ABSTRACT
Increasing soil organic carbon (SOC) content in agricultural fields is one of the strategies for mitigating climate change through carbon (C) sequestration, and could improve soil fertility. However, only a few field experimental studies have been conducted to determine the SOC stock changes in tropical areas. A field experiment was conducted under various soil management approaches in a maize-mung bean double cropping system in Lopburi, Thailand, from 2011 to 2015. The following treatment combinations were applied: 1) rice straw mulch (RS) and no organic matter application (NoOM); 2) tillage (Till) and no-tillage (NoTill); and 3) chemical fertilizer application (CF) and no chemical fertilizer application (NoCF). Soil samples were obtained at 0–15 cm depths before plowing and after maize harvest. SOC stock change was higher in RS than in NoOM. These results were attributable to the high organic C input with rice straw in the soil, although the conversion rate of total organic C input to SOC stock change with rice straw mulch did not increase. SOC stock change in NoTill did not differ from that in Till, which might be caused by no increase in the conversion rate of total organic C to SOC stock change in NoTill in this field. In addition, SOC stock change in CF was not significantly different from the stock change in NoCF. Although a large amount of organic C was supplied to the CF soil, the conversion rate of organic C to SOC decreased. The results of this study could contribute to clarifying SOC stock changes in upland crop fields in tropical monsoon conditions.
1. Introduction
High concentrations of carbon dioxide (CO2) in the atmosphere have altered the global climate system resulting in extreme weather and climatic events, such as extremely hot and cold temperatures, heavy precipitation, and drought. Such changes could adversely affect food production, human health, and economies (IPCC Citation2014a). The sequestration of atmospheric CO2 into the soil as soil carbon (C) is a strategy for mitigating climate change (IPCC Citation2014b).
Fossil fuel-related CO2 emissions reached 32 G t CO2 year−1 in 2010 (IPCC Citation2014a). The ‘4 per 1000: Soils for Food Security and Climate’ initiative was launched at the 21st Conference of the Parties to the United Nations Framework Convention on Climate Change to increase global soil organic matter stocks by 4/1000 per year to counteract the global greenhouse gas emissions from anthropogenic sources (Minasny et al. Citation2017).
Numerous management practices in agricultural crop production, such as conservation tillage, cover crop cultivation, nutrient management, manure application, soil water management, and agroforestry increase C stocks in the soil (Jarecki and Lal Citation2003; Lal Citation2003). Many field experiments have been conducted to investigate the increase in soil C associated with such management practices globally. Jarecki and Lal (Citation2003) reviewed many studies related to SOC sequestration and synthesized that soil C sequestration ranged from 0.03 to 4.2 Mg C ha−1 year−1 with organic matter input over 5 to 90 years, −0.2 to 0.6 Mg C ha−1 year−1 with no tillage or conservation tillage over 3 to 44 years, and 0.1 to 1.1 Mg C ha−1 year−1 with crop residue return estimated that 15% of C contained in the residue can be sequestered. In addition, Minasny et al. (Citation2017) surveyed case studies on soil organic C (SOC) stock and sequestration potentials from 20 regions worldwide and showed that soil C sequestration in arable land was 0.1 to 1.0 Mg C ha−1 year−1 with organic matter input over 3 to 50 years, 0.0 to 0.5 Mg C ha−1 year−1 with no-tillage or reduced tillage over 4 to 42 years, and 0.2 to 0.7 Mg C ha−1 year−1 with NPK fertilizer application over 6 to 36 years. However, the most previous studies have been conducted in temperate areas, and relatively few studies have been conducted in tropical regions.
Fujisaki et al. (Citation2018) reviewed 214 cases in 48 studies in 13 different countries on changes in soil C stocks in tropical croplands in studies published from 1960 to 2016 using web search engines (Google Scholar and Web of Science) and compiled that soil C stocks in tropical regions increased by 0.45 ± 0.14 (mean ± standard error) Mg C ha−1 year−1 with organic matter input over 18.2 ± 1.7 years, 0.32 ± 0.06 Mg C ha−1 year−1 with reduced tillage over 12.3 ± 1.0 years, and 0.24 ± 0.06 Mg C ha−1 year−1 with mineral fertilization over 17.2 ± 1.6 years. They recommended more investigations on the factors influencing soil C accumulation rates, considering that the variance in SOC accumulation rates under different soil management systems remains largely unexplained.
Therefore, in the present study, we conducted a field experiment in maize fields in Thailand from 2011 to 2015 to clarify the effects of organic matter input, tillage and no-tillage, and chemical fertilizer application on SOC change in upland crop fields in tropical areas.
2. Material and methods
2.1. Experimental site description
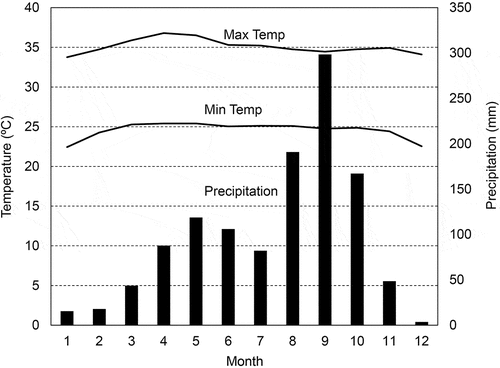
The experiment was performed in a long-term experimental field at the Lopburi Seed Research and Development Center (14°47.9ʹ N, 100°48.0ʹ E; altitude: 86 m). Annual mean maximum and minimum temperatures and annual precipitation from 1986 to 2015 at the Lopburi Seed Research and Development Center were 34.6°C, 24.2°C, and 1221 mm, respectively.
The experimental field was flat with Typic Paleustults, Ultisols according to the USDA Soil Taxonomy System. Soil texture at 0–13 cm depths in 2010 was 13.8% clay, 35.2% silt, and 51.0% sand, respectively. Soil chemical properties at 0–13 cm depth in 2010 were pH 5.8, 10.7 g kg−1 organic matter, 1.7 total nitrogen g kg−1, and 15 mg kg−1 available P (Sugino et al. Citation2013).
Field experiments were performed for 5 years from 2011 to 2015. The monthly maximum and minimum temperatures and monthly precipitation during the period at the Lopburi Seed Research and Development Center are presented in . The year 2011 had high precipitation, 2013 and 2015 had high temperatures, and 2014 had low precipitation and cool temperatures.
2.2. Experimental design
The experimental field consisted of 8 treatments and three replicates, yielding 24 plots. Each plot measured 5.25 m × 6 m. The eight treatments were as follows: 1) rice straw mulch (3.125 t ha−1 as dry weight) (RS) and no extra organic matter input (NoOM); 2) Tillage (Till) and no-tillage (NoTill); and 3) chemical fertilizer application (93.75–31.25-31.25 kg N-P2O5-K2O ha−1) (CF) and no chemical fertilizer application (NoCF) ().
Table 1. Field experiment treatments from 2011 to 2015
Tillage was carried out 1 week before maize sowing. Chemical fertilizer was applied at 20 cm from the rows and at a depth of 5 cm after maize sowing on the same day. The rice straw mulch was added 3 days after maize sowing. Rice straw mulch, chemical fertilizer application, and tillage were not undertaken in mung bean cultivation.
Crops were cultivated under a maize-mung bean double cropping system. Maize (Zea mays, variety: Nakhon Sawan 3) was sown with two to three seeds per hole with 75 cm row intervals and 25 cm hole intervals from the end of May to the beginning of June, and the seedlings were thinned to one plant per hole 2 weeks after sowing. Herbicides were sprayed at the beginning of May and 2 days after sowing. Hand weeding was performed 2 weeks after sowing. Ears and stover were harvested in an area of 3.75 m (five rows) × 4 m in the center of the plot during the middle of September. The stover was returned to the field.
Mung bean (Vigna radiata, variety: Chainat 84–1) was sown with three to five seeds per hole at 60 cm row and 15 cm hole intervals at the end of September. Herbicides were sprayed 2 days after sowing. Hand weeding was performed 2 weeks after sowing. Pods were harvested in an area measuring 3 m (six rows) x 4 m at the center of the plot until the end of November. The leaves and stems were returned to the field.
2.3. Measurement of the dry weight of maize and mung bean
The weights of the harvested seeds and cobs of maize and seeds and pod shells of mung bean from each plot were measured after air drying. The weights of the leaves and stems of the maize and mung bean in the harvested area were measured at harvest for each crop in each plot. To calculate the dry weight ratio, a sample of each part was collected at harvest or after air drying, and the samples were weighed. The dry weights of the samples were measured after oven drying at 65°C for 36 h. Dry matter production of each part was calculated by the dry weight ratio of each part and the weights of each part at harvest or after air drying in each plot.
2.4. Soil sampling and analysis and calculation of soil organic carbon and stock changes
Surface layer soil samples were obtained at depth of 0–15 cm using a 2-inch diameter soil sampler at five points in each plot at harvest in 2010 and 2011, before plowing and at harvest from 2012 to 2015, and before plowing in 2016. Soil samples were air dried and then sieved through a 2 mm sieve to remove any gravel, large roots, and organic matter, which included fine roots and small-sized organic matter. Organic C content in the soil samples was determined using the Walkley-Black method (Nelson and Sommers Citation1996).
Soil core samples were obtained using a core sampler from depths of 0 to 10 cm and 10 to 20 cm in each plot in 2010 and from depths of 0 to 15 cm and 15 to 30 cm in each plot at harvest time in 2015. The core samples were oven-dried at 105°C for 48 h. The bulk density of the soil was determined based on the dry weight of the soil core samples.
The rate of change in SOC content was calculated as a slope using linear regression with the SOC data. The analysis was performed 11 times from the harvest in 2010 to before plowing in 2016 in each plot. The SOC stock was calculated by multiplying the SOC content, soil sampling depth (15 cm), and bulk density in each plot.
2.5. Applied organic matter analysis and the calculation of organic carbon input in the soil
The C input of the maize and mung bean stems and leaves returned to the field was calculated by multiplying the dry matter production of their stems and leaves with their C content, which was assumed to be 42.3% (Ma et al. Citation2018). The root C amount was estimated to be 16% that of stems and leaves, based on Amos and Walters (Citation2006).
The rice straw used in the present study was obtained from farmers who sold rice straw for animal feed. The C content of the rice straw was determined according to Tyurin’s method (Japan Soil Association Citation2000). Based on the results of the analyses, the C content in the rice straw was 43.5%. The C input amounts associated with rice straw mulch were calculated by multiplying the amounts applied (3.125 Mg ha−1 of rice straw mulch) by the respective organic C content.
2.6. Statistical analysis
Differences in crop production, averages and changes in SOC content and stocks, total C input, and conversion rates of total C input to SOC stock change were evaluated using a Student’s t-test for paired samples to determine the effect of organic matter input, no-tillage cultivation, and chemical fertilizer application using JMP v10.0.0 (SAS Institute Inc., Cary, NC, USA). A three-way ANOVA using R software v3.6.3 (R Core Team Citation2020) was performed to investigate the effects of different factors (organic matter input, no-tillage cultivation, and chemical fertilizer application) and their interactions on SOC change from 2011 to 2015. For factor(s) with significant differences, the difference in treatment was evaluated using Turkey’s HSD test.
3. Results
3.1. Crop production
Maize seed production was 3.90 and 4.16 Mg DM ha−1, and maize stem and leaf production, which were returned to the field, were 5.10 and 6.07 Mg DM ha−1 in NoOM and RS, respectively. There was significant difference in RS compared to in NoOM (). Maize seed production was 4.43 and 3.63 Mg DM ha−1, and maize stem and leaf production were 5.85 and 5.32 Mg DM ha−1 in Till and NoTill, respectively, with no significant differences. Maize seed production was 2.98 and 5.09 Mg DM ha−1, and maize stem and leaf production were 4.27 and 6.90 Mg DM ha−1 in NoCF and CF, respectively, with production in CF significantly higher than that in NoCF.
Table 2. Crop dry matter production from 2011 to 2015
Mung bean seed production was 0.27 and 0.32 Mg DM ha−1, and mung bean stem and leaf production, which were returned to the field, were 0.50 and 0.67 Mg DM ha−1 in NoOM and RS, respectively, (). Mung bean seed production in RS was not significantly different to that in NoOM, and mung bean stem and leaf production in RS were significantly higher than those in NoOM. Mung bean seed production was 0.30 and 0.30 Mg DM ha−1, and mung bean stem and leaf production were 0.60 and 0.57 Mg DM ha−1 in Till and NoTill, respectively, with no significant differences. Mung bean seed production was 0.27 and 0.32 Mg DM ha−1, and mung bean stem and leaf production were 0.53 and 0.64 Mg DM ha−1 in NoCF and CF, respectively. Mung bean seed production in CF was not significantly different than that in NoCF, and mung bean stem and leaf production were significantly higher.
3.2. Soil organic carbon change and organic carbon input
Based on the analysis of the three treatment factors and interaction of the factors on SOC stock change, the organic matter input treatment significantly affected the SOC change; however, other treatment factors did not affect SOC change, and interactions between the three factors were not recognized (). Therefore, we evaluated the effect of treatment on each treatment factor. Additionally, the analysis of the effect of each treatment on the organic matter input showed that SOC stock change was significantly different between RS and NoOM (p < 0.05).
Table 3. Three-way ANOVA in SOC change from 2011 to 2015 in the experiment in Lopburi
The SOC content change from the harvest in 2010 to before plowing in 2016 was 0.40 g C kg−1 year−1 in RS, which was significantly higher than the 0.23 g C kg−1 year−1 in NoOM (). The SOC stock change was 0.91 Mg C ha−1 year−1 in RS, which was significantly higher than the 0.52 Mg C ha−1 year−1 observed in NoOM. Changes in SOC content and stocks were not significantly different between Till (content: 0.32 g C kg−1 year−1, stock: 0.75 Mg C ha−1 year−1) and NoTill (content: 0.30 g C kg−1 year−1, stock: 0.69 Mg C ha−1 year−1). In addition, changes in the SOC content and stock were not significantly different between NoCF (content: 0.30 g C kg−1 year−1, stock: 0.68 Mg C ha−1 year−1) and CF (content: 0.33 g C kg−1 year−1, stock: 0.76 Mg C ha−1 year−1).
Table 4. SOC change, organic C input, and ratio of SOC stock change per total organic C input to soil from 2011 to 2015 in the experiment in Lopburi
Most of the total C inputs to the soil originated from the stems and leaves returned to the field, 2.62 Mg C ha−1 year−1 on average. The C input of rice straw was 1.36 Mg C ha−1 year−1. The total C inputs in NoOM and RS were 3.11 and 5.06 Mg C ha−1 year−1, respectively. The total C input in RS was significantly higher, i.e., 1.6 times higher than that in NoOM. However, there were no differences in the dry matter production of stems and leaves between NoOM and RS (). Thus, the higher C input in RS was caused by the rice straw input. The total C inputs in Till and NoTill were 4.26 and 3.92 Mg C ha−1 year−1, respectively, and were not significantly different. The total C inputs in NoCF and CF were 3.33 and 4.84 Mg C ha−1 year−1, respectively, and were significantly higher in CF than in NoCF.
The conversion rates of total C inputs to SOC stock changes (Change of SOC stock/Total organic C input to soil; calculated in each plot; ) were 0.175 and 0.181 in NoOM and RS, respectively, with no significant difference among the treatments. Therefore the rice straw mulch did not improve the efficiency of transforming the applied organic matter into SOC. The increase in SOC in RS was caused solely by the high amount of organic matter. The conversion rates were 0.182 and 0.174 in Till and NoTill, respectively, with no differences among the treatments, which suggested that no-tillage did not improve the efficiency of transforming input organic matter into SOC. The conversion rates were 0.203 and 0.153 in NoCF and CF, respectively. The conversion rate in CF was 0.75 lower than that of the rate in NoCF; however, there was no significant difference. The decomposition rate of the organic matter in the soil might have increased under chemical fertilizer application.
4. Discussion
In Thailand, the maize seed yield in Lopburi Province from 2011 to 2015 was 3.6–4.5 Mg ha−1 (Office of Agricultural Economics, Citation2009-2016). Farmers usually cultivate maize without organic matter input, under tillage, and using chemical fertilizer application rates (personal observation). In the present study, maize seed yield (15% moisture) in CF, NoOM, and Till (treatment 5 in ), similar to farmers’ practices, from 2011 to 2015 was 6.09 t ha−1 on average and ranged from 5.0 to 7.3 t ha−1 (data not shown), which were 1.4- to 1.6-fold higher than the yield reported for Lopburi Province from 2011 to 2015. Therefore, maize production in the present study was better than regular farmer production levels due to optimal management in the experiment. Maize seed production did not exhibit any increasing or decreasing trends over the 5-year study period. Therefore, we did not consider the annual cumulative effects. Mung bean seed yield in Lopburi Province from 2008 to 2012 (mung bean data were not available from 2013) was 0.64 to 0.72 Mg ha−1 (Office of Agricultural Economics, 2009–2013). Mung bean seed yield (8% moisture) in CF, NoOM, and Till (treatment 5 in ) in the study was 0.43 t ha−1 on average and ranged from 0.00 to 1.05 t ha−1 from 2011 to 2015, which was lower than the yields reported in Lopburi Province from 2008 to 2012.
The present study demonstrated that SOC stock change in RS over 5 years was higher (0.39 [= 0.91–0.52] Mg C ha−1 year−1) than in NoOM. Minasny et al. (Citation2017) surveyed case studies and showed C sequestration rates with straw return were 0.47 to 0.89 Mg C ha−1 year−1 in 3–40 years experiment, in which the reviewed studies were mostly conducted in temperate regions. Takakai et al. (Citation2020) analyzed changes in soil C storage at depth of 0–15 cm in a long-term experimental paddy field in Japan, and estimated C sequestration at 0.487 and 0.152 Mg C ha−1 year−1 for 3 and 32 years under rice straw application at 6 Mg fresh weight ha−1 year−1, respectively. In tropical regions, Fujisaki et al. (Citation2018) reviewed 214 experimental cases on changes in soil C stocks and compiled SOC stock changes of +0.14 ± 0.02 Mg C ha−1 year−1 (mean ± standard error) under crop residue inputs for 18.5 ± 3.5 years of experimental duration. Sugino et al. (Citation2013) analyzed SOC change at depth of 0–15 cm over 25 years in a maize field in Thailand and estimated SOC stock changes with rice straw mulch at 4 Mg DM ha−1 year−1 were 0.1 to 0.2 Mg C ha−1 year−1 higher than that without organic matter input. Therefore, C sequestration rates in tropical regions were lower than those in temperate regions. However, the C sequestration rate in the present study, at 0.39 Mg C ha−1 year−1 in RS over 5 years, was higher than that in tropical regions, but it was lower than that in temperate regions. The high amount of C sequestration in the present study might have been caused by the short duration (i.e., 5 years) of the experiment. Minasny et al. (Citation2017) showed that the C sequestration rate decreased as time progressed. Thus, C sequestration with rice straw application for longer field experiment is required. A high amount of C input to the soil contributes to an increase in SOC stock. Nakamura et al. (Citation2012) suggested that a C input of 0.8 Mg C ha−1 year−1 should be applied to maintain the SOC stocks in the Sahel after analyzing SOC change using the Roth-C model. In the present study, high total C inputs of 4.59 to 7.63 Mg C ha−1 year−1, which are higher than 0.8 Mg C ha−1 year−1, might facilitate positive C sequestration in the soil.
The conversion rates of total C inputs to SOC stock changes were 0.175 and 0.181 in NoOM and RS, respectively, in the present study. They were not different among the treatments because rice straw was relatively similar to maize stems and leaves, which only increased the input amount. Fujisaki et al. (Citation2018) estimated the C input to SOC conversion rate ranged from −0.07 to 0.36 in tropical regions. Based on this, the results of the present study are consistent with the conclusion of Fujisaki et al. (Citation2018). Yoneyama, Ohkura, and Matsumoto (Citation2015) suggested rapid organic matter decomposition in tropical regions. Lee, Suzuki, and Inubushi (Citation2018) showed that labile SOC decomposition was different in various vegetation types; therefore, the analysis of labile organic C and stable C is for clarifying carbon dynamics in the soil. Shirato et al. (Citation2005) suggested that half of the C from plant materials added to fields is consumed by termites in tropical regions. In addition, according to Wiesmeier et al. (Citation2019), the role of soil fauna, which decomposes organic matter in soil, should be considered in soil C dynamics. Measurements to determine the decomposition rate of organic matter returned or applied to the field by soil fauna using the mesh-bag method in tropical regions will clarify the soil C dynamics in tropical regions.
No-tillage cultivation did not influence C sequestration in the soil compared with tillage cultivation in the present study, in which the SOC stock changes were 0.75 and 0.69 Mg C ha−1 year−1 with Till and NoTill, respectively. In addition, the conversion rates of total C inputs to SOC stock changes were not significantly different (0.174 and 0.182) in NoTill and Till, respectively. However, in general, no-tillage cultivation, including reduced tillage and conservation tillage, increases C sequestration in the soil. According to a review by Jarecki and Lal (Citation2003), C sequestration in the soil increased by −0.2 to 0.6 Mg C ha−1 year−1 over the 3–44 year experiment with no-tillage or conservation tillage, and according to the collected case studies in Minasny et al. (Citation2017), SOC change increased by 0.0 to 0.5 Mg C ha−1 year−1 over the 3–42 year experiment with no-tillage or reduced tillage. According to the results of the case studies in Fujisaki et al. (Citation2018), in tropical areas, the rate of SOC change increased by 0.32 ± 0.06 Mg C ha−1 year−1 over 12.3 ± 1.0 years of experiment duration with reduced tillage. Matsumoto, Paisancharoen, and Hakamata (Citation2008) reported that the SOC stock increased 0.8 Mg C ha−1 year−1 in the no-tillage cultivation which was higher than 0.1 Mg C ha−1 year−1 in the conventional tillage in the 3-year experiment in northeast Thailand, and the conversion rate of total C input to SOC stock was 0.28 in the no-tillage treatment which was higher than 0.03 in the conventional tillage treatment. Parihar et al. (Citation2018) demonstrated that soil organic C change in the zero-tillage treatment was 1.44 Mg C ha−1 year−1 which was higher than 0.18 Mg C ha−1 year−1 in the conventional tillage in the 5-year experiment in India. In addition, the conversion rate of total C input to SOC stock in the zero-tillage treatment was higher (0.44) than that in the conventional tillage treatment (0.06). In the present study, there was no effect of no-tillage cultivation on SOC stock change, and the conversion rate of total C inputs to SOC stock changes was higher in Till and lower in NoTill compared with the results of previous studies. No-tillage cultivation improves soil aggregation, which is a key factor that increases C sequestration in the soil (Goh Citation2004; Six and Paustian Citation2014). However, the experimental field in the present study had poor soil aggregate formation (personal observation, no data), which might be related to the low SOC stock change in the no-tillage cultivation. Nakamoto et al. (Citation2012) showed that water-stable aggregates were larger in the no-tillage cultivation than in the tillage cultivation, related to fungal activities. Six et al. (Citation2004) reviewed soil aggregate and biota and showed that soil aggregate formation was dependent on soil microbial activity and its products, but was independent in Oxisol dominated by 1:1 minerals and oxides. Future studies should investigate why SOC did not increase in the no-tillage cultivation.
In the present study, the SOC change rate was not significantly different between CF and NoCF. In CF, higher amounts of maize stems and leaves were returned to the soil; however, the conversion rate of total C inputs to SOC stock changes remained low, which suggests that the chemical fertilizer application increased the decomposition rates of the maize stems and leaves. However, previous studies have reported that chemical fertilizer application facilitates SOC accumulation because high crop biomass is returned to the soil. Minasny et al. (Citation2017) showed that the SOC change rate increased by 0.2 to 0.7 Mg C ha−1 year−1 during the 6–36 year experiment with NPK fertilizer application. However, Biederman and Harpole (Citation2013) found that the total C content in the soil decreased with chemical fertilizer application. Kumar and Goh (Citation1999) concluded that nitrogen addition did not influence the decomposition of crop residues. Nutrients of applied chemical fertilizers could increase microbial biomass, in turn, increasing organic matter decomposition rates. Further studies should investigate how chemical fertilizer application influences organic matter dynamics in the soil.
The present study showed SOC stock changes under various treatments; however, it did not analyze any organic matter dynamics in the soil. Purwanto and Alam (Citation2020) reviewed on the effect of agricultural management on C and nitrogen dynamics in the soil in the humid tropics, and concluded factors such as labile fraction in soil, light soil fraction, soil aggregation, soil microbial, and soil fauna. C supply from the root should also be considered. Iimura et al. (Citation2019) showed that soil C was derived from root C using δ13C analysis in mangrove ecosystems. Furthermore, Shirato (Citation2020) suggested that the validation of model analysis using field experiment data could improve the understanding of C dynamics in the soil. Research collaboration between long-term field experiments, analysis of C dynamics using new technologies, and model analysis would advance the clarification of soil C dynamics and contribute to reduce the uncertainty of C sequestration.
5. Conclusion
SOC stock change was analyzed under various treatments in an experimental field in Lopburi from 2011 to 2015. Rice straw mulch increased SOC stock change, which was caused by an increase in the amount of organic matter input into the soil by the addition of rice straw to maize stover and not by a change in the conversion rate of the total C input to SOC stock changes from the rice straw mulch. The SOC stock change did not increase in NoTill compared to in Till, and the conversion rate was not different between Till and NoTill. In general, no-tillage cultivation increased SOC by improving soil physical and biological properties such as an increase in soil aggregation. Further studies are required to clarify the SOC dynamics under no-tillage cultivation regarding tropical monsoonal condition and soil properties. The chemical fertilizer application increased the amount of C input from the maize stover to the soil. However, the SOC stock change in CF was not different to that of NoCF. The conversion rate of total organic C inputs to SOC stock in CF was relatively lower than that in NoCF; therefore, the decomposition rate of organic matter, such as maize stover, in the soil increased.
Acknowledgments
This study was undertaken for the research project entitled, “Development of agricultural technologies based on sustainable management of environment and natural resources in developing regions” in collaboration with JIRCAS and DOA. We would like to thank the staff of the Soil Science Research Group and the Lopburi Seed Research and Development Center, DOA, Thailand, for their kind support in managing the long-term experimental fields. We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amos, B., and D. T. Walters. 2006. “Maize Root Biomass and Net Rhizodeposited Carbon: An Analysis of the Literature.” Soil Science Society of America Journal 70: 1489–1503. doi:10.2136/sssaj2005.0216.
- Biederman, L. A., and W. S. Harpole. 2013. “Biochar and Its Effects on Plant Productivity and Nutrient Cycling: A Meta-analysis.” GCB Bioenergy 5: 202–214. doi:10.1111/gcbb.12037.
- Fujisaki, K., T. Chevallier, L. Chapuis-Lardy, A. Albrecht, T. Razafimbelo, D. Masse, Y. B. Ndour, and J. L. Chotte. 2018. “Soil Carbon Stock Changes in Tropical Croplands are Mainly Driven by Carbon Inputs: A Synthesis.” Agriculture, Ecosystems & Environnment 259: 147–158. doi:10.1016/j.agee.2017.12.008.
- Goh, K. M. 2004. “Carbon Sequestration and Stabilization in Soils: Implications for Soil Productivity and Climate Change.” Soil Science and Plant Nutrition 50: 467–476. doi:10.1080/00380768.2004.10408502.
- Iimura, Y., K. Kinjo, M. Kondo, and T. Ohtsuka. 2019. “Soil Carbon Stocks and Their Primary Origin at Manure Mangrove Ecosystems in the Estuary of Fukido River, Ishigaki Island, Southwestern Japan.” Soil Science and Plant Nutrition 65: 435–443. doi:10.1080/00380768.2019.1660589.
- IPCC. 2014a. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. R. K. Pachauri and L. A. Meyer, edited by. Geneva, Switzerland: IPCC.
- IPCC. 2014b. Climate Change 2014: Mitigation of Climate Change. Contribution of Working Groups III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. O. Edenhofer, R. Pichs-Madruga, Y. Sokona, E. Farahani, S. Kadner, K. Seyboth, A. Adler, et al., edited by. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press.
- Japan Soil Association. 2000. “Methods of Organic Matter Analysis -organic Carbon-.” In Methods of Organic Matter in Compost and Manure Analysis. 140–147. Tokyo: Japan Soil Association. (in Japanese).
- Jarecki, M. K., and R. Lal. 2003. “Crop Management for Soil Carbon Sequestration.” Critical Reviews in Plant Sciences 22: 471–502. doi:10.1080/713608318.
- Kumar, K., and K. M. Goh. 1999. “Crop Residues and Management Practices: Effects on Soil Quality, Soil Nitrogen Dynamics, Crop Yield, and Nitrogen Recovery.” Advances in Agronomy 68: 197–319. doi:10.1016/S0065-2113(08)60846-9.
- Lal, R. 2003. “Global Potential of Soil Carbon Sequestration to Mitigate the Greenhouse Effect.” Critical Reviews in Plant Sciences 22 (2): 151–184. doi:10.1080/713610854.
- Lee, C. G., S. Suzuki, and K. Inubushi. 2018. “Temperature Sensitivity of Anaerobic Labile Soil Organic Carbon Decomposition in Brackish Marsh.” Soil Science and Plant Nutrition 64: 443–448. doi:10.1080/00380768.2018.1464374.
- Ma, S., F. He, D. Tian, D. Zou, Z. Yan, Y. Yang, T. Zhou, K. Huang, H. Shen, and J. Fang. 2018. “Variations and Determinants of Carbon Content in Plants: A Global Synthesis.” Biogeosciences 15: 693–702. doi:10.5194/bg-15-693-2018.
- Matsumoto, N., K. Paisancharoen, and T. Hakamata. 2008. “Carbon Balance in Maize Fields under Cattle Manure Application and No-tillage Cultivation in Northeast Thailand.” Soil Science and Plant Nutrition 54: 277–288. doi:10.1111/j.1747-0765.2007.00223.x.
- Minasny, B., B. P. Malone, A. B. McBratney, D. A. Angers, D. Arrouays, A. Chambers, V. Chaplot, et al. 2017. “Soil Carbon 4 per Mille.” Geoderma 292: 59–86. doi:10.1016/j.geoderma.2017.01.002.
- Nakamoto, T., M. Komatsuzaki, T. Hirata, and H. Araki. 2012. “Effects of Tillage and Winter Cover Cropping on Microbial Substrate-induced Respiration and Soil Aggregation in Two Japanese Fields.” Soil Science and Plant Nutrition 58: 70–82. doi:10.1080/00380768.2011.650134.
- Nakamura, S., K. Hayashi, H. Omae, D. Fatondji, R. Tabo, H. Shinjo, A. K. Saidou, and S. Tobita. 2012. “Rothamsted Carbon Model Reveals Technical Options to Maintain Soil Organic Carbon under Semi-arid Climate.” Agronomy for Sustainable Development 32: 865–872. doi:10.1007/s13593-012-0100-2.
- Nelson, D. W., and L. E. Sommers. 1996. “Total Carbon, Organic Carbon, and Organic Matter.” In Methods of Soil Analysis. Part 3 - Chemical Methods, edited by D. L. Sparks, 961–1010. Madison, Wisconsin, USA: Soil Science Society of Amearica.
- Office of Agricultural Economics. 2009-2016. Agricultural Statistics of Thailand 2008-2015. Thailand: Ministry of Agriculture Cooperatives.
- Parihar, C. M., M. D. Parihar, T. B. Sapkota, R. K. Nanwal, A. K. Singh, H. S. Nayak, D. M. Mahala, et al. 2018. “Long-term Impact of Conservation Agriculture and Diversified Maize Rotations on Carbon Pools and Stocks, Mineral Nitrogen Fractions and Nitrous Oxide Fluxes in Inceptisol of India.” Science of the Total Environment 640-641: 1382–1392. doi:10.1016/j.scitotenv.2018.05.405.
- Purwanto, B. H., and S. Alam. 2020. “Impact of Intensive Agricultural Management on Carbon and Nitrogen Dynamics in the Humid Tropics.” Soil Science and Plant Nutrition 66: 50–59. doi:10.1080/00380768.2019.1705182.
- R Core Team. 2020. “R: A Language and Environment for Statistical Computing.” Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/
- Shirato, Y. 2020. “Use of Models to Evaluate Carbon Sequestration in Agricultural Soils.” Soil Science and Plant Nutrition 66: 21–27. doi:10.1080/00380768.2019.1702477.
- Shirato, Y., K. Paisancharoen, P. Sangtong, C. Nakviro, M. Yokozawa, and N. Matsumoto. 2005. “Testing the Rothamsted Carbon Model against Data from Long-term Experiments on Upland Soils in Thailand.” European Journal of Soil Science 56: 179–188. doi:10.1111/j.1365-2389.2004.00659.x.
- Six, J., H. Bossuyt, S. Degryze, and K. Denef. 2004. “A History of Research on the Link between (Micro)aggregates, Soil Biota, and Soil Organic Matter Dynamics.” Soil and Tillage Research 79: 7–31. doi:10.1016/j.still.2004.03.008.
- Six, J., and K. Paustian. 2014. “Aggregate-associated Soil Organic Matter as an Ecosystem Property and a Measurement Tool.” Soil Biology and Biochemistry 68: A4–A9. doi:10.1016/j.soilbio.2013.06.014.
- Sugino, T., W. Nobuntou, N. Srisombut, P. Rujikun, S. Luanmanee, and N. Punlai. 2013. “Effects of Long-term Organic Material Applications and Green Manure Crop Cultivation on Soil Organic Carbon in Rain Fed Area of Thailand.” International Soil and Water Conservation Research 1 (3): 29–36. doi:10.1016/S2095-6339(15)30028-9.
- Takakai, F., Y. Kominami, S. Ohno, and O. Nagata. 2020. “Effect of Long-term Application of Organic Matter on Soil Carbon Accumulation and GHG Emissions from a Rice Paddy Field in a Cool-temperate Region, Japan. -I. Comparison of Rice Straw and Rice Straw Compost-.” Soil Science and Plant Nutrition 66: 84–95. doi:10.1080/00380768.2019.1609335.
- Wiesmeier, M., L. Urbanski, E. Hobley, B. Lang, M. von Lützow, E. Marin-Spiotta, B. van Wesemael, et al. 2019. “Soil Organic Carbon Storage as A Key Function of Soils - A Review of Drivers and Indicators at Various Scales.” Geoderma 333: 149–162. doi:10.1016/j.geoderma.2018.07.026.
- Yoneyama, T., T. Ohkura, and N. Matsumoto. 2015. “Ecosystem Fertility: A New Paradigm for Nutrient Availability to Plants in the Humid Tropics.” Soil Science and Plant Nutrition 61: 698–703. doi:10.1080/00380768.2015.1017439.