ABSTRACT
There are many nitrogen (N) pools in soil, so their availability and different status can give information about bulk soil response to N deposition. However, the different size of N pools in forest soils and the relationship between them have not been well studied under N deposition when considering the role of litter. Here soil in an N-deposition experiment carried out for 5 years in a broad-leaved forest was used as an object to study the response of N pools to N deposition by stepwise extraction using water or solutions containing 0.5 M K2SO4, 2.5 M H2SO4 (LPI), or 13 M H2SO4 (LPII), and calculation of recalcitrant (RC) N pool. Under N control (CT), soil with the presence of litter had a higher N of 23.8–106.8% in the first four pools, but lower of 80.6% in recalcitrant N pool compared with soil with the absence of litter. In the absence of litter, N addition increased soil N in labile pool but decreased N in the RC pool compared to CT and these impacts were greater at high added N (HN) than low-added N (LN) rates. However, in the presence of litter, LN increased the amount of N in the K2SO4- extracted pool and HN reduced that in the water extracted pool. Additionally, LN and HN increased TN in the RC pool and HN increased the total soluble N (TSN) in the LPI and LPII pool. N changes in the water extraction pool were attributed to inorganic N, whereas they were NH4+ and soluble organic N (SON) in the K2SO4-extracted, LPI, and LPII pools. In the presence of litter, HN increased the SON concentration in the K2SO4, LPI, and LPII extractions; thus, SON may be a potentially important N form for N availability. These results suggested that N additions improve the accumulation of N in RC pool with the presence of litter. The different effects of N additions on soil N pool or N form in each pool depend on litter present or not.
1. Introduction
It was well known that most N in soil is in stable and organic forms (Warren Citation2014). Measurements of total N in soil do not reveal the amount of N available for plant and microbial utilization. Therefore, N in several pools and its transformation therein are important considerations in studies of N processes in soil and related ecosystems. In order to assess the turnover among the various N forms, several different methods are used, such as water, K2SO4, KCl, HCl, and H2SO4 extractions, which allow detecting the various forms of N (Rovira and Vallejo Citation2007; Ros et al. Citation2009; Chen et al. Citation2013). The N identified by these methods reflects the N status of water-soluble N (Xing et al. Citation2010; Cavalli et al. Citation2015), extractable organic N (EON) or dissolved organic N (DON) in soil (Ros et al. Citation2009; Ros, Hoffland, and Temminghoff Citation2010). Exchangeable N dominates the N available for plants and microbes and is the form typically used to assess absorbed and exchangeable N in soil solution (Jones and Willet Citation2006). It also is reported that the changes in the turnover rate of DON and EON pools were related to N mineralization and bioavailability (Ros, Hoffland, and Temminghoff Citation2010; Ros Citation2012). Acid-hydrolyzable N reflects the direct N pool for plants and microbes (Rovira and Vallejo Citation2002). The recalcitrant (RC) N pool is large and its turnover is slow, which represents the long-term storage of N in soil (Rovira and Vallejo Citation2007). The composition (Hagedorn, Saurer, and Blaser Citation2004) and dynamics of soluble organic N (Chen et al. Citation2013), the mineralization of organic N (Gao et al. Citation2016b) were paid the attention. In addition, much more N pools, such as soil microbial biomass N, water-extractable organic N, light fraction organic matter N, particulate organic matter N, and mineral N were considered to study the labile organic N transformations in clay and sandy-loam soils (Luce et al. Citation2014). However, the good studies including labile and recalcitrant N pools have not been performed, and the details of N transformation and the roles of the N pools are lacking (Olk et al. Citation2006; Gunina et al. Citation2014).
In the N-limited stage, N deposition and fertilization can act as a stimulator of N mineralization by removing microbial N limitation and reducing the C/N ratios of the substrate being decomposed. In the N-unlimited stage, N added to forest ecosystems can retard N mineralization (Gao et al. Citation2015). N addition decreased MBC, MBN, arbuscular mycorrhizal fungi, and the F/B ratio (ratio of fungi to bacteria biomass) in the subtropical forests (Tian et al. Citation2017). Microbial mineralization of labile organic N and of recalcitrant organic N showed the opposite in response to increasing NO3− additions (Gao et al. Citation2016b). N addition also affects soil-soluble total N and amino-acid-derived N in K2SO4 extracts (Chen et al. Citation2013) and can increase net N mineralization rates without increasing litter N (Brenner, Boone, and Ruess Citation2005). Therefore, N addition might differently decrease N in each soil pools without exogenous supplement, such as litter.
In forest ecosystems, net N transformation rates have been often investigated to characterize the effect of changing organic matter input on soil N cycling (Pan et al. Citation2009). The decomposition of litter is an important source of soil-available N (Li et al. Citation2004). Water-soluble organic matter (WSOM) from decomposing litter with different decomposition stages (Soong et al. Citation2015) and litter fragmentation (Carrillo et al. Citation2016) have a variable size of the flux and characteristics to enhance soil organic matter. Generally, soil inorganic N content increased with increasing organic matter input, and vice versa (Sayer et al. Citation2012; Wieder et al. Citation2013; Xu, Liu, and Sayer Citation2013). Furthermore, N retained in soil was three times from litter decomposition than from N addition (Nair, Perks, and Mencuccini Citation2017). Therefore, litter makes important contributions in maintaining current stocks of soil N (De Marco et al. Citation2013). Litter decomposition will affect N transformation (Booth, Stark, and Rastetter Citation2005) and N dynamics (Versini et al. Citation2014; Zhou et al. Citation2015), and N addition stimulates the decomposition of forest litter and protein (Yan et al. Citation2008; Vivanco and Austin Citation2011). Since the different microbial mineralization of labile organic N and recalcitrant organic N, and latter decreased with time in response to increasing NO3− additions (Gao et al. Citation2016b), N addition might increase recalcitrant N pool coupled with litter present.
Here we present the results of an N deposition experiment carried out for 5 years in a broad-leaf forest. The soil was collected and extracted stepwise using water, 0.5 M K2SO4, 2.5 M H2SO4, and 13 M H2SO4 to obtain the various N forms. The aim of the research was to determine the various forms of N and the effects of litter on N pools and turnover. We hypothesized that (1) the presence of litter increased the amount of N in each pool, (2) N addition decreased the amount of N in each pool in the absence of litter. (3) N addition increased recalcitrant N pool in the presence of litter.
2. Materials and methods
2.1. Site descriptions
The soil used in this study was collected from the Wanmulin Nature Reserve in Jianou, Fujian Province, China (118°09′E, 27°03′N), a subtropical monsoon climate zone, which is defined as Aquic Paleudults with strongly weathered due to high temperature and precipitation (USDA soil taxonomy, Soil Survey Staff Citation2014). The sampling site was on the southeast side of the Wuyi Mountains, at an altitude of 390 m, an aspect of slope 330°, a slope gradient of 20°, and a canopy density of 0.8. Mean annual precipitation in this area is 1731.4 mm; the mean annual air temperature is 19.4°C, the relative humidity 81%, and the annual frost-free period 227 days updated in 2014. The forest is dominated by a variety of evergreen broad-leaf trees, mainly Cinnamomum chekiangense (Nakai) but also Distyliopsis dunnii (Hemsl.), Engelhardtia fenzelii (Merr), Litsea subcoriace (Yang and Huang), and others. The main species of the sparsely distributed shrubs are Maesa japonica (Thunb.), Symplocos anomala (Brand), and Ardisia punctata (Lindl.). Herbaceous species are Sarcandra glabra (Thunb.), Euporbia hirta, and Woodwardia japonica (L.f.Sm.). There are no agriculture activities or anthropogenic N inputs within 1 km of the sampling site. The experiment site area is about 30 m × 40 m. The basic soil properties at the study site were collected and analyzed before N deposition experiment ().
Table 1. Basic soil properties (0–15-cm depth) at the study site on the Wanmulin Nature Reserve in Jian’ou, Fujian Province, China.
The N deposition experiment was carried out beginning in September 2009. Three N addition rates, control (CT, 0 kg N ha−1 yr−1), low N (LN, 30 kg N ha−1 yr−1), and high N (HN, 100 kg N ha−1 yr−1), and two litter conditions (absence of litter, A and Presence of litter, P) were considered in a randomized block design. Thus, there were 18 plots for six treatments with three replicates each. Each plot was 2 m × 2 m and separated from the others by >10 m to avoid mutual influences. NH4NO3 was used as the N source and prepared for the solution. The solution was spraying to the soil surface of the plots after diluted for 1or 2 L based on soil moisture in May, September, and December. Water was used in the control plots. The CT, LN, and HN application rates from September 2012 were adjusted to 0, 75, and 150 kg N ha−1 yr−1, respectively. The treatment in absence of litter was attained by putting up the nylon net above and aslant soil surface 1.5 m at least, which was fixed as 3 m × 3 m to avoid litter falling in treatment. In addition, we clear the litter on nylon net or soil surface every 2 months. We tried to place each plot between large trees, so only litter on soil was considered, not for floor plants in treatment.
2.2. Soil sampling and chemical analyses
Soil samples were collected for the N deposition experiment in August 2014 at a 15-cm soil depth using a 5-cm diameter corer. Three soil samples were taken randomly from within each of four 4-m2 blocks at each forest plot and then pooled. Therefore, each plot had three replicate bulk samples. After stones and visible plant material were removed, the soil was passed through a 2-mm sieve and then stored cool (4°C) until the analysis.
Water-soluble N: 8 g of dry soil was placed in each of three 50-mL tubes as fresh soil together with 40 mL of deionized water. After 1 h of shaking (250 rpm) on a vibrator, the tubes were centrifuged for 10 min (4000 rpm). The supernatant fluid was collected for N analysis; the soil residue in the tube was dried and used to obtain exchangeable N, as follows.
Exchangeable N: 4 g of the soil residue was placed in a 50-mL tube and mixed with 20 mL of 0.5 M K2SO4. The sample was then processed as described above. The soil residue was air-dried and used to obtain acid-hydrolyzable N as follows.
Acid-hydrolyzable N: The method was followed as a report in Rovira and Vallejo (Citation2007). Briefly, 500 mg of the soil residue from the exchangeable N procedure was mixed with 20 mL of a 2.5 M H2SO4 solution in a glass test tube and then incubated for 3 h at 105°C. After the contents had cooled, the tube was washed with 20 mL of deionized water, which together with the acid-treated soil was transferred to a 50-mL centrifuge tube. After 1 h of shaking (250 rpm) in a vibrator, the tubes were centrifuged for 10 min (4000 rpm) and the supernatant fluid was collected by passing filter paper as the first labile pool of N (LPI) for N analysis. The remaining residue in the tube was extracted for 12 h with 2 mL of a 13 M H2SO4 solution, with the samples placed on a vibrator. Deionized water was added to dilute the H2SO4 solution to 1 M, followed by a hydrolysis step for 3 h at 105°C and further processing as described above. This fraction is referred to herein as the labile pool of N II (LPII)
The total soluble N (TSN), ammonium (NH4+), and nitrate (NO3−) concentrations in each of the extracts were analyzed in a continuous flowing analyzer (Skalar SAN++, Netherlands). SON was calculated as the difference between TSN and inorganic N (NH4+ and NO3−). Soil total organic C and total N were determined by the combustion (Elementar Vario EL III, Elementar, Germany) of air-dried (40°C) milled samples passed through a 0.015-mm sieve. Recalcitrant C and N were calculated based on the difference between soil total C or N and the sum of C or N from the water and K2SO4 extracts and the acid hydrolysis.
2.3. Statistical analysis
All data were processed using Excel 2010 and graphs were plotted using the Origin 8.0 software. The N effect under litter treatment and the effect of litter treatment under N addition were statistically analyzed using SPSS 19.0 in a single-factor analysis of variance (one-way ANOVA) and the least significant difference (LSD) method. The effect of N, litter treatment, and their interaction effect on total N or each N form in each pool were performed by univariate analysis in generalized linear model.
3. Results
3.1. Effects of N addition on TSN in N pools
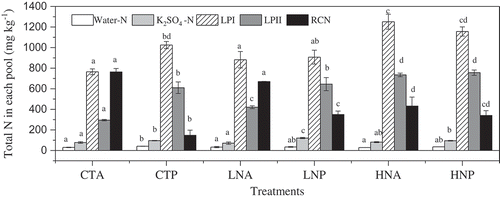
In the absence of litter, both the LN and HN treatments increased TSN in LPI, by 15.3% (p = 0.076) and 63.6% (p < 0.001), and in LPII, by 42.5% (p < 0.01) and 149.1% (p < 0.001), respectively, compared to the CT treatment. By contrast, TN in the RC pool decreased by 12.2% (p = 0.068) and 43.4% (p < 0.001), respectively (). In the presence of litter, LN and HN decreased TSN in water by 16.7% and 13.0% but increased TN in the RC pool by 136.4% (p < 0.05) and 129.9% (p < 0.01). Only HN increased TSN in the LPI and LPII extracts, by 12.8% (p = 0.07) and 24.0% (p < 0.01), respectively, compared to the CT. A significant (p < 0.05) increase in TSN of 17.0–106.8% in the presence vs. the absence of litter was determined for four extracts pools, mainly in the CT treatment but also in the K2SO4 extracts. However, a decrease in TN in the RC pool of 21.2–80.6% was measured and was also the largest in the CT treatment with litter remained compared to removal. These results suggest that the impact of N addition on soil N in each pool was related to the presence of litter ().
Table 2. Results of ANOVA comparing N additions, litter treatments, and their interaction on total N or each N form in each pool.
Figure 1. Effects of nitrogen deposition and litter on total N in the different N pools. CT indicated control treatment; LN and HN indicated nitrogen deposition treatment, such as low and high rates, respectively; A and P indicated two litter conditions, the absence of litter and presence of litter, respectively. Different small letters indicate significant differences (P < 0.05) among different treatments in the same N pool.
3.2. Effects of N addition on NH4+, NO3−, and SON in each pool
In the water extracts ()), NO3− was the dominant form, accounting for 56.3–73.7% of TSN. Compared with CT, NO3− was decreased by 15.8% and 22.3% (p < 0.05) in the LN and HN treatments, respectively, in the presence of litter. However, in the absence of litter NH4+ was increased by 33.1% and 120.7% (p < 0.01) in the LN and HN treatments, respectively. In the presence of litter, NH4+ was increased by 15.0% in the LN treatment and SON by 49.7% in the HN treatment compared to CT. Thus, compared to the litter removal treatments, in treatment with litter remained, inorganic N increased by 36.3–74.7% (p < 0.05) in the CT and LN treatments, and only NO3− by 19.2% and SON by 51.0% in the HN treatment, in which these findings contribute to the increase in TSN (). It was obvious that N addition or litter as main factor had a different effect on NO3− and NH4+ ().
Figure 2. Effects of nitrogen deposition and litter on the different N forms in the different N pools. CT indicated control treatment; LN and HN indicated nitrogen deposition treatment, such as low and high rates, respectively; A and P indicated two litter conditions, the absence of litter and presence of litter, respectively. Different small letters indicate significant differences (P < 0.05) among different treatments in the same N form.
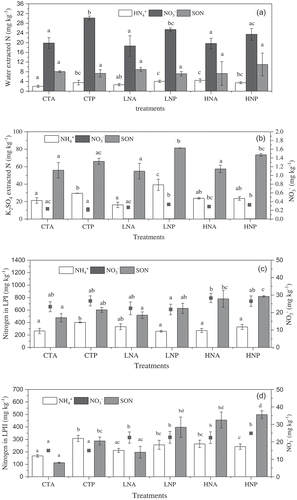
In the K2SO4 extracts ()), the proportion of NO3− in TSN was the smallest (0.3%), followed by NH4+ (27.9% on average), and SON (67.4–77.3%). Compared to CT, in the presence of litter LN treatment increased NH4+, NO3−, and SON by 32.9% (p < 0.05), 54.1% (p < 0.01), and 23.1% (p < 0.05), respectively, whereas in the absence of litter LN treatment decreased NH4+ by 23.8%. In the presence of liter, HN treatment decreased NH4+ by 20.3% and increased NO3− by 49.7% (p < 0.01) compared to CT. Also, compared to litter removal, in the presence of litter, NH4+ and SON increased by 38.8% (p < 0.05) and 18.2% in the CT treatment, NH4+, NO3−, and SON increased by 141.9% (p < 0.001), 25.5% (p < 0.05), and 48.4% (p < 0.01) in the LN treatment, and SON increased by 28.2% (p < 0.05) in the HN treatment. It was observed that N addition had a different effect on each N form, although the presence of litter increased N in this pool ().
When the samples were extracted by 2.5 M H2SO4 ()), NH4+ accounted for 21.7–39.2% and SON for 58.8–70.8% of TSN. NH4+ increased by 25.7% in the absence of litter and by 35.3% (p < 0.01) in the presence of litter in the LN vs. the CT treatment. In the absence of litter, SON increased by 63.4% (p < 0.01) whereas in the presence of litter NH4+ decreased by 18.0% (p < 0.05) and SON increased by 35.8% (p < 0.05) in the HN vs. CT treatment. Thus, compared to litter removal, in the CT treatment with litter remained NH4+ increased by 51.6% (p < 0.01) and SON by 26.2% while in the LN treatment NH4+ decreased by 22.0% and SON increased by 18.6%. It was found that the variable effect of N addition on NH4+ was dependent on litter present or not ().
In 13 M H2SO4 solution ()), NH4+ accounted for 32.0–56.8% and SON for 38.1–65.9% of TSN. With N addition, the proportion of NH4+ declined but that of SON increased. In the absence of litter, the amounts of all N forms increased compared to the CT sample: by 26.2–74.8% in the LN treatment and by 49.2% (p < 0.01) to 304.4% (p < 0.001) in the HN treatment. The increase in SON was the largest. However, in the presence of litter, NH4+ decreased by 16.8% and 21.4% (p < 0.05) in the LN and HN treatments, respectively, while both NO3− and SON increased, by 38.3–73.3%. Compared to the litter removal treatment, significant increases in NH4+ of 83.6% (p < 0.01) and in SON of 155.6% (p < 0.01) in the CT treatment exposed to litter remained. The corresponding values in the LN treatment were 21.1% and 102.1% (p < 0.01), respectively. By contrast, it was not observed in the HN treatment. It was also found that the variable effect of N addition on NH4+ was dependent on litter present or not in this pool ().
4. Discussion
It was reported that the compounds of the litter reflect the decomposition process, which in turn determines the type of matter subsequently released, as larger carbon inputs induce the mineralization and utilization of organic N (Li et al. Citation2004). And then some microbes use labile C to decompose recalcitrant organic matter by depolymerization to release some small molecular nitrogen-containing compound in order to acquire N (Moorhead and Sinsabaugh Citation2006; Craine, Morrow, and Fierei Citation2007). A similar study showed that carbon from litter contributes to the decomposition of recalcitrant organic matter under low soil N conditions (Craine, Morrow, and Fierei Citation2007). Therefore, in the CT soil, the presence of litter increased TSN in each pool, except for a decrease in the RC pool, which partially supported the first hypothesis.
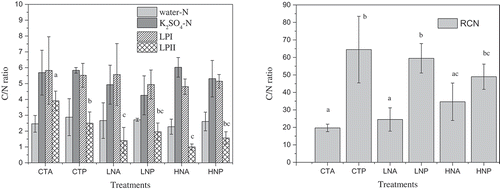
N inputs often accelerate net N mineralization rates, as a sequence: organic matter, organic nitrogen protein, and ammonium by depolymerization (Brenner, Boone, and Ruess Citation2005). Furthermore, the net N mineralization is negatively correlated with soil C/N ratio (Springob and Kirchmann Citation2003). Therefore, N addition induced the breakdown of recalcitrant organic matter, which had a high C/N ratio (). In the absence of litter, N addition improved the mineralization of N in RC pool to increase N in other pools, because of the lack of organic matter input. However, in the presence of litter, N addition not only increased N in the K2SO4, LPI, and LPII pool, but also LN and HN increased TN in the RC pool (). These results indicated that in the presence of litter, N addition is needed for increasing of N pool in RC, which was consistent with hypothesis three. Moreover, it was reported that N could induce the decomposition of litter (Liu et al. Citation2011) rather than mineralization of RC because litter has the smaller molecular and easily depolymerized matter, which may be the reason for increase of TN in RC due to N addition under litter present.
Figure 3. Carbon to nitrogen ratio of each N pool under different litter and N addition treatments. Different small letters indicate significant differences (P < 0.05) among different treatments in the same N pool.
The results in the present study showed that soil NO3− in the water pool and NH4+ in each pool increased in the presence of litter (), which was consistent with the report that increasing organic matter input increased soil inorganic N content (Sayer et al. Citation2012; Wieder et al. Citation2013; Xu, Liu, and Sayer Citation2013). Furthermore, litter remained was shown to be a significant source of water-soluble and acid hydrolysis, besides extractable inorganic nitrogen discussed by Xu, Liu, and Sayer (Citation2013) inputs to the mineral soil in (sub-) tropical forest, where rapid decomposition result in rapid turnover of mineral N. N in K2SO4 extracts is exchangeable N and mainly derives from the release of N absorbed in soil colloids and from organic-mineral compounds (Chen et al. Citation2013). Exchangeable N is the form typically used to assess absorbed and exchangeable N in soil solution (Jones and Willet Citation2006). Therefore, these results suggested the important role of litter was to enhance the N pools for their close relationship of water-soluble and exchangeable N pools. However, Wang et al. (Citation2015) reported soil N transformations were not significantly affected by decomposing litters during the 9-month of decomposition. Hu et al. (Citation2014) only found that the double residue treatment significantly increased the soil N concentration compared with the slash burning treatment in the 0–10 cm layer at year 3. Therefore, since the decomposition rates of organic matter in subtropical forests were faster, little remaining on the soil surface a few years after falling could provide a readily available N supply to the mineral soil (Roberts, Harrington, and Terry Citation2005).
In the presence of litter, N addition increased NH4+ in K2SO4 extracts in LN compared to CT and HN treatments, but NH4+ was decreased in the absence of litter, which suggests moderate N addition stimulated litter decomposition or soil N mineralization with litter input. Additionally, N addition decreased NO3− in water which may be due to its increase in LPII in the presence of litter (). Similarly, Gao et al. (Citation2016a) reported that NH4+ additions did not stimulate soil NO3− production and weaken soil retention of NO3− in the short term. These results suggested that increased NH4+ might slow down leaching losses of NO3− in the subtropical acidic forest. Using pyrolysis-field ionization mass spectrometry, Eshetu, Baum, and Leinweber (Citation2013) analyzed organic matter protected in clay and soil aggregates to show that they are mostly from the breakdown of litter or organic matter. And the decomposition of native organic matter in soil was shown to be induced by litter and inorganic N addition (Zhang and Wang Citation2012; Wang, He, and Liu Citation2016). According to this information, we hypothesize that both litter and inorganic N addition are important in the decomposition of different N sources, soil native N, and litter N (). And, it remains to be seen if this ‘replacement’ of older soil N with fresh N from increased litter inputs will have consequences for soil N stability in the longer term.
Xing et al. (Citation2010) found a significant positive correlation between microbial activity and SON, thus demonstrating the importance of the microbial community in SON production and its mineralization. In addition, since SON pool can be rapidly mineralized by soil microorganisms into mineral N (NH4+ and NO3−) (Jones et al. Citation2005), the higher SON in each pool except in water pool in the presence of litter treatment may be a drive to increase inorganic N. Zhong and Makeschin (Citation2003) studied soluble organic N in a German temperate forest soil and showed that one-third of the SON extracted by K2SO4 was utilized by microbes. However, we did not find a significant difference of SON in water following N addition or litter treatment, which suggested that SON is utilized by microbes rather than persisting in the water extract. This finding is consistent with the result reported by Haynes (Citation2000), who showed that SON is much more easily decomposed in water and can improve microbial activity. HN addition combined with litter present enhanced the SON in the K2SO4, LPI, and LPII extracts. Thus, the retained N in LPI and LPII pools may have been non-extractable as a report by Zhu and Wang (Citation2011), thereby providing a potentially important pool for N availability when the soluble and exchangeable pools are depleted or poor. Hence, it was reported that in the forest ecosystem, the decomposition of litter was a key factor to impact soil N dynamics (Zhou et al. Citation2015). Moreover, these effects of N addition on each pool may explain the non-changes of bulk soil in nitrogen acquisition enzymes and inconsistent results in terms of the effects of N deposition on soil N mineralization (Chen et al. Citation2018).
5. Conclusions
This study demonstrated the importance of both litter and N in soil N dynamics of each pool. In the presence of litter, recalcitrant N pools decreased but the other pools increased compared to litter removal. However, N addition increased the recalcitrant N pool compared to CT in the litter present. N changes in the water-soluble pool were attributed to inorganic N, but they were NH4+ and SON in the K2SO4, LPI, and LPII extracts. N addition to litter present decreased the leaching loss of soil NO3− in water. Under high nitrogen addition with litter present, SON in K2SO4 extracts, LPI and LPII pool may be an important form for N availability. Therefore, in decision-making on N management, the effects of N addition or deposition cannot be estimated based on a single factor but need to be adjusted according to the dynamics of litter decomposition. In addition, both the composition of SON in each N pool during different litter degradation stages and changes in N dynamic should be studied.
Highlights
Litter remained decreased soil recalcitrant nitrogen pool.
In the absence of litter, the higher was N addition, the more soil recalcitrant nitrogen pool was decreased.
In the presence of litter, N addition increased soil recalcitrant nitrogen pool.
Under high nitrogen addition with litter present, SON in LPI and LPII pools may be one potentially important form for nitrogen availability.
The different effects of N additions on soil N pool or N form in each pool depends on litter present or not.
Acknowledgments
This research is supported by the National Natural Science Foundation of China (Grant No. 31770659, 31170578, 31570607, and 31470628) and by the Natural Science Foundation of Fujian Province (2018J01716). The authors are very grateful to anonymous reviewers and responsible editors of this journal for valuable comments and suggestions to improve this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Booth, M. S., J. M. Stark, and E. Rastetter. 2005. “Controls on Nitrogen Cycling in Terrestrial Ecosystems: A Synthetic Analysis of Literature Data.” Ecological Monographs 75: 139–157. doi:10.1890/04-0988.
- Brenner, R. E., R. D. Boone, and R. W. Ruess. 2005. “Nitrogen Additions to Pristine, High-latitude, Forest Ecosystems: Consequences for Soil Nitrogen Transformations and Retention in Mid and Late Succession.” Biogeochemistry 72: 257–282. doi:10.2307/20055169.
- Carrillo, Y., B. A. Ball, and M. Molina. 2016. “Stoichiometric Linkages between Plant Litter, Trophic Interactions and Nitrogen Mineralization across the Litter–Soil Interface.” Soil Biology and Biochemistry 92: 102–110. doi:10.1016/j.soilbio.2015.10.001.
- Cavalli, D., G. Consolati, P. Marino, and L. Bechini. 2015. “Measurement and Simulation of Soluble, Exchangeable, and Non-exchangeable Ammonium in Three Soils.” Geoderma 259–260: 116–125. doi:10.1016/j.geoderma.2015.05.011.
- Chen, H., D. J. Li, J. Zhao, K. C. Xiao, and K. L. Wang. 2018. “Effects of Nitrogen Addition on Activities of Soil Nitrogen Acquisition Enzymes: A Meta-analysis.” Agriculture, Ecosystems & Environment 252: 126–131. doi:10.1016/j.agee.2017.09.032.
- Chen, X. Y., L. H. Wu, X. C. Cao, and Y. H. Zhu. 2013. “Organic Nitrogen Components in Soils from Southeast China.” Journal of Zhejiang University SCIENCE B 14: 259–269. doi:10.1631/jzus.B1200104.
- Craine, J. M., C. Morrow, and N. Fierei. 2007. “Microbial Nitrogen Limitation Increases Decomposition.” Ecology 88: 2105–2113. doi:10.1890/06-1847.1.
- De Marco, A., F. Esposito, B. Berg, M. Giordano, and A. Virzo De Santo. 2013. “Soil C and N Sequestration in Organic and Mineral Layers of Two Coeval Forest Stands Implanted on Pyroclastic Material (Mount Vesuvius, South Italy).” Geoderma 209–210: 128–135. doi:10.1016/j.geoderma.2013.06.011.
- Eshetu, B., C. Baum, and P. Leinweber. 2013. “Compost of Different Stability Affects the Molecular Composition and Mineralization of Soil Organic Matter.” Open Journal of Soil Science 3: 58–69. doi:10.4236/ojss.2013.31007.
- Gao, W., L. Kou, H. Yang, J. Zhang, C. Müller, and S. G. Li. 2016a. “Are Nitrate Production and Retention Processes in Subtropical Acidic Forest Soils Responsive to Ammonium Deposition?” Soil Biology and Biochemistry 100: 102–109. doi:10.1016/j.soilbio.2016.06.002.
- Gao, W., L. Kou, J. Zhang, C. Müller, H. Wang, H. Yang, and S. Li. 2016b. “Enhanced Deposition of Nitrate Alters Microbial Cycling of N in a Subtropical Forest Soil.” Biology and Fertility of Soils 52: 977–986. doi:10.1007/s00374-016-1134-4.
- Gao, W., H. Yang, L. Kou, and S. G. Li. 2015. “Effects of Nitrogen Deposition and Fertilization on N Transformations in Forest Soils: A Review.” Journal of Soils and Sediments 15: 863–879. doi:10.1007/s11368-015-1064-z.
- Gunina, A., M. A. Dippold, B. Glaser, and Y. Kuzyakov. 2014. “Fate of Low Molecular Weight Organic Substances in an Arable Soil: From Microbial Uptake to Utilisation and Stabilization.” Soil Biology and Biochemistry 77: 304–313. doi:10.1016/j.soilbio.2014.06.029.
- Hagedorn, F., M. Saurer, and P. Blaser. 2004. “A 13C Tracer Study to Identify the Origin of Dissolved Organic Carbon in Forested Mineral Soils.” European Journal of Soil Science 55: 91–100. doi:10.1046/j.1365-2389.2003.00578.x.
- Haynes, R. J. 2000. “Labile Organic Matter as an Indicator of Organic Matter Quality in Arable and Pastoral Soils in New Zealand.” Soil Biology and Biochemistry 32: 211–219. doi:10.1016/s0038-0717(99)00148-0.
- Hu, Z. H., Z. M. He, Z. Q. Huang, S. H. Fan, Z. P. Yu, M. H. Wang, X. H. Zhou, and C. M. Fang. 2014. “Effects of Harvest Residue Management on Soil Carbon and Nitrogen Processes in a Chinese Fir Plantation.” Forest Ecology and Management 326: 163–170. doi:10.1016/j.foreco.2014.04.023.
- Jones, D. L., D. Shannon, T. Junvee-Fortune, and J. F. Farrar. 2005. “Plant Capture of Free Amino Acids Is Maximized under High Soil Amino Acid Concentrations.” Soil Biology and Biochemistry 37: 179–181. doi:10.1016/j.soilbio.2004.07.021.
- Jones, D. L., and V. B. Willet. 2006. “Experimental Evaluation of Methods to Quantify Dissolved Organic Nitrogen (DON) and Dissolved Organic Carbon (DOC) in Soil.” Soil Biology and Biochemistry 38: 991–999. doi:10.1016/j.soilbio.2005.08.012.
- Li, Y., M. Xu, O. J. Sun, and W. C. Cui. 2004. “Effects of Root and Litter Exclusion on Soil CO2 Efflux and Microbial Biomass in Wet Tropical Forests.” Soil Biology and Biochemistry 36: 2111–2114. doi:10.1016/j.soilbio.2004.06.003.
- Liu, X. J., L. Duan, J. M. Mo, E. Z. Du, J. L. Shen, X. K. Lu, Y. Zhang, X. B. Zhou, C. E. He, and F. S. Zhang. 2011. “Nitrogen Deposition and Its Ecological Impact in China: An Overview.” Environmental Pollution 159: 2251–2264. doi:10.1016/j.envpol.2010.08.002.
- Luce, M. S., J. K. Whalen, Z. Noura, B. J. Zebarth, and M. H. Chantigny. 2014. “Labile Organic Nitrogen Transformations in Clay and Sandy-loam Soils Amended with 15N-labelled Faba Bean and Wheat Residues.” Soil Biology and Biochemistry 68: 208–218. doi:10.1016/j.soilbio.2013.09.033.
- Moorhead, D. L., and R. L. Sinsabaugh. 2006. “A Theoretical Model of Litter Decay and Microbial Interaction.” Ecological Monographs 76: 151–174. doi:10.1890/0012-9615(2006)076[0151:ATMOLD]2.0.CO;2.
- Nair, R. K., M. P. Perks, and M. Mencuccini. 2017. “Decomposition Nitrogen Is Better Retained than Simulated Deposition from Mineral Amendments in a Temperate Forest.” Global Change Biology 23: 1711–1724. doi:10.1111/gcb.13450.
- Olk, D. C., K. G. Cassman, K. Schmidt-Rohr, M. M. Anders, J. D. Mao, and J. L. Deenik. 2006. “Chemical Stabilization of Soil Organic Nitrogen by Phenolic Lignin Residues in Anaerobic Agroecosystems.” Soil Biology and Biochemistry 38: 3303–3312. doi:10.1016/j.soilbio.2006.04.009.
- Pan, K. W., Z. H. Xu, T. Blumfield, S. Tutua, and M. X. Lu. 2009. “Application of (15NH4)2SO4 to Study N Dynamics in Hoop Pine Plantation and Adjacent Native Forest of Subtropical Australia: The Effects of Injection Depth and Litter Addition.” Journal of Soils and Sediments 9: 515–525. doi:10.1007/s11368-009-0131-8.
- Roberts, S. D., C. A. Harrington, and T. A. Terry. 2005. “Harvest Residue and Competing Vegetation Affect Soil Moisture, Soil Temperature, N Availability, and Douglas-fir Seedling Growth.” Forest Ecology and Management 205: 333–350. doi:10.1016/j.foreco.2004.10.036.
- Ros, G. H. 2012. “Predicting Soil N Mineralization Using Organic Matter Fractions and Soil Properties: A Re-analysis of Literature Data.” Soil Biology and Biochemistry 45: 132–135. doi:10.1016/j.soilbio.2011.10.015.
- Ros, G. H., E. Hoffland, and E. J. M. Temminghoff. 2010. “Dynamics of Dissolved and Extractable Organic Nitrogen upon Soil Amendment with Crop Residues.” Soil Biology and Biochemistry 42: 2094–2101. doi:10.1016/j.soilbio.2010.08.004.
- Ros, G. H., E. Hoffland, C. van Kessel, and E. J. M. Temminghoff. 2009. “Extractable and Dissolved Soil Organic nitrogen-A Quantitative Assessment.” Soil Biology and Biochemistry 41: 1029–1039. doi:10.1016/j.soilbio.2009.01.011.
- Rovira, P., and V. R. Vallejo. 2002. “Labile and Recalcitrant Pools of Carbon and Nitrogen in Organic Matter Decomposing at Different Depths in Soil: An Acid Hydrolysis Approach.” Geoderma 107: 109–141. doi:10.1016/s0016-7061(01)00143-4.
- Rovira, P., and V. R. Vallejo. 2007. “Labile, Recalcitrant, and Inert Organic Matter in Mediterranean Forest Soils.” Soil Biology and Biochemistry 39: 202–215. doi:10.1016/j.soilbio.2006.07.021.
- Sayer, E. J., S. J. Wright, E. V. J. Tanner, J. B. Yavitt, K. E. Harms, J. S. Powers, M. Kaspari, M. N. Garcia, and B. L. Turner. 2012. “Variable Responses of Lowland Tropical Forest Nutrient Status to Fertilization and Litter Manipulation.” Ecosystems 15: 387–400. doi:10.2307/41507786.
- Soil Survey Staff. 2014. Keys to Soil Taxonomy. 12th ed. Washington, DC: USDA-Natural Resources Conservation Service.
- Soong, J. L., W. J. Parton, F. Calderon, E. E. Campbell, and M. F. Cotrufo. 2015. “A New Conceptual Model on the Fate and Controls of Fresh and Pyrolized Plant Litter Decomposition.” Biogeochemistry 124: 27–44. doi:10.1007/s10533-015-0079-2.
- Springob, G., and H. Kirchmann. 2003. “Bulk Soil C to N Ratio as a Simple Measure of Net N Mineralization from Stabilized Soil Organic Matter in Sandy Arable Soils.” Soil Biology and Biochemistry 35: 629–632. doi:10.1016/s0038-0717(03)00052-x.
- Tian, D., L. Jiang, S. H. Ma, W. J. Fang, B. Schmid, L. C. Xu, J. Zhu, et al. 2017. “Effects of Nitrogen Deposition on Soil Microbial Communities in Temperate and Subtropical Forests in China.” Science of the Total Environment 607–608: 1367–1375. doi:10.1016/j.scitotenv.2017.06.057.
- Versini, A., L. Mareschal, T. Matsoumbou, B. Zeller, J. Ranger, and J. P. Laclau. 2014. “Effects of Litter Manipulation in a Tropical Eucalyptus Plantation on Leaching of Mineral Nutrients, Dissolved Organic Nitrogen and Dissolved Organic Carbon.” Geoderma 232-234: 426–436. doi:10.1016/j.geoderma.2014.05.018.
- Vivanco, L., and A. T. Austin. 2011. “Nitrogen Addition Stimulates Forest Litter Decomposition and Disrupts Species Interactions in Patagonia, Argentina.” Global Change Biology 17: 1963–1974. doi:10.1111/j.1365-2486.2010.02344.x.
- Wang, Q. K., T. X. He, and J. Liu. 2016. “Litter Input Decreased the Response of Soil Organic Matter Decomposition to Warming in Two Subtropical Forest Soils.” Scientific Reports 6: 33814. doi:10.1038/srep33814.
- Wang, Y. Z., Z. H. Xu, J. Q. Zheng, K. M. Abdullah, and Q. X. Zhou. 2015. “δ 15N of Soil Nitrogen Pools and Their Dynamics under Decomposing Leaf Litters in a Suburban Native Forest Subject to Repeated Prescribed Burning in Southeast Queensland, Australia.” Journal of Soils and Sediments 15: 1063–1074. doi:10.1007/s11368-015-1117-3.
- Warren, C. R. 2014. “Organic N Molecules in the Soil Solution: What Is Known, What Is Unknown and the Path Forwards.” Plant and Soil 375: 1–19. doi:10.1007/s11104-013-1939-y.
- Wieder, W. R., C. C. Cleveland, P. G. Taylor, D. R. Nemergut, E. L. Hinckley, L. Philippot, D. Bru, S. R. Weintraub, M. Martin, and A. R. Townsend. 2013. “Experimental Removal and Addition of Leaf Litter Inputs Reduces Nitrate Production and Loss in a Lowland Tropical Forest.” Biogeochemistry 113: 629–642. doi:10.1007/s10533-012-9793-1.
- Xing, S., C. Chen, B. Zhou, H. Zhang, Z. M. Nang, and Z. H. Xu. 2010. “Soil Soluble Organic Nitrogen and Active Microbial Characteristics under Adjacent Coniferous and Broadleaf Plantation Forests.” Journal of Soils and Sediments 10: 748–757. doi:10.1007/s11368-009-0159-9.
- Xu, S., L. L. Liu, and E. J. Sayer. 2013. “Variability of Above-ground Litter Inputs Alters Soil Physicochemical and Biological Processes: A Meta-analysis of Litterfall-manipulation Experiments.” Biogeosciences 10: 7423–7433. doi:10.5194/bg-10-7423-2013.
- Yan, E. R., X. H. Wang, J. J. Huang, and W. Zhou. 2008. “Decline of Soil Nitrogen Mineralization and Nitrification during Forest Conversion of Evergreen Broad-leaved Forest to Plantations in the Subtropical Area of Eastern China.” Biogeochemistry 89: 239–251. doi:10.1007/s10533-008-9216-5.
- Zhang, W. D., and S. L. Wang. 2012. “Effects of NH4+ and NO3− on Litter and Soil Organic Carbon Decomposition in a Chinese Fir Plantation Forest in South China.” Soil Biology & Biochemistry 47: 116–122. doi:10.1016/j.soilbio.2011.12.004.
- Zhong, Z., and F. Makeschin. 2003. “Soluble Organic Nitrogen in Temperate Forest Soils.” Soil Biology and Biochemistry 35: 333–338. doi:10.1016/s0038-0717(02)00252-3.
- Zhou, W. J., L. Q. Sha, D. A. Schaefer, Y. P. Zhang, Q. H. Song, Z. H. Tan, Y. Deng, X. B. Deng, and H. L. Guan. 2015. “Direct Effects of Litter Decomposition on Soil Dissolved Organic Carbon and Nitrogen in a Tropical Rainforest.” Soil Biology and Biochemistry 81: 255–258. doi:10.1016/j.soilbio.2014.11.019.
- Zhu, W. X., and W. W. Wang. 2011. “Does Soil Organic Matter Variation Affect the Retention of 15NH4+ and 15NO3− in Forest Ecosystems?” Forest Ecology and Management 261: 675–682. doi:10.1016/j.foreco.2010.11.024.
