9 and 45.1 mg C kg−1, respectively. In contrast, 35 S + M did not show significantly positive PE, unlike 15 S + M and 25 S + M, but the cumulative soil-derived CO2 emission of 35 S + M was higher trend than those of 35 S. The amount of primed CO2 emission decreased in the following order: 15 S + M > 25 S + M > 35 S + M. In particular, the amount of primed CO2 emission at 15 S + M was significantly higher than that at 25 S + M and 35 S + M.
3.3. Temperature sensitivity
shows the CO2 emissions for each treatment and temperature sensitivity as the Q10 value of control soil and soil with added maize leaf. CO2 emission from control soil increased with increasing temperature, for a Q10 value of 2.6. In contrast, CO2 emission from the soil with added maize leaf did not clearly change with increasing temperature, with Q10 values of 1.0 for total emission (soil plus maize), 0.9 for soil-derived emission, and 1.1 for maize-derived emission.
3.4. Microbial biomass
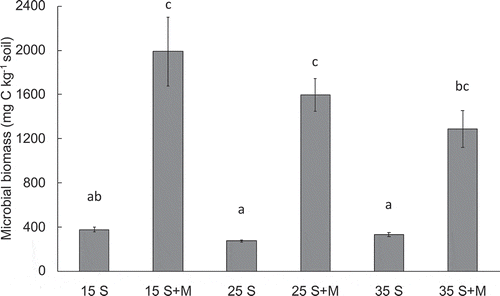
shows the microbial biomass at the end of the incubation period for each treatment. Although these values did not significantly differ with temperature, the microbial biomass in soil with added maize leaf was significantly higher than that in soil without maize leaf. There was no regular relationship between microbial biomass and temperature in the control soil. On the other hand, microbial biomass gradually decreased with increasing temperature in soil with added maize leaf.
Figure 2. Microbial biomass from soil and soil with added maize at the end of the incubation period for different temperature conditions
4. Discussion
4.1. Possible mechanisms of PE in volcanic ash soil
Abiotic factors such as temperature, soil moisture, and pH are the main drivers of C turnover in soil. They act indirectly, mainly by affecting microbial activity that drives the mineralization of SOM and plant residues (Blagodatskaya & Kuzyakov Citation2008). In addition to these abiotic factors, many biotic factors directly affect C mineralization in soil (e.g., PE) (Blagodatskaya & Kuzyakov Citation2008). The term PE was introduced to describe changes in SOM decomposition effected by the addition of organic or mineral substances (Kuzyakov, Friedel, and Stahr Citation2000). In this study, we investigated the direction and magnitude of the PE in volcanic ash soil with added maize leaf under different temperatures. We found that the PE was positive during 25 days of incubation at temperatures of 15°C, 25°C, and 35°C and increased with decreasing temperature (). Mineralization of native SOM through the PE under sterile conditions has never been observed. It is essentially the result of the activity of microorganisms growing on the decomposed substrate (Kuzyakov, Friedel, and Stahr Citation2000; Chen et al. Citation2014). In addition, PE is consistent well with microbial biomass at the end of the incubation period ( and ). Therefore, it was suggested that the PE can occur in a volcanic ash soil as a result of microbial activity, regardless of abiotic factors such as temperature and soil moisture.
Our understanding of the PE is incomplete, but consistent elements are emerging from many recent studies (e.g., Fontaine et al., Citation2003; Kuzyakov, Citation2010; Chen et al. Citation2014). In particular, a PE mechanism based on microbial succession hypothesis is currently accepted (Fontaine et al. Citation2011). This mechanism is based on competition for energy and nutrient acquisition and on shifts between microbial communities specialized for utilizing various sources: fast-growing r-strategists that benefit by utilizing easily available substrates and slow-growing K-strategists that benefit by utilizing recalcitrant SOM. This microbial succession occurred in a short-term (about 1 week) incubation study (Chen et al. Citation2014). Although we did not distinguish the domination of r-strategists and K-strategists in the microbial biomass community, biomass clearly increased with the addition of maize leaf at all temperature conditions (). In addition, our results showed that cumulative maize-derived CO2 emission tended to increase with increasing temperature, whereas soil-derived CO2 emission did not (). Bradford (Citation2013) reported that soil microbial community composition shifts toward r-strategists as temperature increases, and our result seemed to support them. Moreover, primed CO2 emission tended to increase with decreasing temperature, and these PE results were consistent with the microbial biomass at the end of the incubation period ( and ). An acceleration of recalcitrant SOM decomposition rate can occur if a substantial part of the labile C is absorbed by the K-strategist (Fontaine et al. Citation2004). Thus, K-strategist may have gradually dominated instead of r-strategist, particularly in lower temperature condition, during 25 days of incubation. These results suggest that r-strategists are relatively temperature dependent but K-strategists are less temperature-dependent on growing with labile C supply, and that K-strategists were the main cause of positive PE under the conditions of this study.
It is assumed that microbial succession from r-strategists to K-strategists and their temperature sensitivity may depend on the quantity and quality of the substrate added to the soil as well as soil type. For example, Blagodatskaya & Kuzyakov (Citation2008) pointed out that the magnitude of priming is generally related to the amount of labile C input to the soil. Ghee et al. (Citation2013) reported that the addition of glucose to Cambisol (drained sandy loam soil) resulted in positive PE regardless of the temperature (varying from 15°C to 30°C), but that the absolute magnitude of this response was not affected by temperature. Thus, the mechanisms of the PE and their temperature sensitivity are probably very complicated, and detailed investigations are needed in future studies.
4.2. Temperature sensitivity with and without input of substrate to the soil
The sensitivity of the decomposition of SOM to temperature has recently received considerable interest with the concern for climate change. The Q10 value is often used to express the change in soil respiration rate with a 10°C change in temperature (Davidson and Janssens Citation2006) and thus describes the sensitivity of CO2 efflux to temperature. We should note that the Q10 values shown here are convenient indications for comparing the CO2 emissions at different temperatures, but do not necessarily represent the exactly thermodynamic characteristics of SOM turnover because these values could be changed depending on the incubation period.
CO2 emission from control soil (i.e., without added maize leaf) increased with an increase in air temperature from 15℃ to 35°C, with a Q10 value of 2.6 (). This relationship between CO2 emission from soil and temperature is confirmed by many studies (e.g., Zhu and Cheng Citation2011; Ghee et al. Citation2013; Meyer et al. Citation2018). It is often explained that the Q10 value increases with increasing molecular complexity of the substrate (i.e., organic compounds with recalcitrant molecular structure) based on the kinetics of enzymatic reactions, which are materially analogous to conventional Arrhenius kinetic theory (Davidson and Janssens Citation2006; Craine, Fierer, and Mclauchlan Citation2010; Ghee et al. Citation2013). This conventional enzyme-kinetic hypothesis was recently supported by a combination study of physical fractionation and analysis of molecular structure (Wagai et al. Citation2013). Our results showed that the Q10 value of soil with added maize leaf (Q10 = 1.0) was clearly lower than that of control soil (Q10 = 2.6). This result seemed to support the conventional enzyme-kinetic hypothesis. However, the soil-derived Q10 value (Q10 = 0.9) in soil with added maize leaf was clearly lower than that of control soil (). This result cannot be explained by the conventional enzyme-kinetic hypothesis. We found that the addition of maize leaf to soil stimulated SOM mineralization (i.e., PE), particularly at lower temperatures (). This PE phenomenon clearly contributed to the decline in the Q10 value in soil with added maize leaf relative to control soil in this study. In addition to our result, there are many contradictory results concerning the enzyme-kinetic hypothesis for Q10 values (e.g., Fang et al. Citation2005; Fissore et al. Citation2008; Conant, Ryan, and Ågren et al. Citation2011). According to our results, the direction and magnitude of the PE could be factors controlling the Q10 values in many soil types. Therefore, it may be necessary to investigate these factors in future studies.
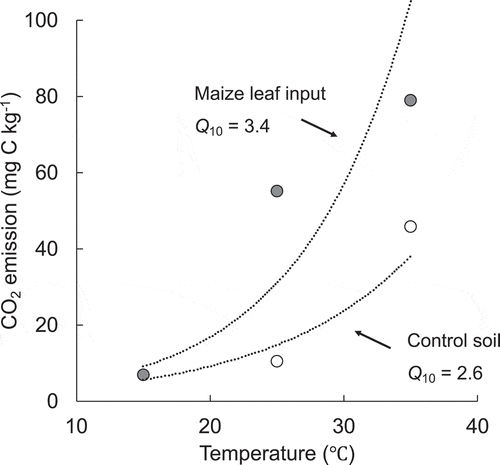
It is generally considered that global warming increases the input of labile C (e.g., from leaf litter deposition, root exudation, fine root biomass and their detritus, etc.) to soil through increased photosynthesis and C allocation below ground (Yin et al. Citation2013; Leppalammi-kujansuu et al. Citation2014; Karhu et al. Citation2017). Therefore, we need to clarify the responses of soil not only to temperature increase but also to changes in belowground C allocation. We calculated Q10 values from the soil as affected by the increase in temperature, and by the simultaneous increase in temperature and labile C (maize leaf) input (). We found that the increase in Q10 values in soil with added maize leaf (soil-derived) with temperature is higher (Q10 = 3.4) than that in control soil (Q10 = 2.6). Zhu and Cheng (Citation2011), in studies of planted sandy loam soil, reported that rhizosphere PE increased with increased temperature, a result similar to ours. These results suggest that not only the temperature increase but also the simultaneous changes in belowground C allocation is likely to be important for temperature sensitivity of SOM turnover.
Figure 3. CO2 emissions and Q10 values from the soil as affected by the increase in temperature, and by the simultaneous increase in temperature and labile C (maize leaf) input
Wagai et al. (Citation2013) found that Q10 value for short-term incubation (during 1 month) was significantly correlated with the abundance of aromatic plus alkyl-C relative to O-alkyl C groups in low-density fraction but not in bulk soil fraction in Japanese volcanic ash soils and supported the enzyme-kinetic hypothesis. It is well known that Japanese volcanic ash soils often show a dark-colored A horizons with large amounts of black SOM (Kuwatsuka, Tsutsuki, and Kumada Citation1978; Yonebayash and Hattori Citation1988), which are characterized by their extremely high aromatic C content and stabilities such as black carbon (Watanabe et al. Citation2006; Ikeya et al. Citation2011; Shindo and Honma Citation2001). Therefore, this soil type may be relatively susceptible to decomposition with global warming compared to the other soil types, based on the enzyme-kinetic hypothesis. Any consensus has not yet emerged on the difference in the direction and magnitude of the PE, including their temperature sensitivity, among soil types. In the long run, greater uncertainty is the warming response of the SOM fractions with decadal turnover rate (Davidson and Janssens Citation2006; Wagai et al. Citation2013). Including the PE reaction, therefore, more detailed studies of the temperature sensitivity of SOM turnover at Japanese volcanic ash soils, particularly in long-term decomposition Q10, are needed in the future.
5. Conclusions
In this study, we focused on the temperature sensitivity of primed C release from SOM (the PE) in a Japanese volcanic ash soil with added maize leaf. We found that the PE was positive during 25 days of incubation at temperatures of 15°C, 25°C, and 35°C and increased with decreasing temperature. The temperature sensitivity (Q10 value) of soil with added maize leaf was lower than that of control soil, and soil-originated C release contributed to it highly. In contrast, soil-originated CO2 emission increased with increasing temperature on the assumption that the simultaneous increase in temperature and labile C (maize leaf) input. These results strongly suggest that not only temperature increase, but also the changes in belowground C allocation, may be important for the temperature sensitivity of SOM turnover in Japanese volcanic ash soil.
Acknowledgments
We thank the members of Laboratory of Soil Science, The University of Shiga Prefecture. The study was supported by JSPS KAKENHI Grant Number 17K00524.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Asano, M., and R. Wagai. 2014. “Evidence of Aggregate Hierarchy at Micro- to Submicron Scales in an Allophanic Andisol.” Geoderma 216: 62–74. doi:10.1016/j.geoderma.2013.10.005.
- Batjes, N. H. 1996. “The Carbon and Nitrogen in the Soils of the World.” European Journal of Soil Science 47: 151–163. doi:10.1111/j.1365-2389.1996.tb01386.x.
- Blagodatskaya, E., and Y. Kuzyakov. 2008. “Mechanisms of Real and Apparent Priming Effects and Their Dependence on Soil Microbial Biomass and Community Structure: Critical Review.” Biology and Fertility of Soils 45: 115–131. doi:10.1007/s00374-008-0334-y.
- Bradford, M. A. 2013. “Thermal Adaptation of Decomposer Communities in Warming Soils.” Frontiers in Microbiology 333: 1–16.
- Chen, R., M. Senbayram, S. Blagodatsky, O. Myachina, K. Dittert, X. Lin, E. Blagodatskaya, and Y. Kuzyakov. 2014. “Soil C and N Availability Determine the Priming Effect: Microbial N Mining and Stoichiometric Decomposition Theories.” Global Change Biology 20: 2356–2367. doi:10.1111/gcb.2014.20.issue-7.
- Chen, S., S. Yoshitake, Y. Iimura, C. Asai, and T. Ohtsuka. 2017. “Dissolved Organic Carbon (DOC) Input to the Soil: DOC Fluxed and Their Partitions during the Growing Season in a Cool-temperate Broad-leaved Deciduous Forest, Central Japan.” Ecological Research 32: 724–731. doi:10.1007/s11284-017-1488-6.
- Conant, R. T., M. G. Ryan, G. I. Ågren, et al. 2011. “Temperature and Soil Organic Matter Decomposition Rates-synthesis of Current Knowledge and a Way Forward”. Global Change Biology 17: 3392–3404. doi:10.1111/j.1365-2486.2011.02496.x.
- Craine, J. M., N. Fierer, and K. K. Mclauchlan. 2010. “Widespread Coupling between the Rate and Temperature Sensitivity of Organic Matter Decay.” Nature Geoscience 3: 854–857. doi:10.1038/ngeo1009.
- Davidson, E. Q., and I. A. Janssens. 2006. “Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change.” Nature 440: 165–173. doi:10.1038/nature04514.
- Fang, C., P. Smith, J. B. Moncrieff, and J. U. Smith. 2005. “Similar Response of Labile and Resistant Soil Organic Matter Pools to Changes in Temperature.” Nature 433: 57–59. doi:10.1038/nature03138.
- FAO, ISRIC, ISSS. 2014. “World Reference Base for Soil Resources.” 106 World soil resources reports. FAO, Rome.
- Fissore, C., C. P. Giardina, R. K. Kolka, C. C. Trettin, G. M. King, M. F. Jurgensen, C. D. Barton, and S. D. McDowell. 2008. “Temperature and Vegetation Effects on Soil Organic Carbon Quality along a Forested Mean Annual Temperature Gradient in North America.” Global Change Biology 14: 193–205.
- Fontaine, S., A. Mariotti, and L. Abbadie. 2003. “The Priming Effect Of Organic Matter: A Question Of Microbial Competition?” Soil Biology and Biochemistry 35: 837–843. doi: 10.1016/s0038-0717(03)00123-8.
- Fontaine, S., C. Henault, A. Aamor, N. Bdioui, J. M. G. Bloor, V. Maire, B. Mary, S. Revaillot, and P. A. Maron. 2011. “Fungi Mediate Long Term Sequestration of Carbon and Nitrogen in Soil through Their Priming Effect.” Soil Biology and Biochemistry 43: 86–96. doi:10.1016/j.soilbio.2010.09.017.
- Fontaine, S., G. Bardoux, L. Abbadie, and A. Mariotti. 2004. “Carbon Input to Soil May Decrease Soil Carbon Content.” Ecology Letters 7: 314–320. doi:10.1111/ele.2004.7.issue-4.
- Fontaine, S., S. Barot, P. Barre, N. Bidioui, B. Mary, and C. Rumpel. 2007. “Stability of Organic Carbon in Deep Soil Layers Controlled by Fresh Carbon Supply.” Nature 450: 277–280. doi:10.1038/nature06275.
- Fröberg, M., D. Berggren, B. Bergkvist, C. Bryant, and H. Knicker. 2003. “Contributions of Oi, Oe and Oa Horizons to Dissolved Organic Matter in Forest Floor Leachates.” Geoderma 113: 311–322. doi:10.1016/S0016-7061(02)00367-1.
- Ghee, C., R. Neilson, P. D. Hallett, D. Robinson, and E. Paterson. 2013. “Priming of Soil Organic Matter Mineralization Is Intrinsically Insensitive to Temperature.” Soil Biology and Biochemistry 66: 20–28. doi:10.1016/j.soilbio.2013.06.020.
- Han, B., K. Kitamura, M. Hirota, H. Shen, Y. Tang, T. Suzuki, and N. Fujitake. 2019. “Humus Composition and Humification Degree Pf Humic Acids of Alpine Meadow Soils in the Northeastern Part of the Qinghai-Tibet Plateau.” Soil Science and Plant Nutrition 65: 11–19. doi:10.1080/00380768.2018.1547098.
- Han, M., and G. Jin. 2018. “Seasonal Variations of Q10 Soil Respiration and Its Components in the Temperate Forest Ecosystems, Northern China.” European Journal of Soil Biology 85: 36–42. doi:10.1016/j.ejsobi.2018.01.001.
- Heater, M., J. A. Throckmorton, N. M. Bird, N. Monte, T. Doane, M. K. Firestone, and W. R. Horwath. 2015. “The Soil Matrix Increases Microbial C Stabilization in Temperatute and Tropical Forest Soils.” Biogeochemistry 122: 35–45. doi:10.1007/s10533-014-0027-6.
- Ikeya, K., and A. Watanabe. 2003. “Direct Expression of an Index for the Degree of Humification of Humic Acids Using Organic Carbon Concentration.” Soil Science and Plant Nutrition 49: 47–53. doi:10.1080/00380768.2003.10409978.
- Ikeya, K., T. Hikage, S. Arai, and A. Watanabe. 2011. “Size Distribution of Condensed Aromatic Rings in Various Soil Humic Acids.” Organic Geochemistry 42: 55–61. doi:10.1016/j.orggeochem.2010.10.006.
- Inoue, K. 1990. “Active Aluminium and Iron Components in Andisols and Related Soils.” Transactions of 14th International Congress of Soil Science, Vol. VII. International Society of Soil Science, Kyoto, pp. 153–158.
- Jobbágy, E. G., and R. B. Jackson. 2010. “The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation.” Ecological Applications 10: 2356–2367.
- Karhu, K., E. Hilasvuori, H. Friza, C. Biasi, H. Nykänen, J. Liski, P. Vanhala, J. Heinonsalo, and J. Pumpanen. 2017. “Priming Effect Increases with Depth in a Boreal Forest Soil.” Soil Biology and Biochemistry 99: 104–107. doi:10.1016/j.soilbio.2016.05.001.
- Kumada, K. 1987. Chemistry of Soil Organic Matter, 181–204. Amsterdam: Japan Scientific Societies Press, Elsevier.
- Kuwatsuka, S., K. Tsutsuki, and K. Kumada. 1978. “Chemical Studies on Soil Humic Acids: I. Elementary Composition.” Soil Science and Plant Nutrition 24: 337–347. doi:10.1080/00380768.1978.10433113.
- Kuzyakov, Y. 2010. “Priming Effects: Interaction between Living and Dead Organic Matter.” Soil Biology and Biochemistry 42: 1363–1371. doi: 10.1016/j.soilbio.2010.04.003.
- Kuzyakov, Y., J. K. Friedel, and K. Stahr. 2000. “Review of Mechanisms and Quantication of Priming Effects.” Soil Biology and Biochemistry 32: 1485–1498. doi:10.1016/S0038-0717(00)00084-5.
- Leppalammi-kujansuu, J., M. Salemma, D. B. Kleja, S. Linder, and H. S. Heimisaari. 2014. “Fine Root Turover and Litter Production of Norway Spruce in a Long-term Temperature and Nutrient Manipulation Experiment.” Plant and Soil 374: 73–88. doi:10.1007/s11104-013-1853-3.
- Meyer, N., H. Meyer, G. Welp, and W. Amelung. 2018. “Soil Respiration and Its Temperature Sensitivity (Q10): Rapid Acquisition Using Mid-infrared Spectroscopy.” Geoderma 323: 31–40. doi:10.1016/j.geoderma.2018.02.031.
- Ohtsuka, T., T. Akiyama, Y. Hashimoto, M. Inatomi, T. Sakai, S. Jia, W. Mo, S. Tsuda, and H. Koizumi. 2005. “Biometric Based Estimates of Net Primary Production (NPP) in a Cool-temperate Deciduous Forest Stand beneath a Flux Tower.” Agricultural and Forest Meteorology 134: 27–38. doi:10.1016/j.agrformet.2005.11.005.
- Schmidts, N. W., M. S. Torn, S. Abiven, T. Dittmar, G. Guggenberger, I. A. Janssens, M. Kleber, et al. 2011. “Persistence of Soil Organic Matter as an Ecosystem Property.” Nature 478: 49–56. doi:10.1038/nature10386.
- Shindo, H., and H. Honma. 2001. “Significance of Burning Vegetation in the Formation of Black Humic Acids in Japanese Volcanic Ash Soils.” In Humic Substances, Structures, Models and Function, edited by E. A. Ghabbour and G. Davies, 293–306. Cambridge, UK: Royal Society of Chemistry.
- Sollins, P., M. G. Kramer, C. Swanston, K. Lajtha, T. Filley, A. K. Aufdenkampe, R. Wagai, and R. D. Bowden. 2009. “Sequential Density Fractionation across Soils of Contrasting Mineralogy: Evidence for Both Microbial- and Mineral-controlled Soil Organic Matter Stabilization.” Biogeochemistry 96: 209–231. doi:10.1007/s10533-009-9359-z.
- Wada, K., and T. Higashi. 1976. “The Categories of Aluminium- and Iron-humus Complexes in Ando Soils Determined by Selective Dissolution.” Journal of Soil Science 27: 357–368. doi:10.1111/ejs.1976.27.issue-3.
- Wagai, R., A. W. Kishimoto-Mo, S. Yonemura, Y. Shirato, S. Hiradate, and Y. Yagasaki. 2013. “Linking Temperature Sensitivity of Soil Organic Matter Decomposition to Its Molecular Structure, Accessibility, and Microbial Physiology.” Global Change Biology 19: 1114–1125. doi:10.1111/gcb.12112.
- Wang, Q., Y. Wang, S. Wang, T. He, and L. Liu. 2014. “Fresh Carbon and Nitrogen Inputs Alter Organic Carbon Mineralization and Microbial Community in Forest Deep Soil Layers.” Soil Biology and Biochemistry 72: 145–151. doi:10.1016/j.soilbio.2014.01.020.
- Watanabe, A., D. B. McPhail, N. Maie, S. Kawasaki, H. A. Anderson, and M. V. Cheshire. 2006. “Electron Spin Resonance Characteristics of Humic Acids from a Wide Range of Soil Types.” Organic Geochemistry 36: 981–990. doi:10.1016/j.orggeochem.2005.03.002.
- Yashiro, Y., Y. Shizu, T. Adachi, T. Ohtsuka, N.Y.Lee, Y. Iimura, and H. Koizumi. 2012. “The Effect Of Dense Understory Dwarf Bamboo (Sasa Senanensis) on Soil Respiration before and after Clearcutting Of Cool Temperate Deciduous Broad-leaved Forest.” Ecological Research 27: 577–586. doi: 10.1007/s11284-012-0925-9.
- Yin, H., Y. Li, J. Xiao, Z. Xu, X. Cheng, and Q. Liu. 2013. “Enhanced Root Exudation Stimulates Soil Nitrogen Transformations in a Subalpine Coniferous Forest under Experimental Warming.” Global Change Biology 19: 2158–2167. doi:10.1111/gcb.12161.
- Yonebayash, K., and T. Hattori. 1988. “Chemical and Biological Studies on Environmental Humic Acids: I. Composition of Elemental and Functional Groups of Humic Acids.” Soil Science and Plant Nutrition 34: 571–584. doi:10.1080/00380768.1988.10416472.
- Zhu, B., and W. Cheng. 2011. “Rhizosphere Priming Effect Increases the Temperature Sensitivity of Soil Organic Matter Decomposition.” Global Change Biology 17: 2172–2183. doi:10.1111/gcb.2011.17.issue-6.