ABSTRACT
Phosphorus (P) is a macronutrient essential for plant growth and productivity. Plants uptake P as inorganic phosphate (Pi); however, in the natural ecosystem, Pi availability is frequently a severe limiting factor for plant growth. Thus, plants have evolved several mechanisms, such as the expression of Pi starvation-responsive genes, to adapt to Pi deficient conditions. Although we recently reported that phytochrome (Phy)-mediated red light signaling promotes Pi uptake by increasing expression levels of such genes in the model plant Arabidopsis, it remains elusive whether a similar mechanism exists in agricultural crops. In the present study, we analyzed the effects of red light signaling on Pi uptake in rice (Oryza sativa L.) using osphyA and osphyB single mutants, and the osphyA osphyB double mutant. Unlike osphyA seedlings, osphyB seedlings showed a reduction in Pi uptake and Pi content. Furthermore, illumination of wild-type seedlings with red light significantly promoted Pi uptake, whereas illumination with blue or far-red light did not. The osphyB mutant showed reduced expression levels of several Pi starvation-responsive genes including Pi transporter genes. Additionally, these phenotypes of osphyB knockout mutants were much more evident under Pi deficient conditions than under Pi sufficient conditions. Moreover, red light promoted Pi uptake in seedlings of other plant species including broccoli (Brassica oleracea L. var. italica) and lettuce (Latuca sativa L.). These results suggest that OsPhyB-mediated red light signaling promotes Pi uptake in rice by up-regulating the expression of Pi starvation response-associated genes, and this phenomenon may be conserved in a wide range of plant species.
1. Introduction
Phosphorus (P) is an essential macronutrient and a constituent of essential organic compounds, including adenosine triphosphate (ATP), nucleic acids, nicotinamide adenine dinucleotide phosphate (NADP+), phospholipids, and phosphoproteins. Plants acquire P from the soil as inorganic phosphate (Pi) (Schachtman, Reid, and Ayling Citation1998). In the natural ecosystem, availability of Pi is frequently lower than the amount needed to sustain plant growth and development, since Pi ions are immediately immobilized in the soil, given their reactivity with cations such as calcium (Ca2+) and magnesium (Mg2+) ions in alkaline soil, and iron (Fe3+) and aluminum (Al3+) ions in acidic soil (López-Arredondo et al. Citation2014). Although Pi fertilizers are applied to an agricultural field, only 20–30% of the applied Pi is absorbed by plant roots, while the remaining is retained in the soil in a passive state. Therefore, Pi fertilizers are applied in excess, which causes environmental problems such as eutrophication (Correl Citation1998). Therefore, understanding the regulatory mechanisms underlying Pi acquisition and utilization in plants is important for developing sustainable agriculture and maintaining crop productivity in highly diverse Pi environments.
In the past few decades, a number of genes associated with Pi acquisition and starvation responses have been identified, thus advancing our understanding of the molecular mechanism underlying Pi availability-dependent modulation of Pi acquisition (Nussaume et al. Citation2011; Rouached, Arpat, and Poirier Citation2010). Besides Pi availability, other soil nutrients and environmental stimuli also affect Pi acquisition and utilization to optimize plant growth. For instance, high availability of nitrate (NO3−) induces Pi acquisition through the NIN-LIKE PROTEIN (NLP)-NITRATE-INDUCIBLE GARP-TYPE TRANSCRIPTIONAL REPRESSOR1 (NIGT1)-SPX transcriptional cascade (Ueda, Kiba, and Yanagisawa Citation2020) and through post-translational regulation of NIGT1 (Medici et al. Citation2019). However, such mechanisms remain largely elusive.
Light is one of the many critical environmental factors affecting plant growth and development. Plants possess several types of photoreceptors that perceive light across a broad spectrum, ranging from 300 to 800 nm, and induce wavelength-specific responses (Möglich et al. Citation2010). Among the plant photoreceptors, phytochromes (Phys) function as receptors for red light (approximately 600–700 nm) and far-red light (700–800 nm) (Li et al. Citation2011). Phys were intensively characterized in the model plant Arabidopsis thaliana. The Arabidopsis genome encodes five Phy proteins (PhyA–E) (Sharrock and Clack Citation2002). Among these, PhyA and PhyB play major roles in regulating a variety of light-responsive processes including seed germination (Reed et al. Citation1994), de-etiolation (McNellis and Deng Citation1995), shade avoidance (Smith and Whitelam Citation1997), leaf senescence (Sakuraba et al. Citation2014), and flowering (Reed et al. Citation1994). However, since PhyA and PhyB are responsible for responses to far-red light and red light, respectively (Reed et al. Citation1994), they play different roles in light responses. Recently, we showed that PhyB-mediated red light signaling stimulates Pi acquisition in Arabidopsis roots (Sakuraba et al. Citation2018). Red light signaling elevated the expression level of a major Pi transporter gene, PHOSPHATE TRANSPORTER1;1 (PHT1;1), with the help of PHYTOCHROME INTERACTING FACTR 4 (PIF4), PIF5, and ELONGATED HYPOCOTYL 5 (HY5) transcription factors (Sakuraba et al. Citation2018). However, the promotion of Pi acquisition by PhyB-mediated red light signaling has not yet been demonstrated in economically important agricultural crops.
In rice (Oryza sativa L.), three Phy genes, OsPhyA–C, have been identified (Kay et al. 1989), and physiological functions of the encoded proteins have been investigated using their knockout mutants (Takano, Inagaki, and Xie et al. Citation2005; Jeong et al. Citation2007). Similar to Arabidopsis Phys, OsPhyA and OsPhyB regulate seedling de-etiolation (Takano, Inagaki, and Xie et al. Citation2005), leaf elongation (Takano, Inagaki, and Xie et al. Citation2005), anther and pollen development (Sun et al. Citation2017), and flowering (Takano, Inagaki, and Xie et al. Citation2005). However, it has also been proposed that OsPhyA and OsPhyB exhibit both overlapping and distinct roles in light-dependent biological processes (Takano, Inagaki, and Xie et al. Citation2005; Piao et al. Citation2015).
In this study, we examined the effects of Phy-mediated red light signaling on Pi uptake and accumulation in rice using osphyA and osphyB single mutants and the osphyA osphyB double mutant. We also investigated the effects of mutations in OsPhyA and OsPhyB genes on the expression of Pi starvation response-associated genes. The results of these analyses, together with the detection of red light-induced promotion of Pi uptake in broccoli (Brassica oleracea L. var. italica) and lettuce (Latuca sativa L.) seedlings, suggest that the promotion of Pi acquisition by Phy-mediated red light signaling is conserved across diverse plant species.
2. Materials and methods
2.1. Plant materials and growth conditions
Rice (Oryza sativa L.) cultivar Dong-jin was used as the wild type (WT) in this study. Rice T-DNA insertion mutant lines in Dong-jin background, osphyB-1 (PFG_2D-20,484.R), osphyB-2 (PFG_4A-02226.R), and osphyA-3 (PFG_3A-16,812.R) were obtained from the Crop Biotech Institute at Kyung Hee University, Republic of Korea (Jeon et al. 2000; Piao et al. Citation2015). The transcript levels of OsphyA in osphyA-3 and OsphyB in osphyB-1 and osphyB-2 were previously examined, revealing that they are osphyA and osphyB knockout mutants (Jeong et al. Citation2007; Piao et al. Citation2015). The osphyB-2 osphyA-3 double mutant line was generated by crossing osphyB-2 and osphyA-3 single mutants. WT and mutant seedlings were grown hydroponically in a growth chamber at 28°C under continuous white light (100 μmol m−2s−1) in Yoshida nutrient solution (Yoshida et al. Citation1976). To conduct Pi sufficient or deficient treatments, the concentration of Pi in the nutrient solution was changed to 100 or 5 μM, respectively.
2.2. Measurement of Pi uptake activity using 33P-labeled Pi
Rice seedlings were grown hydroponically in Pi sufficient nutrient solution for 5 days. Subsequently, seedlings were transferred to either Pi deficient or Pi sufficient solution, and grown for five more days. To determine the effects of different light wavelengths on Pi uptake, rice seedlings were grown in Pi sufficient solution for 5 days and then in Pi deficient solution for 5 days under continuous white light (100 μmol m−2 s−1). Then, seedlings were either illuminated (with white, red, far-red, or blue light) or maintained in the dark for 1 day under Pi deficient conditions. The intensity of white, red, far-red, and blue light was adjusted to 70 μmol m−2 s−1 using light-emitting diodes (LEDs) (Bio Medical Science Co., Tokyo, Japan). Then, rice seedlings were transferred to 1/10 Yoshida nutrient solution containing 100 kBq ml−1 33P-labeled Pi and incubated for 60 min under 70 μmol m−2 s−1 light intensity. Since Pi uptake in the seedlings incubated with 33P-labeled Pi solution for different periods revealed that Pi uptake was not saturated within 60 min (Supplementary Figure 1), incubation for 60 min was applied to the measurement of Pi uptake activity in the current study.
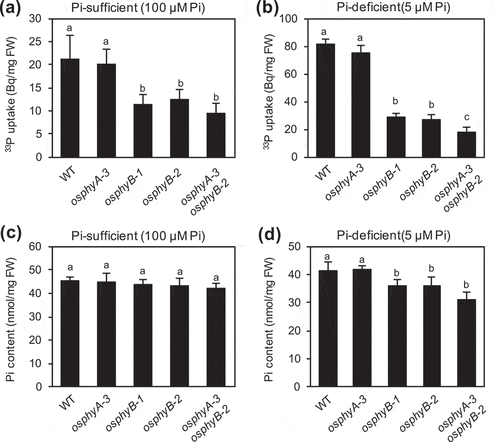
Figure 1. Effects of OsPhyB knockout mutations on the uptake and content of Pi in rice seedlings. (a, b) Quantification of 33P-labeled Pi uptake in wild-type (WT), osphyA-3, osphyB-1, osphyB-2, and osphyA-3 osphyB-2 seedlings grown under Pi sufficient conditions for 5 days and then under Pi sufficient (a) or Pi deficient (b) conditions for 5 days. Data represent mean ± standard deviation (SD) of four biological replicates. (c, d) Pi contents of WT, osphyA-3, osphyB-1, osphyB-2, and osphyA-3 osphyB-2 seedlings grown under Pi sufficient conditions for 5 days and then under Pi sufficient (c) or Pi deficient (d) conditions for 5 days. Data represent mean ± SD of five biological replicates. Different lowercase letters above each bar indicate significant differences between means (P < 0.05; Tukey’s multiple comparison test)
To measure Pi uptake activity in other plant species, seedlings of broccoli (Brassica oleracea L. var. italica), spinach (Spinacia oleracea), and lettuce (Latuca sativa L.) were grown hydroponically in Pi sufficient liquid medium containing half-strength N-, P-, and Fe-free Murashige and Skoog (MS) salts, 2 mM KNO3, 2 mM NH4NO3, 500 mM NaH2PO4, 1 mM FeSO4, and 0.5% (w/v) sucrose for 5 days, followed by Pi deficient liquid medium (i.e., Pi sufficient liquid medium lacking NaH2PO4) for 5 days, and were then illuminated with white or red light (70 μmol m−2 s−1) for 1 day. Then, the seedlings were transferred to Pi deficient liquid medium containing 50 kBq ml−1 33P-labeled Pi and incubated for 1 h under 70 μmol m−2 s−1 light intensity. To monitor the uptake of 33P-labeled Pi, images of seedling roots were captured using an imaging plate (Fujifilm Co., Tokyo, Japan) and FLA-5000 Fluorescent Image Analyzer (Fujifilm Co.), as described previously (Sakuraba et al. Citation2018). The amount of radiolabeled Pi in roots was quantified using the ImageQuant TL software (GE Healthcare Japan Co., Tokyo, Japan), as described previously (Sakuraba et al. Citation2018).
2.3. Measurement of Pi concentration by ion chromatography
WT, osphyB-1, osphyB-2, osphyA-3, and osphyA-3 osphyB-2 seedlings, grown under Pi sufficient conditions for 5 days and then under Pi sufficient or deficient conditions for five additional days, were frozen in liquid nitrogen. The frozen seedlings were ground to a fine powder using zirconia beads and a vortex mixer, and 20 mg of the powder was suspended in 1 ml Milli-Q water. After centrifugation at 12,000 rpm for 10 min, the supernatant was transferred to a clean tube, and Pi concentration was measured using the ICS-3000 ion chromatography system (DIONEX Co., Sunnyvale, CA), according to the manufacturer’s protocol.
2.4. RNA extraction and gene expression analysis
Total RNA was isolated from rice seedling roots using the ISOSPIN Plant RNA Kit (Nippon Gene Co., Ltd., Tokyo, Japan), according to the manufacturer’s protocol. First-strand cDNA was synthesized from 1 μg total RNA using SuperScript™ II reverse transcriptase and oligo(dT)15 primers (Invitrogen, Thermo Fisher Scientific, Inc., Waltham, MA) in 20 μl reverse transcriptase reaction mixture. Then, quantitative PCR coupled with reverse transcription (RT-qPCR) was performed on the StepOnePlus Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific, Inc., Waltham, MA) in 10 μl PCR reaction mixture that contained 1 μl of the reverse transcriptase reaction mixture, 5 μl of 2 × KAPA SYBR Fast qPCR mixture (Nippon Gene Co. Ltd.), and 0.25 mM each of forward and reverse gene-specific primer (Supplementary Table 1). The transcript level of each gene was normalized relative to that of OsUBQ5.
3. Results
3.1. Pi uptake efficiency is reduced inosphyB mutants
We previously showed significantly reduced Pi uptake activity in the phyB knockout mutant of Arabidopsis compared with the WT. Lower Pi uptake activity was also observed in the rice Phy mutant osphyB-2 compared with the WT (Sakuraba et al. Citation2018). To establish the Phy-dependent regulation of Pi uptake in rice, we examined Pi uptake activity in the seedlings of two independent osphyB knockout mutants, osphyB-1 and osphyB-2 (Jeong et al. Citation2007), the osphyA knockout mutant osphyA-3 (Piao et al. Citation2015), and the osphyB-2 osphyA-3 double mutant ( and ). WT and mutant seedlings were grown hydroponically under the Pi sufficient condition (100 μM Pi) for 5 days and then under Pi sufficient or deficient (5 μM Pi) conditions for 5 days. Growth rates of WT and mutant seedlings were similar in both conditions, although shoot heights of osphyB-1, osphyB-2, and osphyB-2 osphyA-3 seedlings were very slightly lower than that of WT seedlings (Supplementary Figure 2). Pi uptake activity in osphyB-1 and osphyB-2 seedlings was lower than that in WT seedlings under both conditions, while no significant reduction in Pi uptake activity was detected in osphyA-3 seedlings under either condition. Nevertheless, Pi uptake activity in osphyB-2 osphyA-3 double mutant seedlings was slightly lower than that in osphyB single mutants, indicating that both OsPhyA and OsPhyB are involved in the promotion of Pi uptake in rice, although OsPhyB rather than OsPhyA contributes to this promotion.
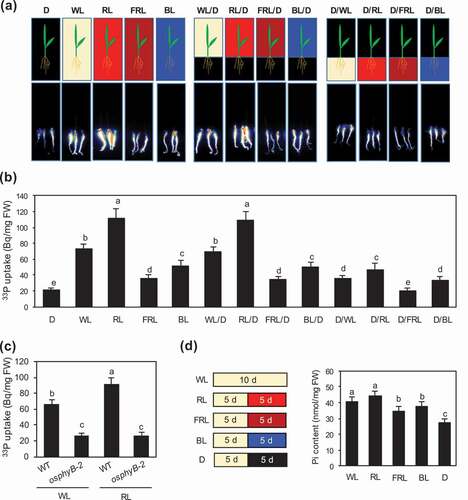
Figure 2. Light wavelength-dependent enhancement of Pi uptake and accumulation in rice seedlings. (a, b) Visualization (a) and quantification (b) of 33P-labeled Pi uptake in WT seedlings grown under Pi sufficient conditions for 5 days, followed by Pi deficient conditions for 5 days, and then treated with white light (WL), red light (RL), far-red light (FRL), blue light (BL), or darkness (D) for 24 h. WL/D, RL/D, FRL/D, and BL/D are seedlings whose roots were covered with aluminum foil during various light treatments; D/WL, D/RL, D/FRL, and D/BL are seedlings whose shoots were covered with aluminum foil during various light treatments. Data represent mean ± SD of four biological replicates. (c) Quantification of 33P-labeled Pi uptake in WT and osphyB-2 seedlings grown under Pi sufficient conditions for 5 days, followed by Pi deficient conditions for 5 days, and then exposed to WL or RL for 24 h. Data represent mean ± SD of four biological replicates. (d) Pi contents of WT seedlings grown under Pi sufficient conditions and WL for 5 days, and then under Pi deficient conditions and WL, RL, FRL, BL, or D for 5 days. Data represent mean ± SD of five biological replicates. In (b–d), different lowercase letters indicate significant differences (P < 0.05; Tukey’s multiple comparison test)
Next, we examined Pi contents of osphyB-1, osphyB-2, osphyA-3, and osphyA-3 osphyB-2 seedlings grown under Pi sufficient conditions for 5 days and then under Pi sufficient or deficient conditions for 5 days ( and ). Except osphyA-3 seedlings, all mutant seedlings showed significantly lower Pi contents than WT seedlings under Pi deficient conditions. However, Pi contents of mutant and WT seedlings grown under Pi sufficient conditions were comparable, consistent with the observation that the effect of knockout mutations of OsPhyB on Pi uptake activity was more evident under Pi deficient conditions than under Pi sufficient conditions ( and ).
3.2. Red light enhances Pi uptake and accumulation in rice seedlings
Since OsPhyB is the major red light receptor (Takano, Inagaki, and Xie et al. Citation2005), we examined the effects of red light on Pi uptake using WT seedlings grown under Pi sufficient conditions for 5 days, followed by Pi deficient conditions and white light (70 μmol m−2 s−1) for 5 days, and then either under light (100 μmol m−2 s−1 of white, red, far-red, or blue light) or in the dark for 1 day ( and ). Compared with seedlings treated with white light, those treated with dark, blue light, and far-red light showed significantly lower Pi uptake, whereas those treated with red light showed significantly higher Pi uptake. Moreover, red light irradiation did not affect Pi uptake activity in osphyB-2 seedlings (), indicating that PhyB-mediated red light signaling promotes Pi uptake in rice.
We previously showed that Arabidopsis seedlings showed significantly reduced Pi uptake when aboveground parts were wrapped in aluminum foil to block the light (Sakuraba et al. Citation2018). To test whether blocking light has a similar effect in rice, we covered the aboveground parts of rice seedlings with aluminum foil and exposed these rice seedlings to red or white light. The results showed decreased Pi uptake activity when aboveground parts of rice seedlings were covered with aluminum foil ( and ). However, light shielding of roots did not affect Pi uptake activity ( and ). Additionally, we confirmed that the effect of red light on Pi uptake activity depends on OsPhyB, based on the results of the osphyB-2 mutant ().
Next, to examine the effect of red light on Pi accumulation in rice seedlings, we measured the Pi content of WT seedlings grown under Pi sufficient conditions and constant white light for 5 days, and then under Pi deficient conditions with or without illumination (white, red, far-red, blue light, or dark) for 5 days (). The Pi content of WT seedlings was significantly reduced in the dark. Compared with white light, red light irradiation appeared to be more effective in increasing Pi accumulation, whereas far-red and blue light were less effective in increasing Pi accumulation. Collectively, these results suggest that the perception of red light by OsPhyB in aboveground plant parts is important for promoting Pi uptake and accumulation in rice seedlings.
3.3. Knockout mutation of OsPhyB reduces Pi deficiency-induced expression of Pi transporter genes and Pi starvation-responsive genes
Since Pi uptake and accumulation in osphyB-2 and osphyA-3 osphyB-2 seedlings were significantly lower than those in WT seedlings (), we performed quantitative real-time PCR (RT-qPCR) to investigate the expression levels of several Pi transporter genes and Pi starvation-responsive genes in the roots of WT, osphyB-2, osphyA-3, and osphyA-3 osphyB-2 seedlings grown under Pi sufficient conditions for 7 days, and then under Pi sufficient or deficient conditions for 5 days ( and ). In rice, 13 Pi transporters (OsPT1–13) are phylogenetically classified into the PHOSPHATE TRANSPORTER1 (PHT1) family, and some of the rice PHT1 family proteins, such as rice PHOSPHATE TRANSPORTER1 (OsPT1), OsPT2, OsPT4, and OsPT6, are involved in Pi uptake from the soil (Sun et al. Citation2012; Ai et al. Citation2009; Ye et al. Citation2015), similar to Arabidopsis PHT1 family proteins (Misson et al. Citation2004; Shin et al. Citation2004). In agreement with previous results (Gho and Jung Citation2019), expression levels of six Pi transporter genes, OsPT1–PT4, OsPT6, and OsPT8, in WT, osphyA-3, osphyB-2, and osphyA-3 osphyB-2 rice seedlings grown under Pi deficient conditions were much higher than those grown under Pi sufficient conditions (). However, we found that the osphyB knockout mutants showed a reduction in the Pi deficiency-induced expression level of these genes to different extents. Among the six Pi transporter genes, OSPT1 and OsPT6 were especially affected in the osphyB mutants (), with a slight decrease in expression in the osphyB-2 and osphyB-2 osphyA-3 seedlings grown under Pi sufficient conditions (Supplementary Figure 3). On the other hand, expression levels of these Pi transporter genes in osphyA-3 seedlings were almost comparable to those in WT seedlings, independent of the Pi treatment ().
Figure 3. Expression analysis of Pi transporter genes in WT, osphyA-3, osphyB-2, and osphyA-3 osphyB-2 seedlings. (a–f) Expression levels of OsPT1 (a), OsPT2 (b), OsPT3 (c), OsPT4 (d), OsPT6 (e), and OsPT8 (f). Total RNA was prepared from roots of seedlings grown under Pi sufficient conditions for 8 days and then under Pi sufficient (black bars) or deficient (white bars) conditions for 5 days. Gene expression levels were normalized first against OsUBQ5 transcript levels and then against the value obtained from root samples of WT seedlings not treated with Pi deficiency. Data represent mean ± SD of four biological replicates. Asterisks indicate significant differences between mutant and WT seedlings (*P < 0.05, **P < 0.01)
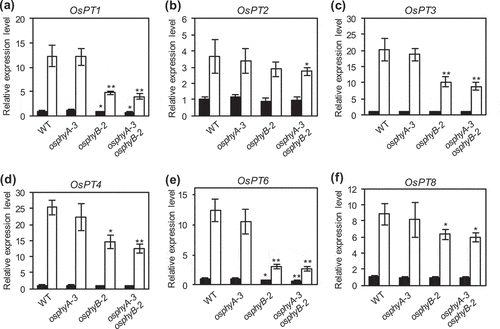
Figure 4. Expression analysis of Pi starvation-responsive genes in WT, osphyA-3, osphyB-2, and osphyA-3 osphyB-2seedlings. (a–i) Expression levels of OsPHR1 (a), OsPHR2 (b), OsPHR3 (c), OsPHR4 (d), OsIPS1 (e), OsIPS2 (f), OsPAP10 (g), OsSQD1 (h), and OsPHO2 (i). Total RNA was prepared from roots of seedlings grown under Pi sufficient conditions for 8 days and then under Pi sufficient (black bars) or deficient (white bars) conditions for 5 days. Transcript levels of genes were normalized first against OsUBQ5 transcript levels and then against the value obtained from root samples of WT seedlings not treated with Pi deficiency. Data represent mean ± SD of four biological replicates. Asterisks indicate significant differences between mutant and WT seedlings (*P < 0.05, **P < 0.01)
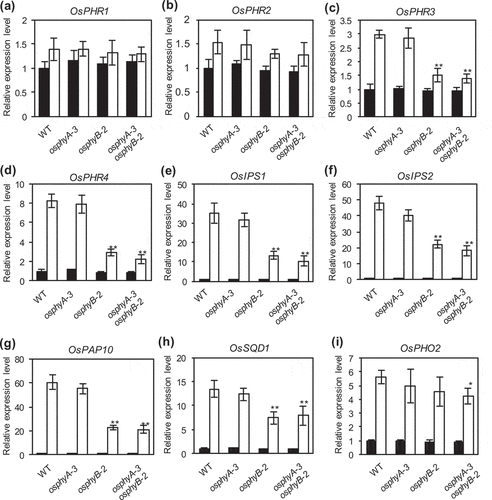
Next, we examined expression levels of several Pi starvation-responsive rice genes, including PHOSPHATE STARVATION RESPONSE1 (OsPHR1), OsPHR2, OsPHR3, OsPHR4, INDUCED BY PHOSPHATE STARVATION1 (OsIPS1), OsIPS2, SULPHOQUINOVOSE SYNTHASE1 (OsSQD1), PURPLE ACID PHOSPHATASE 1 (OsPAP10), and PHOSPHATE2 (OsPHO2) (). OsPHR1–4 are GARP-type transcription factors that play a central role in Pi signaling and homeostasis (Guo, Ruan, and Li et al. Citation2015; Ruan et al. Citation2017). OsIPS1 and OsIPS2 are members of the TOMATO PHOSPHATE STARVATION-INDUCED1 (TPSI1)/Mt4 family; expression of OsIPS1 and OsIPS2 is strongly induced under Pi deficient conditions (Hou et al. Citation2005). OsPAP10 and OsSQD1 are also induced by Pi deficiency (Wang et al. Citation2006). OsPHO2 encodes a ubiquitin-conjugating E2 enzyme involved in the Pi starvation response (Wang et al. Citation2009). Consistent with previous results, Pi deficiency treatment slightly induced the expression of OsPHR1 and OsPHR2, but strongly induced the expression of OsPHR3, OsPHR4, OsIPS1, OsIPS2, OsSQD1, OsPAP10, and OsPHO2 in WT seedlings. Interestingly, knockout mutation of the OsPhyB gene decreased the expression of all Pi starvation-responsive genes (except OsPHO2) under Pi deficient conditions, but did not affect the expression of OsPHR1 and OsPHR2. Notably, the knockout mutation of OsPhyA alone did not affect the expression of the analyzed genes under Pi sufficient and deficient conditions.
3.3. Red light radiation induces the expression of OsPT1, OsPT6, and OsPHR4
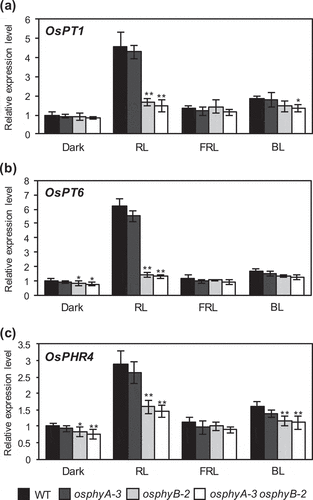
Next, to determine whether OsPT1, OsPT6, and OsPHR4 are red light-responsive genes, we examined the expression levels of OsPT1, OsPT6, and OsPHR4 in WT, osphyB-2, osphyA-3, and osphyA-3 osphyB-2 seedlings under Pi deficient conditions (). These seedlings were grown in the dark for 6 days and then exposed to red, far-red, or blue light for 24 h. In WT seedlings, expression levels of OsPT1, OsPT6, and OsPHR4 were induced upon exposure to red light for 24 h, while the effects of blue and far-red light exposure appeared to be negligible. Furthermore, red light-induced expression of these genes was largely diminished in osphyB-2 and osphyB-2 osphyA-3 seedlings. These results indicated that OsPhyB-mediated red light signaling enhances the expression of OsPT1, OsPT6, and OsPHR4, which are associated with Pi acquisition and Pi deficiency responses.
Figure 5. RT-qPCR analysis of OsPT1, OsPT6, and OsPHR4 expression in WT, osphyA-3, osphyB-2, and osphyA-3 osphyB-2 seedling exposed to red light. (a) OsPT1; (b) OsPT6; (c) OsPHR4. Total RNA was prepared from the roots of 5-day-old dark-grown seedlings after exposure to red light (RL), far-red light (FRL), blue light (BL), or darkness for 24 h. Transcript levels of genes were normalized first against OsUBQ5 transcript levels and then against the value obtained from the roots of WT seedlings grown in the dark. Data represent mean ± SD of four biological replicates. Asterisks indicate significant differences between mutant and WT seedlings (*P < 0.05, **P < 0.01)
3.4. Red light enhances Pi uptake in other plant species
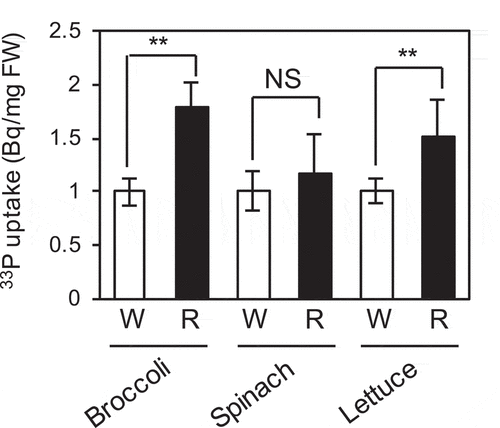
The results of this study and our previous study (Sakuraba et al. Citation2018) indicated that PhyB-mediated red light signaling elevates Pi uptake in Arabidopsis and rice seedlings by modulating the expression levels of Pi uptake and starvation response-related genes. To examine whether red light signaling also enhances Pi uptake in other plant species, we monitored Pi uptake activity in broccoli, spinach, and lettuce seedlings. Like Arabidopsis, broccoli belongs to Brassicaceae, whereas spinach and lettuce are classified into Amaranthaceae and Asteraceae, respectively. These seedlings that were grown for 5 days under Pi sufficient conditions and then for 5 days under Pi deficient and continuous white light conditions were illuminated by white or red light for 24 hours before the measurement of Pi uptake activity. Broccoli and lettuce seedlings treated with red light showed approximately 1.7- and 1.4-fold higher Pi uptake, respectively, than those treated with white light (). No significant difference in Pi uptake activity was detected between red light- and white light-treated spinach seedlings. Overall, these results suggest that red light illumination enhances Pi uptake in a variety of plant species, although the magnitude of the effect might vary among different plant species.
Figure 6. Effect of red light on Pi uptake activity in broccoli, spinach, and lettuce seedlings. Seedlings were grown under Pi sufficient conditions for 5 days, followed by Pi deficient conditions for 5 days, and were exposed to white light (W) or red light (R) for 1 day under Pi deficient conditions. Pi uptake was quantified using 33P-labeled Pi. Data represent mean ± SD of five biological replicates. Asterisks indicate significant differences between WL- and RL-treated seedlings (*P < 0.05, **P < 0.01). NS, not significant
4. Discussion
We recently showed that PhyB-mediated red light signaling promotes Pi uptake in Arabidopsis (Sakuraba et al. Citation2018); however, whether Phys play a similar role in other plant species was unclear. In this study, we demonstrated that OsPhyB-mediated red light signaling also enhances Pi uptake in rice seedlings. OsPhyA and OsPhyB are orthologous to Arabidopsis PhyA and PhyB, respectively (Takano et al. Citation2001; Takano, Inagaki, and Xie et al. Citation2005). In this study, OsPhyB played a major role in the promotion of Pi uptake, while OsPhyA played only an auxiliary role. Additionally, red light, but not far-red light, promoted Pi uptake in rice. These results are consistent with our previous findings in Arabidopsis (Sakuraba et al. Citation2018).
Expression levels of several Pi transporter genes and Pi starvation-responsive genes were significantly reduced in the roots of osphyB-2 and osphyB-2 osphyA-3 mutant seedlings, especially under Pi deficient conditions ( and ), implying that Phy-mediated red light signaling amplifies Pi starvation responses. In agreement with this implication, red light enhanced the expression of OsPHR3 and OsPHR4 genes, which encode transcription factors that play vital roles in the regulation of Pi starvation responses in rice (Guo et al. Citation2015; Ruan et al. Citation2017). In Arabidopsis, PHR1 and PHR1-LIKE (PHL) transcriptional activators play similar roles (Bustos et al. Citation2010), and we previously showed that PhyB-mediated red light signaling upregulates PHL1 in Arabidopsis (Sakuraba et al. Citation2018). Thus, Phy-mediated red light signaling likely promotes Pi uptake in Arabidopsis and rice, at least in part, through transcriptional activation of PHR/PHL genes. Because of post-translational regulation, PHR/PHL transcription factors activate their target genes only under Pi deficient conditions (Puga, Mateos, and Charukesi et al. Citation2014). Thus, the effect of Phy-mediated red light signaling on Pi uptake may be more evident under Pi deficient conditions than under Pi sufficient conditions.
Currently, it is unclear how red light signaling regulates Pi starvation-responsive genes and PHR genes in rice. In Arabidopsis, PIF4, PIF5, and HY5 transcription factors modulate the expression of genes associated with Pi uptake and Pi starvation response (Sakuraba et al. Citation2018). Although the function of rice PIF-LIKE1 (OsPIL1), the closest homolog of Arabidopsis PIF4 and PIF5 (Nakamura et al. 2007), is different from that of Arabidopsis PIF4 and PIF5 (Sakuraba et al. Citation2014, Citation2017; Sakuraba, Kim, and Paek Citation2017), we cannot eliminate the possibility that OsPIL1 regulates the expression of Pi starvation-responsive genes and PHR genes in rice. On the other hand, Arabidopsis HY5 and its three homologs in rice (OsbZIP01, OsbZIP18, and OsbZIP48) play similar roles (Ang et al. Citation1998; Burman, Bhatnagar, and Khurana Citation2018). Thus, OsbZIP01/18/48 likely regulate the expression of Pi starvation response-associated genes in rice. Since we demonstrated red light-dependent promotion of Pi uptake in Arabidopsis (previously), and in rice, broccoli, and lettuce (in this study), the identification of transcription factors that regulate the expression of Pi starvation response-associated genes in rice and other plant species would help to enhance Pi uptake in the field.
Red light irradiance elevated Pi uptake activity not only in rice seedlings but also in broccoli and lettuce seedlings. This is consistent with a previous study in lettuce, which showed that illumination using red light-rich LED (more than 80% red light) promotes plant growth and increases the content of several macronutrients, including P, in lettuce leaves (Pinho, Jokinen, and Halonen Citation2017). Furthermore, our results possibly explain why red light irradiation, rather than blue light irradiation, had positive effects on the agronomical traits of broccoli, including plant height, yield, and seed number per plant, in a previous study (Uddin et al. Citation2017). Thus, it is possible that Phy-mediated red light signaling enhances the uptake and utilization of nutrients, including P, leading to better growth in a wide range of plant species. In most agricultural and natural ecosystems, plants are grown at high densities and are exposed to varying red light to far-red light ratios because of shading caused by neighboring plants. Since our results showed that red light, but not far-red light, enhances Pi uptake activity in the roots of rice and Arabidopsis seedlings (; Sakuraba et al. Citation2018), a balance between red and far-red light is one of the key determinants of Pi acquisition in agricultural and natural ecosystems. Thus, it is important to analyze the roles of Phy-mediated red light signaling in the acquisition of nutrients, including P.
Supplemental Material
Download Zip (144.1 KB)Acknowledgments
We thank the Crop Biotech Institute at Kyung Hee University (Republic of Korea) for providing seeds of rice T-DNA insertion mutant lines. We also thank Sachiyo Nagumo for technical assistance during plant cultivation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ai, P., S. Sun, J. Zhao, X. Fan, W. Xin, Q. Guo, L. Yu, et al. 2009. “Two Rice Phosphate Transporters, OsPht1;2 and OsPht1;6, Have Different Functions and Kinetic Properties in Uptake and Translocation.” The Plant Journal 57 (5): 798–809. doi:https://doi.org/10.1111/j.1365-313X.2008.03726.x.
- Ang, L. H., S. Chattopadhyay, N. Wei, T. Oyama, K. Okada, A. Batschauer, and X. W. Deng. 1998. “Molecular Interaction between COP1 and HY5 Defines a Regulatory Switch for Light Control of Arabidopsis Development.” Molecular Cell 1: 213–222. doi:https://doi.org/10.1016/S1097-2765(00)80022-2.
- Burman, N., A. Bhatnagar, and J. P. Khurana. 2018. “OsbZIP48, a HY5 Transcription Factor Ortholog, Exerts Pleiotropic Effects in Light-Regulated Development.” Plant Physiology 176: 1262–1285. doi:https://doi.org/10.1104/pp.17.00478.
- Bustos, R., G. Castrillo, F. Linhares, M. I. Puga, V. Rubio, J. Parez-Perez, R. Solano, A. Leyva, and J. Paz-Ares. 2010. “A Central Regulatory System Largely Controls Transcriptional Activation and Repression Responses to Phosphate Starvation in Arabidopsis.” PloS Genetics 6: e1001102. doi:https://doi.org/10.1371/journal.pgen.1001102.
- Correl, D. L. 1998. “The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review.” Journal of Environmental Quality 27: 261–266. doi:https://doi.org/10.2134/jeq1998.00472425002700020004x.
- Gho, Y. S., and K. H. Jung. 2019. “Comparative Expression Analyses of Rice and Arabidopsis Phosphate Transporter Families Revealed Their Conserved Roles for the Phosphate Starvation Response.” Plant Breeding and Biotechnology 7: 42–49. doi:https://doi.org/10.9787/PBB.2019.7.1.42.
- Guo, M., W. Ruan, C. Li, F. Huang, M. Zeng, Y. Liu, Y. Yu, et al. 2015. “Integrative Comparison of the Role of the PHOSPHATE RESPONSIVE1 Subfamily in PHOSPHATE Signaling and Homeostasis in Rice.” Plant Physiology 168: 1762–1776. doi:https://doi.org/10.1104/pp.15.00736.
- Hou, X. L., P. Wu, F. C. Jiao, Q. J. Jia, H. M. Chen, J. Yu, X. W. Song, and K. K. Yi. 2005. “Regulation of the Expression of OsIPS1 and OsIPS2 in Rice via Systemic and Local Pi Signalling and Hormones.” Plant, Cell and Environment28 353–364. doi:https://doi.org/10.1111/j.1365-3040.2005.01272.x.
- Jeon, J. S., S. Lee, K. H. Jung, S. H. Jun, D. H. Jenog, J. Lee, C. Kim, et al. 2000. “T-DNA Insertional Mutagenesis for functional genomics in rice.” The Plant Journal. 22: 561–570 doi:https://doi.org/10.1046/j.1365-313x.2000.00767.x.
- Jeong, D. H., S. Lee, S. L. Kim, I. Hwang, and G. An. 2007. “Regulation of Brassinosteroid Responses by Phytochrome B in Rice.” Plant, Cell and Environment. doi:https://doi.org/10.1111/j.1365-3040.2007.01644.x.
- Kay, S. A., B. Keith, K. Shinozaki, and N. H. Chua1989. “The sequence of the rice phytochrome gene.” Nucleic Acid Research. 17: 2865–2866 doi:https://doi.org/10.1093/nar/17.7.2865.
- Li, J., G. Li, H. Wang, and X. W. Deng. 2011. “Phytochrome Signaling Mechanisms.” The Arabidopsis Book 9: e0148. doi:https://doi.org/10.1199/tab.0148.
- López-Arredondo, D. L., M. A. Leyva-González, S. I. González-Morales, J. López-Bucio, and L. Herrera-Estrella. 2014. “Phosphate Nutrition: Improving Low-Phosphate Tolerance in Crops.” Annual Review of Plant Biology 65: 95–123. doi:https://doi.org/10.1146/annurev-arplant-050213-035949.
- McNellis, T. W., and X. W. Deng. 1995. “Light Control of Seedling Morphogenetic Pattern.” The Plant Cell 7: 1749–1761. doi:https://doi.org/10.1105/tpc.7.11.1749.
- Medici, A., W. Szponarski, P. Dangeville, A. Safi, I. M. Dissanayake, C. Saenchai, A. Emanuel, et al. 2019. “Identification of Molecular Integrators Shows that Nitrogen Actively Controls the Phosphate Starvation Response in Plants.” The Plant Cell 31: 1171–1184. doi:https://doi.org/10.1105/tpc.18.00656.
- Misson, J., M. C. Thibaud, N. Bechtold, K. Raghothama, and L. Nussaume. 2004. “Transcriptional Regulation and Functional Properties of Arabidopsis Pht1;4, a High Affinity Transporter Contributing Greatly to Phosphate Uptake in Phosphate Deprived Plants.” Plant Molecular Biology 55: 727–741. doi:https://doi.org/10.1007/s11103-004-1965-5.
- Möglich, A., X. Yang, R. A. Ayers, and K. Moffat. 2010. “Structure and Function of Plant Photoreceptors.” Annual Review of Plant Biology 61: 21–47. doi:https://doi.org/10.1146/annurev-arplant-042809-112259.
- Nakamura, Y., T. Kato, T. Yamashino, M. Murakami, and T. Mizuno. 2007. “Characterization of a Set of Phytochrome-Interacting Factor-Like bHLH Proteins in Oryza sativa.” Bioscience, Biotechnology, and Biochemistry 71: 1183–1191. doi:https://doi.org/10.1271/bbb.60643.
- Piao, W., E. Y. Kim, S. H. Han, Y. Sakuraba, and N. C. Paek. 2015. “Rice Phytochrome B (Osphyb) Negatively Regulates Dark- and Starvation-Induced Leaf Senescence.” Plants 4: 644–663. doi:https://doi.org/10.3390/plants4030644.
- Pinho, P., K. Jokinen, and L. Halonen. 2017. “The Influence of the LED Light Spectrum on the Growth and Nutrient Uptake of Hydroponically Grown Lettuce.” Lighting Research & Technology 49: 866–881. doi:https://doi.org/10.1177/1477153516642269.
- Puga, M. I., I. Mateos, R. Charukesi, et al. 2014. “SPX1 Is a Phosphate-Dependent Inhibitor of Phosphate Starvation Response 1 in Arabidopsis.” Proceedings of the National Academy of Sciences of the United States of America 111: 14947–14952. doi:https://doi.org/10.1073/pnas/1404654111.
- Puag, M. I., I. Mateos, R. Charukesi, Z. Wang, J. M. Franco-Zorrilla, L. de Lorenzo, M. L. Irigoyen, et al. 2014. “SPX1 Is a Phosphate-Dependent Inhibitor of Phosphate Starvation Response 1 in Arabidopsis.” Proceedings of the National Academy of Sciences of the United States of America. 111: 14947–14952 doi:https://doi.org/10.1073/pnas/1404654111.
- McNellis, T. W., and X. W. Deng. 1995. “Light Control of Seedling Morphogenetic Pattern.” The Plant Cell 7: 1749–1761. doi:https://doi.org/10.1105/tpc.7.11.1749.
- Reed, J. W., A. Nagatani, T. D. Elich, M. Fagan, and J. Chory. 1994. “Phytochrome A and Phytochrome B Have Overlapping but Distinct Functions in Arabidopsis Development.” Plant Physiology 104: 1139–1149. doi:https://doi.org/10.1104/pp.104.4.1139.
- Rouached, H., A. B. Arpat, and Y. Poirier. 2010. “Regulation of Phosphate Starvation Responses in Plants: Signaling Players and Cross-Talks.” Molecular Plant 3: 288–299. doi:https://doi.org/10.1093/mp/ssp120.
- Ruan, W., M. Guo, P. Wu, and K. Yi. 2017. “Phosphate Starvation Induced OsPHR4 Mediates Pi-Signaling and Homeostasis in Rice.” Plant Molecular Biology 93: 327–340. doi:https://doi.org/10.1007/s11103-016-0564-6.
- Sakuraba, Y., E. Y. Kim, and N. C. Paek. 2017. “Roles of Rice PHYTOCHROME-INTERACTING FACTOR-LIKE1 (Ospil1) in Leaf Senescence.” Plant Signaling and Behavior 12: e1362522. doi:https://doi.org/10.1080/15592324.2017.1362522.
- Sakuraba, Y., E. Y. Kim, S. H. Han, W. Piao, G. An, D. Todaka, K. Yamaguchi-Shinozaki, and N. C. Paek. 2017. “Rice Phytochrome-Interacting Factor-Like1 (Ospil1) is Involved in the Promotion of Chlorophyll Biosynthesis through Feed-Forward Regulatory Loops.” Journal of Experimental Botany 68: 4103–4114. doi:https://doi.org/10.1093/jxb/erx231.
- Sakuraba, Y., J. Jeong, M. Y. Kang, J. Kim, N. C. Paek, and G. Choi. 2014. “Phytochrome-Interacting Transcription Factors PIF4 and PIF5 Induce Leaf Senescence in Arabidopsis.” Nature Communications 5: 4636. doi:https://doi.org/10.1038/ncomms5636.
- Sakuraba, Y., S. Kanno, A. Mabuchi, K. Monda, K. Iba, and S. Yanagisawa. 2018. “A Phytochrome-B-Mediated Regulatory Mechanism of Phosphorus Acquisition.” Nature Plants 4: 1089–1101. doi:https://doi.org/10.1038/s41477-018-0294-7.
- Schachtman, D. P., R. J. Reid, and S. M. Ayling. 1998. “Phosphorus Uptake by Plants: From Soil to Cell.” Plant Physiology 116: 447–453. doi:https://doi.org/10.1104/pp.116.2.447.
- Sharrock, R. A., and T. Clack. 2002. “Patterns of Expression and Normalized Levels of the Five Arabidopsis Phytochromes.” Plant Physiology 130: 442–456. doi:https://doi.org/10.1104/pp.005389.
- Shin, H., H. S. Shin, G. R. Dewbre, and M. J. Harrison. 2004. “Phosphate Transport in Arabidopsis: Pht1;1 and Pht1;4 Play a Major Role in Phosphate Acquisition from Both Low- and High-Phosphate Environments.” The Plant Journal 39: 629–642. doi:https://doi.org/10.1111/j.1365-313X.2004.02161.x.
- Smith, H., and G. C. Whitelam. 1997. “The Shade Avoidance Syndrome: Multiple Responses Mediated by Multiple Phytochromes.” Plant, Cell and Environment 20: 840–844. doi:https://doi.org/10.1046/j.1365-3040.1997.d01-104.x.
- Sun, S., M. Gu, Y. Cao, X. Huang, X. Zhang, P. Ai, J. Zhao, X. Fan, and G. Xu. 2012. “A Constitutive Expressed Phosphate Transporter, OsPht1;1, Modulates Phosphate Uptake and Translocation in Phosphate-Replete Rice.” Plant Physiology 159: 1571–1581. doi:https://doi.org/10.1104/pp.112.196345.
- Sun, W., X. H. Xu, X. Lu, L. Xie, B. Bai, C. Zheng, H. Sun, Y. He, and X. Z. Xie. 2017. “The Rice Phytochrome Genes, PHYA and PHYB, Have Synergistic Effects on Anther Development and Pollen Viability.” Scientific Reports 7: 6439. doi:https://doi.org/10.1038/s41598-017-06909-2.
- Takano, M., H. Kanegae, T. Shinomura, A. Miyao, H. Hirochika, and M. Furuya. 2001. “Isolation and Characterization of Rice Phytochrome A Mutants.” The Plant Cell 13: 521–534. doi:https://doi.org/10/1105/tpc.13.3.521.
- Takano, M., N. Inagaki, X. Xie, N. Yuzurihara, F. Hihara, T. Ishizuka, M. Yano, et al. 2005. “Distinct and Cooperative Functions of Phytochromes A, B, and C in the Control of Deetiolation and Flowering in Rice.” The Plant Cell 17: 3311–3325. doi:https://doi.org/10.1105/tpc.105.035899.
- Uddin, A. F. M. J., I. A. Jahan, B. Laila, S. Rini, and H. Ahmad. 2017. “Red Light Supplementation on Growth, Yield and Seed Production of Broccoli.” International Journal of Business, Social and Scientific Research 5: 95–102.
- Ueda, Y., T. Kiba, and S. Yanagisawa. 2020. “Nitrate-Inducible NIGT1 Proteins Modulate Phosphate Uptake and Starvation Signalling via Transcriptional Regulation of SPX Genes.” The Plant Journal. doi:https://doi.org/10.1111/tpj.14637.
- Wang, C., S. Ying, H. Huang, K. Li, P. Wu, and H. Shou. 2009. “Involvement of OsSPX1 in Phosphate Homeostasis in Rice.” The Plant Journal 57: 895–904. doi:https://doi.org/10.1111/j.1365-313X.2008.03734.x.
- Wang, X., K. Yi, Y. Tao, F. Wang, Z. Wu, D. Jiang, X. Chen, L. Zhu, and P. Wu. 2006. “Cytokinin Represses Phosphate-Starvation Response through Increasing of Intracellular Phosphate Level.” Plant, Cell and Environment 29: 1924–1935. doi:https://doi.org/10.1111/j.1365-3040.2006.01568.x.
- Ye, Y., J. Yuan, X. Chang, M. Yang, L. Zhang, K. Lu, and X. Lian. 2015. “The Phosphate Transporter Gene OsPht1;4 Is Involved in Phosphate Homeostasis in Rice.” PloS One 10: e0126186. doi:https://doi.org/10.1371/journal.pone.0126186.
- Yoshida, S., D. A. Forno, J. A. Cock, and K. A. Gomez. 1976. Laboratory Manual for Plaant Physiological Studies of Rice. 3rd ed. Manila, Philippines: International Rice Research Institute.
