ABSTRACT
In this mini review, the importance of rhizosphere is focused. As the rhizosphere is underneath the soil, the analytical approach is still required from the viewpoints of understanding the interaction among root, soil and its interface. For this purpose, multi omics approach has been carried out with the effort to visualize the active rhizosphere area.
1. Introduction
Rhizosphere is known to differ from surrounding soils physically, chemically and biologically. These differences result from how the roots directly affect the soil and microorganisms and from considerable changes in nutrient concentrations due to plant root uptake. Such changes in mineral and organic compound concentrations also drive changes in biological activity in rhizosphere. It is thus demonstrated that biotic (plant and microorganism) and abiotic (soil) entities directly and indirectly develop close mutual relationships within rhizospheres. These relationships render the rhizosphere distinctive (). However, it is difficult to specify an area of soil as a rhizosphere. Most related work has been carried out on areas close to root surfaces.
2. Comparisons of gramineae and fabaceae
Levels of dry matter production per absorbed nitrogen are higher for Gramineae than for Fabaceae (Osaki, Shinano, and Tadano Citation1992). This difference is attributed to higher respiratory rates observed in Fabaceae (Shinano et al. Citation1993; Shinano, Osaki, and Tadano Citation1993). As higher respiratory rates are linked to metabolism, mainly through nitrogenous compounds, the reconstruction of nitrogenous compounds in plants is considered to be a central reason for higher rates of respiration observed in Fabaceae in isotope studies (Shinano, Osaki, and Tadano Citation1994). Based on these findings, we reintroduce the concept of growth efficiency to explain differences in productivity observed between Gramineae and Fabaceae (Osaki et al. Citation1996). The low levels of productivity observed cannot be attributed to strong energy requirements for the synthesis of proteins and/or lipids in Fabaceae, and differences are identified even during the vegetative growth stage when the chemical compositions of Gramenae and Fabacease are similar (Shinano, Osaki, and Tadano Citation1995). Thus, we suggest that the reconstruction of carbon and nitrogen along with retranslocation appear to be important. However, these phenomena indicate that Fabaceae plants require more carbon to produce the same amount of dry matter, and similar tendencies have been observed for other elements. Roots absorb nutrients from the soil in different ways, and for Gramineae, root lengths are important, while for Fabaceae, nutrient uptake levels per unit of root length (or root surface) are critical. Root activity has been thoroughly studied in relation to phosphorus uptake (i.e. in relation to the exudation of organic acid (Li, Shinano, and Tadano Citation1997; Luo et al. Citation1999)), and enzyme-acid phosphatase exudation (Wasaki et al. Citation1999, Citation2000, Citation2003a) plays an important role in the solubilization of less soluble phosphorus compounds in soil. In terms of nutrient use efficiency, it is also important to consider internal phosphorus utilization efficiency levels in relation to the nutrient efficiency of an entire plant (Shinano et al. Citation2001, Citation2005; Nanamori et al. Citation2004; Dissanayaka et al. Citation2018, Nishida et al. Citation2019).
3. Applying omics to the rhizosphere
In addition to conducting a transcriptome analysis on plant root gene expression (Wasaki et al. Citation2003b, Citation2003c), we perform a comprehensive analysis of root exudates. A proteome analysis of root exuding proteins was carried out using rice, as rice genome information has become available. Rice was aseptically grown, and then, protein was collected from the bathing solution; after concentration, a proteome analysis was carried out via nano-LC MS/MS (Nanoscale liquid chromatography coupled to tandem mass spectrometry). More than 100 proteins were detected and identified. Most include a signal peptide involved in secretion and are considered to serve as pathogenesis-related (PR) proteins (Shinano et al. Citation2011, Citation2013). As the expression patterns of some proteins are not known, we identify the rhizosphere as an important area in which plant selectively expresses proteins. While aseptically culturing itself can apply stress to a plant, we suggest that various PR proteins are constantly expressed in the rhizosphere before a pathogen attack. Further research on how these PR proteins fight against soil pathogens is needed.
A metabolite analysis was carried out by applying methods developed for plant metabolomes (Okazaki et al. Citation2008, Citation2009, Citation2010, Citation2012) and root exudates (Suzuki et al. Citation2009) using GC-MS (Gas chromatography-mass spectrometry). To improve the sensitivity of our analysis, CE-MS (Capillary electrophoresis-mass spectrometry) was introduced and responses to phosphorus nutrition were measured (Tawaraya et al. Citation2013, Citation2014, Citation2018). Root exudate is known to play an important role in solubilizing minerals (essential and nonessential elements) from clay and organic materials.
Plant uptake nutrients in the soil, and sometimes nonessential element are also absorbed. The uptake and accumulation of elements is carried out by transporters and through ion homeostasis in the body. To determine how plants regulate ion homeostasis, an ionomics approach was applied. Lotus japonicus has been used as a model plant for Fabaceae, and EMS (ethyl methane sulfonate)-treated seeds with several mutants have been obtained to examine varied behaviors of element accumulation (Chen et al. Citation2009a, Citation2009b). One mutant includes very low molybdenum concentrations with molybdenum transporter mutations (Duan et al. Citation2017). Ionome is also useful for investigating the effects of material application in soils on plant nutrient concentrations, especially for trace elements. The effects of manure application (Sha et al. Citation2012) and temperature on the accumulation of elements have been examined (Quadir et al. Citation2011).
4. Clarifying the role of rhizosphere microorganisms
Though Rhizobium and mycorrhiza are known to develop a close symbiotic relationship with plants, a large number of studies have reported on the role of various microorganisms in the rhizosphere without obvious relationships. Most of such research has been carried out to screen for beneficial microorganisms based on their specific roles as part of an index. Otherwise, microorganism diversity levels have been used by applying DGGE (Denaturing gradient gel electrophoresis) methods and so on. We have also used protease and phosphatase genes of microorganisms as indexes and have applied PCR (Polymerase chain reaction)-DGGE to investigate how soil microorganism behavior changes with the application of organic materials to the soil (Sakurai et al. Citation2007, Citation2008). The activity of such enzymes is enhanced with an increase in diversity and especially in the rhizosphere.
Furthermore, the importance of utilizing unavailable phosphorus resources in soil must also be considered. Phytate is the main organic phosphorus compound and plants cannot decompose and utilize phosphorus from phytate in the soil. Soil microorganisms seem to alter the phytate status of soil (Unno et al. Citation2005; Unno and Shinano Citation2013). When phytate is applied to the soil, in most cases, plants are not able to utilize this compound because plants do not exudate phytase to the rhizosphere. However, sometimes, when soil is used as a medium, plants can grow better with the addition of phytate (Unno et al. Citation2005). It has been speculated the introduction of soil microorganisms may decompose phytate and release phosphorus (mainly using phytate as a carbon source), and several isolates have been shown to exhibit this capacity. For a gnotobiotic environment (when Lotus japonicas and an isolate exhibiting phytase and phosphorus releasing activity are the only organisms in the system), this isolate promotes plant growth with an increase in phosphorus uptake, but this was not confirmed for soil conditions. This means that under actual soil conditions, non-culturable microorganisms and/or microbial complexes may play a role. To determine the role of rhizosphere microorganisms in the utilization of phytate, a metagenome analysis was applied. While several microorganisms have been categorized, differences in phytate utilization have not been determined. We analyzed functional gene categorizations and their relative existence in phytate based on the status of plants. When plant growth was promoted through the application of phytate, rhizosphere soil from the plant contributed more to citrate synthase and alkaline phosphatase (Unno and Shinano Citation2013). As phytic acid binds to metal ions (Al, Fe, etc.) and organic compounds and forms phytate in the soil, complexes such as citric acid are required to solubilize phytate. Furthermore, phytase, as one an alkaline phosphatase that enhances alkaline phosphatase genes, may contribute to the decomposition of phytate in rhizosphere soil. The improvement of information on soil microbial functional genes will further our understanding of the rhizosphere’s biological functions.
Hence, functional analyzes of rhizosphere microorganisms will prove useful. Microorganisms exhibit strong abilities, and it is not necessary for microbial communities to be composed of the same members. It is possible to form a metabolic pathway through the combination of functions. Thus, we must analyze rhizosphere soil and develop technical methods for regulate it from this perspective.
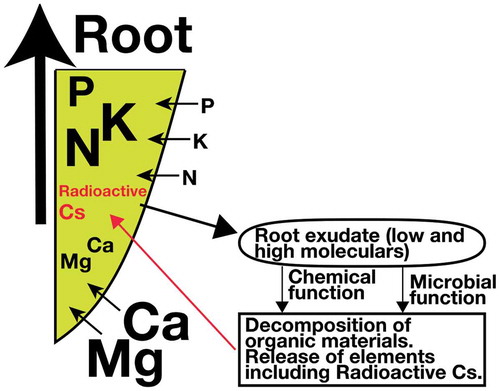
However, though the definition of the rhizosphere is clear (the sphere affected by root activity), it is difficult to precisely define rhizosphere soil. Most related research has been carried out based on the distance from the root surface or from physical strength of attachments to roots. The effects of the root on the soil occur more or less physically, chemically and biologically, but it is not possible to evaluate root activity (by active exudation) in the rhizosphere. Part of a photosynthate is known to translocate from the shoot to the root and then to the rhizosphere. Several trials have used 14CO2 to evaluate the distribution of photosynthate to roots. To specify the area of the rhizosphere, we choose 11C as radioactive carbon with a short half-life (20.334 minutes) to visualize the movement of photosynthate to roots and subsequent distribution to the soil. In examining plant species with strong root exuding capacities, we obtain a clear account of rhizosphere soil of high radioactivity, and we in turn analyze degrees of microbial diversity across different levels of radioactivity. By linking microbial functions, mineral element dynamics in soil and subsequent uptake by roots, we can better define the role of the rhizosphere (Wasaki et al. Citation2018).
5. Links to countermeasures of radioactive cesium
From recent studies developing countermeasures for radio cesium contamination in soil, we are interested in how potassium in clay is solubilized through plant activity. To mitigate radioactive cesium transport from soil to plants, potassium is typically applied (as fertilizer and/or potassium containing resources) in the field (Eguchi et al. Citation2015; Kubo et al. Citation2015, Citation2017; Yamamura et al. Citation2018). Though the mechanism to explain the role fo potassium on 137Cs has been thoroghly studied especially from the viewpoint of soil mineral characteristics (e.g. Kurokawa et al. Citation2019; Nakao et al. Citation2008; Yamaguchi et al. Citation2017; Ogasawara, Nakao, and Yanai Citation2017), it is stil unclear how the plant root interact with the dynamics of 137Cs in the rhizosphere. However, a remaining issue to address in coping with radioactivity in agricultural is high levels of Fabaceae transference. It is known that Lupine has the highest transfer factor, which is roughly 2 to 4 times higher than that for soybeans under the same conditions. The strong exudation capacities of Lupine have been speculated to play a role. As exudation is especially accelerated under phosphorus deficient conditions, we must determine to what extent and how roots exudate and affect soil to release radioactive cesium even under different soil conditions. These mechanisms may facilitate the development of new technologies that regulate radioactive cesium transfer from soils to plants.
Acknowledgments
This research was initiated under the guidance of the late Dr. Tanaka, Dr. Tadano and former Professor of Hokkaido University, Dr. Osaki.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Chen, Z., T. Watanabe, T. Shinano, K. Okazaki, and M. Osaki. 2009b. “Rapid Characterization of Plant Mutants with Altered Ion-profile: A Case Study Using Lotus Japonicus.” New Phytologist 181 (4): 795–801. doi:10.1111/j.1469-8137.2008.02730.x.
- Chen, Z., T. Watanabe, T. Shinano, T. Ezawa, J. Wasaki, K. Kimura, M. Osaki, and Y. G. Zhu. 2009a. “Element Interconnections in Lotus Japonicus: A Systematic Study of the Effects of Elements Additions on Different Natural Variants.” Soil Science and Plant Nutrition 55 (1): 91–101. doi:10.1111/j.1747-0765.2008.00311.x.
- Dissanayaka, D. M. S. B., S. Nishida, K. Tawaraya, and J. Wasaki. 2018. “Organ-specific Allocation Pattern of Acquired Phosphorus and Dry Matter in Two Rice Genotypes with Contrasting Tolerance to Phosphorus Deficiency.” Soil Science and Plant Nutrition 64 (3): 282–290. doi:10.1080/00380768.2018.1436941.
- Duan, G., T. Hakoyama, T. Kamiya, H. Miwa, F. Lombardo, S. Sato, S. Tabata, et al. 2017. “LjMOT1, a High-affinity Molybdate Transporter from Lotus Japonicus, Is Essential for Molybdate Uptake, but Not for the Delivery to Nodules.” The Plant Journal 90 (6): 1108–1119. doi:10.1111/tpj.13532.
- Eguchi, T., T. Ohta, T. Ishikawa, H. Matsunami, Y. Takahashi, K. Kubo, N. Yamaguchi, N. Kihou, and T. Shinano. 2015. “Influence of the Nonexchangeable Potassium of Mica on Radio Cesium Uptake by Paddy Rice.” Journal of Environmental Radioactivity 147: 33–42. doi:10.1016/j.jenvrad.2015.05.002.
- Kubo, K., K. Nemoto, H. Kobayashi, Y. Kuriyama, H. Harada, H. Matsunami, T. Eguchi, et al. 2015. “Analyses and Countermeasures for Decreasing Radioactive Cesium in Buckwheat in Areas Affected by the Nuclear Accident in 2011.” Field Crops Research 170: 40–46. doi:10.1016/j.fcr.2014.10.001.
- Kubo, K., S. Fujimura, H. Kobayashi, T. Ota, and T. Shinano. 2017. “Effect of Soil Exchangeable Potassium Content on Cesium Absorption and Partitioning in Buckwheat Grown in a Radioactive Cesium-contaminated Field.” Plant Production Science 20 (4): 396–405. doi:10.1080/1343943X.2017.1355737.
- Kurokawa, K., A. Nakao, T. Tsukada, Y. Mampuku, and J. Yanai. 2019. “Exchangeability of 137cs and K in Soils of Agricultural Fields after Decontamination in the Eastern Coastal Area of Fukushima.” Soil Science and Plant Nutrition. (online). doi:10.1080/00380768.2019.1622402.
- Li, M., T. Shinano, and T. Tadano. 1997. “Distribution of Exudates of Lupin Roots in the Rhizosphere under Phosphorus Deficient Conditions.” Soil Science and Plant Nutrition 43 (1): 237–245. doi:10.1080/00380768.1997.10414731.
- Luo, H. M., T. Watanabe, T. Shinano, and T. Tadano. 1999. “Comparison of Aluminum Tolerance and Phosphate Absorption between Rape (brassica Napus L.) And Tomato (lycopersicum Esculentum MilL.) In Relation to Organic Acid Exudation.” Soil Science and Plant Nutrition 45 (4): 897–907. doi:10.1080/00380768.1999.10414339.
- Nakao, A., Y. Thiry, S. Funakawa, and T. Kosaki. 2008. “Characterization of the Frayed Edge Site of Micaceous Minerals in Soil Clays Influenced by Different Pedogenetic Conditions in Japan and Northern Thailand.” Soil Science and Plant Nutrition 54 (4): 479–489. doi:10.1111/j.1747-0765.2008.00262.x.
- Nanamori, M., T. Shinano, J. Wasaki, T. Yamamura, I. M. Rao, and M. Osaki. 2004. “Low Phosphorus Tolerance Mechanisms: Phosphorus Recycling and Photosynthate Partitioning in the Tropical Forage Grass, Brachiaria Hybrid Cultivar Mulato Compared with Rice.” Plant and Cell Physiology 45 (4): 460–469. doi:10.1093/pcp/pch056.
- Nishida, S., D. M. S. B. Dissanayaka, S. Honda, Y. Tateishi, M. Chuba, H. Maruyama, K. Tawaraya, and J. Wasaki. 2018. “Identification of Genomic Regions Associated with Low Phosphorus Tolerance in Japonica Rice (oryza Sativa L.) By QTL-Seq.” Soil Science and Plant Nutrition 64 (3): 278–281. doi:10.1080/00380768.2017.1412238.
- Ogasawara, S., A. Nakao, and J. Yanai. 2017. “A Stepwise Change of Frayed Edge Site Content in Biotite in Response to the Gradual Release of Potassium from the Interlayers.” Soil Science and Plat Nutrition 63 (6): 529–535. doi:10.1080/00380768.2017.1402660.
- Okazaki, K., N. Oka, T. Shinano, M. Osaki, and M. Takebe. 2008. “Differences in the Metabolite Profiles of Spinach (spinacia Oleracea L.) Leaf in Different Concentrations of Nitrate in the Culture Solution.” Plant and Cell Physiology 49 (2): 170–177. doi:10.1093/pcp/pcm173.
- Okazaki, K., N. Oka, T. Shinano, M. Osaki, and M. Takebe. 2009. “Metabolic Profiling of Spinach (spinacia Oleracea L.) Leaves by Altering the Ratio of NH4+/NO3− in the Culture Solution.” Soil Science and Plant Nutrition 55 (4): 496–504. doi:10.1111/j.1747-0765.2009.00383.x.
- Okazaki, K., T. Shinano, N. Oka, and M. Takebe. 2010. “Metabolite Profiling of Raphanus Sativus L. To Evaluate the Effects of Manure Amendment.” Soil Science and Plant Nutrition 56 (4): 591–600. doi:10.1111/j.1747-0765.2010.00490.x.
- Okazaki, K., T. Shinano, N. Oka, and M. Takebe. 2012. “Metabolite Profiling of Komatsuna (brassica Rapa L.) Field-grown under Different Soil Organic Amendment and Fertilization Regimes.” Soil Science and Plant Nutrition 58 (6): 696–706. doi:10.1080/00380768.2012.733924.
- Osaki, M., M. Matsumoto, T. Shinano, and T. Tadano. 1996. “A Root-shoot Interaction Hypothesis for High Productivity of Root Crops.” Soil Science and Plant Nutrition 42 (2): 289–301. doi:10.1080/00380768.1996.10415099.
- Osaki, M., T. Shinano, and T. Tadano. 1992. “Carbon-nitrogen Interaction in Field Crop Production.” Soil Science and Plant Nutrition 38 (3): 553–564. doi:10.1080/00380768.1992.10415087.
- Quadir, Q. F., T. Watanabe, Z. Chen, M. Osaki, and T. Shinano. 2011. “Ionomic Response of Lotus Japonicus to Different Root-zone Temperatures.” Soil Science and Plant Nutrition 57 (2): 221–232. doi:10.1080/00380768.2011.555841.
- Sakurai, M., J. Wasaki, Y. Tomizawa, T. Shinano, and M. Osaki. 2008. “Analysis of Bacterial Communities on Alkaline Phosphatase Gene in Organic Matter Applied Soil.” Soil Science and Plant Nutrition 54 (1): 62–71. doi:10.1111/j.1747-0765.2007.00210.x.
- Sakurai, M., K. Suzuki, M. Onodera, T. Shinano, and M. Osaki. 2007. “Analysis of Bacterial Communities in Soil by PCR-DGGE Targeting Protease Genes.” Soil Biology and Biochemistry 39 (11): 2777–2784. doi:10.1016/j.soilbio.2007.05.026.
- Sha, Z., N. Oka, T. Watanabe, B. Tampubolon, K. Okazaki, M. Osaki, and T. Shinano. 2012. “Ionome of Soybean Seed Affected by Previous Cropping with Mycrorrhizal Plant and Manure Application.” Journal of Agricultural and Food Chemistry 60 (38): 9543–9552. doi:10.1021/jf3024744.
- Shinano, T., M. Nanamori, M. Dohi, J. Wasaki, and M. Osaki. 2005. “Evaluation of Phosphorus Starvation Inducible Genes Relating to Efficient Phosphorus Utilization in Rice.” Plant and Soil 269 (1–2): 81–87. doi:10.1007/s11104-004-5026-2.
- Shinano, T., M. Osaki, K. Komatsu, and T. Tadano. 1993. “Comparison of Production Efficiency of the Harvesting Organs among Field Crops. I. Growth Efficiency of the Harvesting Organs.” Soil Science and Plant Nutrition 39 (2): 269–280. doi:10.1080/00380768.1993.10416998.
- Shinano, T., M. Osaki, and T. Tadano. 1993. “Comparison of Production Efficiency among Field Crops Related to Nitrogen Nutrition and Application.” Plant and Soil 155 (1): 207–210. doi:10.1007/BF00025020.
- Shinano, T., M. Osaki, and T. Tadano. 1994. “14c-allocation of 14c-compounds Introduced to a Leaf to Carbon and Nitrogen Components in Rice and Soybean during Ripening.” Soil Science and Plant Nutrition 40 (2): 199–210. doi:10.1080/00380768.1994.10413294.
- Shinano, T., M. Osaki, and T. Tadano. 1995. “Comparison of Growth Efficiency between Rice and Soybean at the Vegetative Growth Stage.” Soil Science and Plant Nutrition 41 (3): 471–480. doi:10.1080/00380768.1995.10419609.
- Shinano, T., R. Yonetani, N. Ushihara, H. Adachi, J. Wasaki, H. Matsui, and M. Osaki. 2001. “Characteristics of Phosphoenolpyruvate Phosphatase Purified from Allium Cepa.” Plant Science 161 (5): 861–869. doi:10.1016/s0168-9452(01)00480-0.
- Shinano, T., S. Komatsu, T. Yoshimura, S. Tokutake, F. J. Kong, T. Watanabe, J. Wasaki, and M. Osaki. 2011. “Proteomic Analysis of Secreted Proteins from Aseptically Grown Rice.” Phytochemistry 72 (4–5): 312–320. doi:10.1016/j.phytochem.2010.12.006.
- Shinano, T., T. Yoshimura, T. Watanabe, Y. Unno, M. Osaki, Y. Nanjo, and S. Komatsu. 2013. “Effect of Phosphorus Levels on the Protein Profiles of Secreted Protein and Root Surface Protein of Rice.” Journal of Proteome Research 12 (11): 4748–4756. doi:10.1021/pr400614n.
- Suzuki, K., K. Okazaki, K. Tawaraya, M. Osaki, and T. Shinano. 2009. “Gas Chromatographymass Spectrometry Associated Global Analysis of Rice Root Exudates under Aseptical Condition.” Soil Science and Plant Nutrition 55 (4): 505–513. doi:10.1111/j.1747-0765.2009.00390.x.
- Tawaraya, K., R. Horie, A. Saito, T. Shinano, T. Wagatsuma, K. Saito, and A. Oikawa. 2013. “Metabolite Profiling of Shoot Extracts, Root Extracts, and Root Exudates of Rice Plant under Phosphorus Deficiency.” Journal of Plant Nutrition 36 (7): 1138–1159. doi:10.1080/01904167.2013.780613.
- Tawaraya, K., R. Horie, T. Shinano, T. Wagatsuma, K. Saito, and A. Oikawa. 2014. “Metabolite Profiling of Soybean Root Exudates under Phosphorus Deficiency.” Soil Science and Plant Nutrition 60 (5): 679–694. doi:10.1080/00380768.2014.945390.
- Tawaraya, K., R. Horie, T. Wagatsuma, K. Saito, and A. Oikawa. 2018. “Metabolite Profiling of Shoot Extract, Root Extract, and Root Exudate of Rice under Nitrogen and Phosphorus Deficiency.” Soil Science and Plant Nutrition 64 (3): 312–322. doi:10.1080/00380768.2018.1476828.
- Unno, Y., K. Okubo, J. Wasaki, T. Shinano, and M. Osaki. 2005. “Plant Growth Promotion Abilities and Microscale Bacterial Dynamics in the Rhizosphere of Lupin Analyses by Phytate Utilization Ability.” Environmental Microbiology 7 (3): 396–404. doi:10.1111/j.1462-2920.2004.00701.x.
- Unno, Y., and T. Shinano. 2013. “Metagenomic Analysis of the Rhizosphere Soil Microbiome with respect to Phytic Acid Utilization.” Microbes and Environment 28 (1): 120–127. doi:10.1264/jsme2.ME12181.
- Wasaki, J., J. Sakaguchi, T. Yamamura, S. Ito, T. Shinano, and M. Osaki. 2018. “P and N Deficiency Change the Relative Abundance and Function of Rhizosphere Microorganisms during Cluster Root Development of White Lupin (lupinus Albus L.).” Soil Science and Plant Nutrition 64 (6): 686–696. doi:10.1080/00380768.2018.1536847.
- Wasaki, J., M. Omura, M. Ando, H. Dateki, T. Shinano, M. Osaki, H. Ito, H. Matsui, and T. Tadano. 2000. “Molecular Cloning and Root Specific Expression of Secretory Acid Phosphatase from Phosphate Deficient Lupin (lupinus Albus L.).” Soil Science and Plant Nutrition 46 (2): 427–437. doi:10.1080/00380768.2000.10408796.
- Wasaki, J., M. Omura, M. Ando, T. Shinano, M. Osaki, and T. Tadano. 1999. “Secreting Portion of Acid Phosphatase in Roots of Lupin (lupinus Albus L.) And a Key Signal for the Secretion from the Roots?” Soil Science and Plant Nutrition 45 (4): 937–945. doi:10.1080/00380768.1999.10414343.
- Wasaki, J., R. Yonetani, S. Kuroda, T. Shinano, J. Yazaki, F. Fujii, K. Shimbo, et al. 2003c. “Transcriptomic Analysis of Metabolic Changes by Phosphorus Stress in Rice Plant Roots.” Plant, Cell and Environment 26 (9): 1515–1523. doi:10.1046/j.1365-3040.2003.01074.x.
- Wasaki, J., R. Yonetani, T. Shinano, and M. Osaki. 2003b. “Expression of the OsPI1 Gene, Cloned from Rice Roots Using cDNA Microarray, Rapidly Responds to Phosphorus Status.” New Phytologist 158 (2): 239–248. doi:10.1046/j.1469-8137.2003.00748.x.
- Wasaki, J., T. Yamamura, T. Shinano, and M. Osaki. 2003a. “Secreted Acid Phosphatase Is Expressed in Cluster Roots of Lupin in Response to Phosphorus Deficiency.” Plant and Soil 248 (1–2): 129–136. doi:10.1023/A:1022332320384.
- Yamaguchi, N., H. Tsukada, K. Kohyama, Y. Takata, A. Takeda, S. Isono, and I. Taniyama. 2017. “Radiocesium Interception Potential of Agricultural Soils in Northeast Japan.” Soil Science and Plant Nutrition 63 (2): 119–126. doi:10.1080/00380768.2017.1294467.
- Yamamura, K., S. Fujimura, T. Ota, T. Ishikawa, T. Saito, Y. Arai, and T. Shinano. 2018. “A Statistical Model for Estimating the Radiocesium Transfer Factor from Soil to Brown Rice Using the Soil Exchangeable Potassium Content.” Journal of Environmental Radioactivity 195: 114–125. doi:10.1016/j.jenvrad.2018.04.026.