ABSTRACT
Graminaceous cereal crops as well as all other green plants require iron (Fe), as Fe is a component of heme and [Fe-S] clusters in the photosystems (PSI, PSII) containing green pigments (chlorophylls). The interveinal yellowing (Fe chlorosis) of growing leaves is caused by insufficient Fe uptake and internal delivery. In the 1970s, Sei-ichi Takagi discovered Fe3+-chelating mugineic acid family phytosiderophores (MAs), which are released from graminaceous roots to directly absorb Fe-MAs complexes. However, the mechanisms underlying the Fe delivery from root cells to terminal interveinal mesophyll cells need intensive investigation. This review first overviews the roles of metal-chelating compounds, i.e., MAs and nicotianamine (NA), and the Fe-chelate transporters involved in primary Fe partitioning; then, the delivery of Fe to the growing leaves via phloem and into the developing chloroplasts/thylakoids via symplastic diffusion and membrane transport is discussed. Fe-MAs are absorbed into the root epidermis cells by YSL transporters and transformed to Fe-NA for symplastic radial movement between root parenchyma cells and into the xylem. If heavy metal ions such as Co2+ and Cu2+ are simultaneously present, competition may occur at their complex formation with NA prior to the radial movement that causes leaf chlorosis because of heavy metal-induced restriction of Fe availability. In xylem saps, large fractions of Fe form Fe-MAs, as in barley plants (Hordeum vulgaris), instead of Fe-citrate, which is predominant in rice plants (Oryza sativa). The former complexes are transferred to the phloem at the stem nodes for the growing leaves, while Fe-citrate is partitioned to the mature leaves by transpiration. Although the major routes of Fe supply to the growing leaves by phloem transport have been confirmed, the mechanisms underlying the synthesis of phloem Fe-compounds, delivery through the phloem system, unloading at the growing sink leaves and Fe trafficking and utilization in the chloroplasts/thylakoids still require intensive investigation. Thus, insufficient root uptake of Fe-MAs and insufficient phloem delivery of Fe to the growing leaves and finally to the chloroplasts may cause the reduced chlorophyll‒Fe-protein assembly (chlorosis). However, the schemes of iron phloem delivery presented in this review must be confirmed in future studies.
1. Introduction: historical review of iron chlorosis in graminaceous plants
Iron (Fe) is an essential nutrient for all plants, including graminaceous cereal crops, such as rice (Oryza sativa L.) and barley (Hordeum vulgare L.). Fe is a cofactor of the respiratory cytochromes and aconitase in mitochondria and a main metal component of photosystems I and II (PSI, PSII) and cytochrome-b6f (Cyt-b6f), which collect solar energy to convert atmospheric CO2 to carbohydrates in the green chloroplasts. A deficiency of Fe causes chlorosis in the growing parts of graminaceous plants ().
Figure 1. Photos of Fe-deficient barley and rice plants grown hydroponically without Fe nutrition after cultivation with a complete set of nutrients to establish early plant development. The Fe concentrations in the shoots of barley and rice plants were 53 and 81 mg kg−1 DW, respectively (photos courtesy of Dr. Takeshi Shimizu)

Although large quantities of Fe are present in soil as ferric (Fe3+) oxides and hydroxides, these forms are insoluble and scarcely available to crops, particularly under H+-deficient neutral and alkaline conditions. Fe in submerged anoxic soil is soluble in ferrous form (Fe2+) and available to plants such as paddy-grown rice.
About 100 years ago, leaf chlorosis, a condition characterized by yellowing of leaves due to a deficiency of chlorophyll, was observed in the young leaves of upland rice cultivated in the calcareous soil in what is now Puerto Rico (Gile and Carrero Citation1920). The chlorosis was induced in calcareous soils containing carbonates and in lime-amended soils. The leaves were cured of leaf chlorosis by spraying with a ferrous sulfate solution, but not by applying the same solution to soil, even in considerable quantities, since the ferrous form was immediately converted to the insoluble ferric form in calcareous soils.
Gile and Carrero (Citation1920) also reported that when rice plants with slight chlorosis were submerged, they showed severe chlorosis several days later but they became a perfectly normal green color after 10 days of continuous flooding. Takagi (Citation1966) later clarified the mechanism underlying such leaf-color change from the yellow of severe chlorosis to the normal green during flooding. Namely, he considered that the initial flooding dilutes the root-excreted Fe-solubilizing substances, while the continuous flooding increases the amounts and concentrations of such substances.
Willis and Carrero (Citation1923) demonstrated that the liming of rice soils makes them alkaline and results in the conversion of the soil water-soluble ferrous form to an insoluble ferric form. Alkalization of the culture-solution pH is also induced in the calcium-nitrate nutrition but not in ammonium-sulfate nutrition. This is because the preferential uptake of nitrate by plants results in an excess of alkaline calcium ions, causing a high pH, whereas the preferential uptake of the ammonium from ammonium-sulfate results in an excess of acid, causing a low pH.
Leaf-interveinal chlorosis of upland rice plants was frequently observed in the farm areas (soil pH 6.5–7.3) near the Ashio Copper Mining site in Japan’s Gunma Prefecture, as well as in the Watarase Riverside fields, which were contaminated by copper drains from the Ashio Copper Mining site (Mitsui et al. Citation1958; Shionoya et al. Citation1959). The copper concentrations in the yellow leaves were as high as 30 mg g−1 (DW), while their Fe concentrations fluctuated widely. The rice plants with chlorosis were occasionally re-greened by foliar sprays of a diluted ferrous sulfate (Shionoya et al. Citation1959).
Interveinal chlorosis developed quickly in the young leaves of hydroponically cultured rice plants when the plants were treated simultaneously with heavy metals and Fe-citrate. The chlorosis was most severe in the plants treated with excess cobalt (Co2+). Plants treated with high doses of Ni2+ or Zn2+ showed less severe chlorosis, whereas those treated with a high dose of toxic Cu, which is known to cause severe water stress (Chino Citation1967), did not exhibit any chlorosis at all. This type of chlorosis is known as ‘heavy metal-induced chlorosis,’ and the plants suffering from this condition are restored to a healthy green color by a foliar spray of diluted ferrous sulfate solution. Such heavy metal-induced chlorosis has also been observed in non-graminaceous plants (Hewitt Citation1948; Dekock Citation1956).
In the 1950s, Sei-ichi Takagi began investigations on the acute chlorosis induced in rice seedlings by water flooding (Takagi Citation1966). His pioneering studies in graminaceous plants led to the finding that under Fe starvation, these plants develop a unique physiological strategy: synthesis and excretion of the phytosiderophores known as mugineic acid family phytosiderophores (MAs) (Takagi, Kamei, and Yu Citation1988; Kawai, Takagi, and Sato Citation1988) from the root surface to the rhizosphere to solubilize insoluble Fe in soils. MAs also solubilize the root-apoplast-localized ferric oxides. Thus, the graminaceous plants developed a specific Fe-uptake system exclusively by forming Fe-MAs complexes (Takagi Citation1976; Takagi, Nomoto, and Takemoto Citation1984). However, due to the limited secretion of MAs, certain graminaceous crops such as rice and corn are susceptible to Fe shortage and subsequent severe chlorosis, whereas Fe-deficiency-tolerant species such as barley excrete large quantities of MAs.
The MAs released were 2ʹ-deoximugineic acid (DMA) from rice and corn plants and mugineic acid (MA) and its family phytosiderophores from barley and other grasses (Takemoto et al. Citation1978; Sugiura et al. Citation1981; Mino et al. Citation1983; Kawai, Takagi, and Sato Citation1988; Murakami et al. Citation1989; Ma and Nomoto Citation1996). MAs have the ability to specifically solubilize Fe3+ in soils at pH 4.0‒9.0, and rice and barley plants take up Fe-DMA and Fe-MAs, respectively, into their roots (Takagi, Nomoto, and Takemoto Citation1984). MAs are also synthesized and accumulated in the leaves for inter-organ Fe mobilization (Higuchi et al. Citation2001).
2. Uptake and transport of iron in the roots
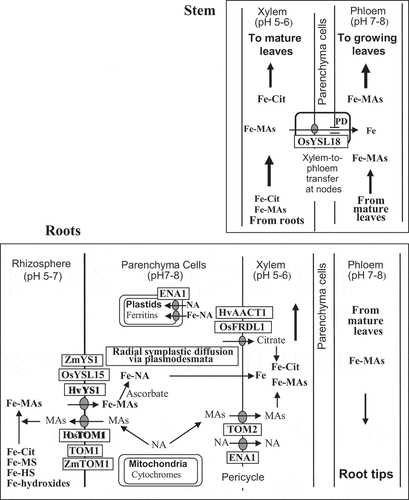
As depicted in , the MAs produced from nicotianamine (NA) in the root cells of graminaceous plants are secreted from the apical zone (Marschner, Römheld, and Kissel Citation1987; Yoshida, Kawai, and Takagi Citation2004) by rice transporter of MAs (TOM1) and HvTOM1 Fe-chelator effluxers (Nozoye et al. Citation2011). The Fe3+-MAs formed via equilibrium complexation with high stability constants (Guelke and von Blanckenburg Citation2007) or via prior exchanges with soil Fe(III)-MSs (microbial siderophores) (Yehuda et al. Citation1996) and Fe3+-HSs (humic substances) (Cesco et al. Citation2002) near the root surfaces are taken up by YS/YSL (yellow stripe/yellow stripe-like) Fe-uptake transporters such as ZmYS1 (Curie et al. Citation2001), HvYS1 (Murata et al. Citation2006), and OsYSL15 (Inoue et al. Citation2009; Lee et al. Citation2009) located in the plasma membrane of epidermal root cells. Leaf interveinal chlorosis appeared in the ZmYS1-deficient mutant (ys1), which releases DMA but cannot absorb Fe-DMA (von Wirén et al. Citation1994), and OsYSL15-less mutants of rice showed chlorosis and reduced Fe concentrations in their shoots (Lee et al. Citation2009). The Fe3+-MAs taken up are reduced to Fe2+-NA by cytosolic ascorbate (Weber, von Wirén, and Hayen Citation2008). The Fe-NA may be transferred via a symplastic radial movement through the plasmodesmata among parenchyma cells (Barberon and Geldner Citation2014) and finally unloaded into the xylem. The main carriers of Fe in the xylem fluids are citrate and MAs (Ariga et al. Citation2014): The citrate and MAs are released to the apoplastic xylem vessels by rice ferric reductase defective-like (OsFRDL1) citrate effluxer (Inoue et al. Citation2004; Yokosho et al. Citation2009) and barley Al-activated citrate efflux transporter (HvAACT1; Furukawa et al. Citation2007), and TOM2 (Nozoye et al. Citation2015), respectively, at the pericycle. The Fe-NA released to the acidic-apoplast xylem (pH 5.5‒6.5) may be ligand-exchanged to Fe-citrate and Fe-MAs as observed in xylem saps (Ariga et al. Citation2014). The NA present in xylem saps (Ariga et al. Citation2014) is released from root parenchyma cells by the NA exporter ENA1 (Nozoye et al. Citation2019).
Figure 2. Fe uptake at the root-cell plasma membranes and transport to xylem vessels in the roots (lower picture); upward transport through the stem xylem to mature leaves and xylem-to-phloem transfer to growing leaves (top picture) in graminaceous plants. AACT, Al-activated citrate efflux transporter; Cit, citrate; ENA, efflux transporter of NA; FRDL, ferric reductase defective-like; HS, humic substances; MAs, mugineic acid family phytosiderophores; MS, microbial siderophores; NA, nicotianamine; PD, plasmodesmata; TOM, transporter of mugineic acid family phytosiderophores; YSL, yellow stripe-like
3. Heavy metal-induced restriction of Fe transport in the root cells, resulting in leaf chlorosis
When rice seedlings grown hydroponically (pH 5.1) were treated with high doses of Co2+, Cu2+, and Mn2+ for 3 days, the treated plants exhibited a decreased transport of taken-up 59Fe [supplied as 59Fe-citrate simultaneously for 3 days] to the plant tops, and the levels of specific activity of 59Fe accumulation in the tops and specifically in the growing leaves became smaller than those in the non-treated control plants (; Chino Citation1967). The appearance of chlorosis in the growing leaves was severe in the Co2+-treated plants and negligible in the Mn2+-treated plants, and was related to the smaller distribution of 59Fe into the growing leaves in the former plants, although the 59Fe accumulation in the roots was little affected by the treatment with high doses of heavy metals (Chino Citation1967).
Table 1. Effect of simultaneous treatment with Co, Cu, and Mn on the partitioning of 59Fe to the roots, tops, and growing leaves
In barley plants grown hydroponically with Fe supplied as Fe-K2-EDTA, treatments with certain heavy metals caused interveinal chlorosis in growing leaves (Agarwala, Bisht, and Sharma Citation1977). The chlorosis was most severe in the plants treated with high doses of Co2+ or Cu2+, and was slight in those treated with high doses of Zn2+ or Mn2+. The extent of chlorosis was inversely related to the quantity of 59Fe [supplied as 59Fe-EDTA], which was partitioned to the shoots, with low levels of Fe concentrations also observed in growing leaves.
Heavy metal excess-induced chlorosis ranging from pale green to yellow/white in growing leaves was previously reported for both non-MAs-synthesizing plants such as sugar beet (Hewitt Citation1948), mustard (Sinapis alba; Dekock Citation1956) and soybean (Brown and Tiffin Citation1960) and MAs-synthesizing plants such as oat (Hunter and Vergnano Citation1953) and rice (Brown et al. Citation1953) by a similar order of heavy metals, i.e., Co > Cu > Zn > Mn, although this order was somewhat dependent on the plant species and heavy-metal physiology. The appearance of the chlorosis was well ordered, with stability constants of the metal complexes declining in the order Cu2+ > Co2+ > Fe2+ > Zn2+ > Mn2+, as given by Mellor and Maley (Citation1948), Hewitt (Citation1951) and Irving and Williams (Citation1953).
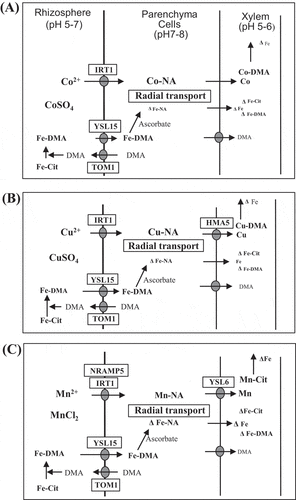
A working model proposed for the heavy metal-induced Fe deficiency in rice seedlings, which was reported in , is depicted in . In the rice plants, the ligand of Fe-citrate supplied to the culture solution is exchanged by the DMA secreted from the roots and the Fe-DMA complex formed is taken up by the OsYSL15 transporter (Murata et al. Citation2006; Inoue et al. Citation2009). Bivalent cations of Co, Cu, and Mn supplied simultaneously with 59Fe may be taken up by IRT1 (Bughio et al. Citation2002; Ishimaru et al. Citation2006; Barberon and Geldner Citation2014) and Mn may be taken up by NRAMP5 (Ishimaru et al. Citation2012; Sasaki et al. Citation2012). No competition would occur between Fe-DMA and either Co2+, Cu2+, or Mn2+ during their uptake processes, as previously inferred by Chino (Citation1967). The Fe3+-DMA taken up into the epidermal cells may be reduced to Fe2+ by ascorbate (Weber, von Wirén, and Hayen Citation2008), and the Fe2+ produced competes against Co2+, Cu2+, and Mn2+ during metal-NA complex formation, probably following the stability constants of the metal complexes stated above under a limited quantity of NA in the root-cell cytosols (Higuchi et al. Citation1999). The metal-NA complexes formed are transported via symplastic diffusion among the parenchyma cells (Barberon and Geldner Citation2014). Thus, the reduced excretion of Fe to the xylem may result in the reduced availability of Fe to the plant tops and finally to the growing leaves, where chlorosis due to the Fe-deficiency may occur (see ).
Figure 3. Working model of heavy metal-induced restriction of iron transport in the radial symplastic diffusion through parenchyma cells in rice plants under treatment with excess doses of Co2+ (A), Cu2+ (B), and Mn2+ (C), which may compete with Fe2+ in the formation of complexes with NA. A decrease in the Fe quantity is marked by Δ. DMA, 2ʹ-deoxymugineic acid; HMA, heavy metal P-type ATPase; IRT, Fe-regulated transporter; NRAMP, natural resistance-associated macrophage protein. For Cit and YSL, see the legend of
4. Iron delivery to the leaves via xylem
Both barley and rice plants exhibit interveinal chlorosis under Fe deficiency, but rice shoots have a higher Fe content than barley shoots (). In a culture experiment by Maruyama et al. (Citation2005), barley plants showed a higher chlorophyll index (SPAD value), i.e., a higher chlorophyll:Fe ratio in the leaves and a more efficient distribution of Fe to the growing leaves than rice plants, indicating that chloroplast Fe in barley leaves functions more efficiently than that in rice leaves (Hirai et al. Citation2007).
Fe-citrate and Fe-MAs in the xylem fluids ascend through the stems. Fe-citrate is largely delivered via transpiration to the mature leaves as in Arabidopsis (Schuler et al. Citation2012) and castor bean (Ricinus communis) (Hazama et al. Citation2015), whereas Fe-MAs may be efficiently transferred via xylem transfer cells to phloem in the stem nodes by YSL transporters such as OsYSL18 (Aoyama et al. Citation2009) and then by plasmodesmata, which are located between adjacent parenchyma and phloem companion cells, to the phloem sieve elements in a manner similar to amino-acid xylem-to-phloem transport by amino acid transporters (Tegeder Citation2014; Yoneyama et al. Citation2016) (). The xylem-to-phloem transfer of Fe at the nodes is more active in barley plants (Tsukamoto et al. Citation2009) than in rice plants, since the quantity of Fe-MAs in barley xylem sap is large (). The preferential transport of Fe-MAs, regulated at nodal transfer cells and destined to the growing leaves in barley may be responsible for the more efficient use of Fe in the growing leaves than in rice. In rice, Fe is predicted to be re-translocated to the growing leaves via phloem, after its delivery to the mature leaves via xylem as citrate-bound Fe.
Table 2. The concentrations of iron (Fe)-containing complexes in xylem saps from young rice, barley, and castor bean plants grown on water-submerged (rice) or upland (barley, castor bean) soils
5. Synthesis and delivery of phloem Fe-compounds
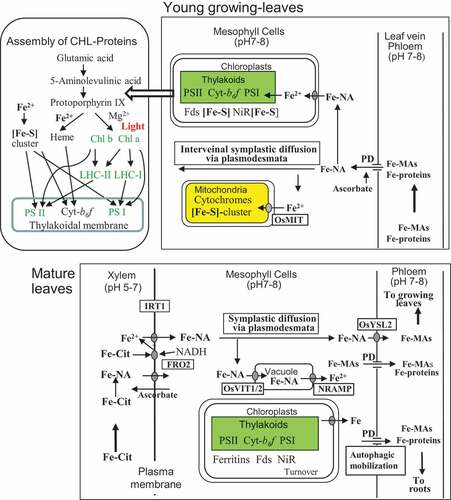
shows functional schemes of Fe nutrition in the mature and growing leaves in graminaceous plants. The site with the greatest demand for Fe nutrition is the growing leaves, where new organelles such as mitochondria and chloroplasts that contain prosthetic groups of heme and [Fe-S] cluster proteins are constructed. Sixty to ninety percent of the leaf iron is allocated to the chloroplasts (Terry and Abadía Citation1986; Wada et al. Citation2015; Kroh and Pilon Citation2020), and most of the Fe required in the growing leaves during their early developing period is actively supplied by phloem transport.
Figure 4. Schemes of the re-mobilization of Fe in mature leaves (lower picture) and utilization of Fe in growing leaves (top picture) in graminaceous plants. Cyt, cytochrome; FRO, ferric-chelate oxidoreductase; Fds, ferredoxins; LHC, light-harvesting chlorophyll (a/b) protein; MIT, mitochondrial Fe transporter; NiR, nitrite reductase; PD, plasmodesmata; VIT, vacuolar iron transporter. For Cit, PD and YSL, see the legend of
5.1. Chemical forms of phloem Fe and other metals
Although the identification of metal compounds in the phloem sap is essential, analysis of the chemical forms (speciation) of the metals is still difficult because the phloem system, a coupling of enucleated sieve elements and companion cells, is usually located at the central sites of stems and leaf veins. We collected 0.5‒2 μL phloem sap from the cut stylets of plant hoppers from young rice plants according to the method of Kawabe, Fukumorita, and Chino (Citation1980) and 5‒20 μL phloem sap of castor bean plants from the surface incision of the stems by the method of Hall and Baker (Citation1972).
Our precise chemical analysis of rice and castor bean phloem saps () showed nearly identical chemical forms (speciation) for Fe as well as Cd, Zn, and Cu. Fe was largely bound to DMA as Fe3+-DMA in rice and to NA as Fe2+/3+-NA in castor bean, and the remaining Fe was bound to 10‒20 kDa proteins and citrate. Large fractions of phloem sap Cd in rice were bound to 13 kDa proteins, and a smaller fraction was bound to thio-compounds such as phytochelatins (PCs). Zn was predominantly bound to NA both in rice and castor bean plants. Cu was dominantly bound to NA with the remainder bound to histidine and proteins.
Table 3. Chemical forms of Cd, Zn, Fe, and Cu in phloem saps from rice and castor bean plants
5.2. Metabolism of Fe in the mature source leaves
In rice, Fe is predicted to be the first allocated to the mature leaves via xylem as Fe-citrate and then re-allocated to the growing leaves through the phloem, as previously discussed in Section 4 and depicted in . The Fe-citrate, which arrives at the xylem apoplasts of mature leaf veins of graminaceous plants, may be reduced to Fe2+ via a light-dependent Fe-chelate oxidoreductase (FRO) system in the mesophyll-cell membranes using an NADH-dependent FRO such as OsFRO2 (Gross et al. Citation2003; Ishimaru et al. Citation2006; Mikami et al. Citation2011), and the Fe2+ produced and present in the xylem apoplast may be absorbed by the iron-regulated transporter (IRT1) into the mesophyll cells (Mikami et al. Citation2011). Such Fe-citrate reduction and Fe2+ transport systems in the leaf mesophyll membranes (Brüggemann, Maas-Kantel, and Moog Citation1993; Mukherjee et al. Citation2006; Feng et al. Citation2006) are operated in non-graminaceous plants, which transport predominantly Fe-citrate in the xylem to the leaves (castor bean in ; Schuler et al. Citation2012). A hypothesis proposed by Grillet et al. (Citation2014) and Grillet and Schmidt (Citation2017) for dicotyledonous plants suggests that apoplastic Fe-citrate may also be reduced to Fe2+ by ascorbate excreted from the mesophyll cells, and the Fe2+ produced is also taken up by membrane-localized IRT1 into the mesophyll cells. In the future, it would be worthwhile to examine the operation of such xylem Fe-citrate reduction with excreted ascorbate in graminaceous plants. The intracellular Fe2+ may bind to NA as Fe-NA. The Fe-NA could then move through the plasmodesmata of mesophyll cells by symplastic diffusion (Madore, Oross, and Lucas Citation1986; Stephan et al. Citation1996; Schuler et al. Citation2012) and, finally, would be loaded into phloem by OsYSL2 (Koike et al. Citation2004) for re-translocation to the growing leaves after ligand exchange to MAs in the phloem. Some Fe-NA in mature leaf cells are stored as ferritins (high-Fe proteins) in the chloroplast stroma (Briat et al. Citation2010) and as Fe-NA in the vacuoles through rice vacuolar iron transporters (OsVIT1/2; Zhang et al. Citation2012). Vacuolar Fe is taken up to the cytoplasm by NRAMPs (natural resistance-associated macrophage proteins) under low-Fe supply (in Arabidopsis; Lanquar et al. Citation2005).
Fe-containing proteins, such as the PSI and PSII proteins assembled in the thylakoid membranes, are the sources of Fe for re-mobilization from the mature leaves. The investigation of protein turnover in barley leaves by a proteome analysis combined with in planta isotope labeling indicated Kd (d−1) values of 0.04 for PSI and 0.10 for PSII (Nelson et al. Citation2014). The ferritin proteins accumulated as soluble storage forms in the chloroplasts are ready for re-mobilization under Fe deficiency (Briat Citation2008; Briat et al. Citation2010). The turnover of these Fe-containing compounds, including autophagy at the stage of leaf senescence (Pottier et al. Citation2014), may also be one of the mechanisms which sustain the re-mobilization and re-cycling of Fe as phloem-delivered Fe-MAs and Fe-proteins. Along with the decreases in leaf Fe and N contents, which are accelerated by senescence in barley plants, the expressions of NAS2 and DMAS1 genes and the synthesis of Fe-chelators DMA and citrate rather than NA are enhanced (Shi et al. Citation2012).
5.3. Synthesis of phloem Fe-compounds
At present, the mechanisms by which Fe-compounds (Fe-MAs and Fe-proteins) in phloem sap are formed in the source leaves and delivered to the sink leaves are largely unknown, although a possible mechanism can be proposed based on the literature of phloem transport of photosynthates, amino acids, and proteins (Oparka and Santa Cruz Citation2000; Van Bel Citation2003; Lee J-Y Citation2018). Namely, Fe-NA in the mesophyll cells may be released to the apoplastic space and taken up by OsYSL2, which is located in the plasma membranes of companion cells. The Fe-NA would then be transformed to Fe-MAs and transferred to the sieve elements. The Fe-MAs present and Fe-proteins re-mobilized in the mature leaves could then be released to sieve elements through the plasmodesmata (). The phloem companion cells are also known as the site of protein synthesis (Van Bel Citation2003). It is possible that certain specific proteins are synthesized in the companion cells by incorporating phloem-present Fe ions, and are then released to the sieve elements. Such metal-binding activity has been observed for Cd in rice phloem sap by 13 kDa proteins (Kato et al. Citation2010) and for Fe by the castor bean iron transport protein (Krüger et al. Citation2002).
5.4. Unloading of phloem Fe-compounds in sink leaves and roots
The phloem Fe-compounds (Fe-MAs and Fe-proteins) delivered to the young-leaf veins may be released to the mesophyll cells through plasmodesmata (Hell and Stephan Citation2003), which are located between phloem companion cells and mesophyll cells (). Upon delivery to the mesophyll cells, such Fe-MAs and Fe-proteins may be utilized as Fe nutrients by converting them to Fe-NA for the synthesis of mitochondria and for the light-involved synthesis of green chloroplasts. Fe import to mitochondria in rice is mediated by the rice mitochondrial Fe transporter (OsMIT), which is localized on the inner mitochondrial membrane (Bashir et al. Citation2011), and Fe import to chloroplasts and its allocation in the chloroplasts may be regulated by PIC1 (permease in chloroplasts) (in Arabidopsis; Duy et al. Citation2011), although the chemical forms of their substrates in vivo are not known.
It is important to note that the phloem transport of Fe-compounds to the root apex has the role of long-distance signaling of the Fe demand of shoots, thereby regulating the Fe-acquisition activity in the roots under the conditions of Fe-deficiency or Fe-excess (Kobayashi et al. Citation2010). In such shoot-to-root signaling, phloem-specific iron transporters such as AtOPT3 (Arabidopsis oligopeptide transporter; Stacey et al. Citation2008; Zhai et al. Citation2014) and potentially OsYSL2 (Ishimaru et al. Citation2010) may play important roles, although the sensing mechanism of the signals, which are unloaded at the root apex (Lee J-Y Citation2018), has not been elucidated and is under active investigation. As a shoot-to-root signal in rice, certain peptides, called IRON MAN (IMA)/Fe uptake-inducing peptides, were recently reported (Kobayashi, Nagano, and Nishizawa Citation2021).
6. Fe utilization in chloroplasts and occurrence of chlorosis
During vegetative growth, Fe is mainly used for the synthesis of chloroplasts and mitochondria in the growing leaves (Álvarez-Fernández et al. Citation2014). As depicted in , solar energy is captured by chlorophylls through the process of excitation and is soon transferred to the complexes of PSII (containing 2‒3 Fe), Cyt-b6f (5 Fe) and PSI (12 Fe) arranged on the chloroplast thylakoids (Raven, Evans, and Korb Citation1999). The chlorophyll-protein assemblies (CPs) in thylakoids contain heme and [Fe-S] cluster proteins, which function as the prosthetic groups. The [Fe-S] moiety is synthesized in the chloroplasts using imported Fe2+ on the SUF (sulfur utilization factor) machinery, which includes scaffold SUFB, and shortage of imported Fe causes downregulation of SUFB expression, resulting in retardation of [Fe-S] synthesis (Liang et al. Citation2014; Hantzis et al. Citation2018; Kroh and Pilon Citation2020). Heme and chlorophyll moieties are formed from a common precursor, protoporphyrin IX (Kroh and Pilon Citation2020). The heme structure is formed when Fe2+, probably supplied as Fe-NA, is inserted into protoporphyrin IX by ferrochelatase. Chlorophylls (a/b) are produced via two major steps: (1) Mg2+ insertion into protoporphyrin IX by magnechelatase and (2) the light-dependent reduction of dark-accumulated protochlorophyllide to chlorophyllide a. The synthesis and accumulation of green pigments in leaf cells may induce the transformation of the etioplasts to chloroplasts (Rhodes and Yemm Citation1966; Bollivar Citation2006).
The chlorophylls are inserted into specific proteins to form light-harvesting chlorophyll (LHC) proteins by binding with galactolipids on the thylakoids (in sugar beet; Nishio, Taylor, and Terry Citation1985); these LHC proteins function as antennae, capturing solar energy and transferring it to the photo-systems where solar energy is converted to chemical energy. Thus, chlorophylls are bound noncovalently to LHC proteins and [Fe-S]-containing PSI and PSII (Markwell, Thornber, and Boggs Citation1979; von Wettstein, Gough, and Kannangara Citation1995); the uncombined free chlorophylls under illumination may be chemically destroyed due to an excess of energy, which may produce toxic radicals (Rodríguez-Celma et al. Citation2013). First PSI and then PSII and Cyt-b6f are assembled on the thylakoid membranes in balanced ratios (Nevo et al. Citation2012). The ratio of the Fe-containing heme moiety to the chlorophyll moiety in leaves is always in balance (Marsh, Evans, and Matrone Citation1963), and the ratio of iron and chlorophylls also appears to be balanced (Oertli and Jacobson Citation1960). The re-arrangement of leaf PSII proteins in barley Fe-deficient young leaves may underlie the reorganization/remodeling of the LHCII proteins, which is unique in Fe-deficient barley leaves and functions to photo-protect the plants by a partial re-allocation of green chlorophylls (Saito et al. Citation2010, Citation2014). This re-modeling does not occur in rice leaves, and thus rice leaves undergo severe yellowing under prolonged iron deficiency. It is noteworthy that the contents of ferredoxin (Fd), a chloroplast-soluble [Fe-S]-containing protein, are correlated with the signs of iron chlorosis (Alcaraz et al. Citation1985). If the amount of Fe2+-NA delivered to the developing mesophyll cells is small, it may cause a decrease in the ratio of the green chlorophyll and Fe-protein (C-P) complexes relative to the structural growth (Machold Citation1971; Spiller and Terry Citation1980), resulting in interveinal chlorosis.
7. Conclusion and future research
Iron chlorosis occurs in growing leaves due to a local shortage of Fe at chlorophyll-PS assembly. To deliver the root-absorbed Fe to the growing leaves, the substrate Fe is chemically transformed so that it can pass the plasmodesmata by diffusion in slightly alkaline cytosols in the root parenchyma and leaf mesophyll cells, and so that it can move around subcellular organelles such as plastids and mitochondria. To draw a clear picture of iron availability to the sink leaves, further studies will be needed to clarify the speciation of intracellularly delivered Fe and to characterize the phloem delivery via loading and unloading, as described in the sections below.
7.1. Mechanisms of heavy metal-induced restriction of iron transport
During the formation of metal-MAs complexes in the rhizosphere, there is a possibility of competition between Fe-MAs and Cu-MAs or between Fe-MAs and Co-MAs (Sugiura et al. Citation1981), and between inhibition of the uptake of Fe-MAs by Cu-MAs or Co-MAs. However, the findings of the study by Chino () showed that 59Fe uptake into the plants was not significantly inhibited by Co2+, Cu2+, or Mn2+, although 59Fe transport from the roots to the plant tops was greatly inhibited. It is also possible that Fe-MAs in the xylem fluid compete against heavy metals, since Cu2+ and Co2+ can form Cu-MAs and Co-MAs (Sugiura et al. Citation1981; Ando et al. Citation2013) (). However, Fe in the xylem can form abundant levels of Fe-citrate complexes (Ariga et al. Citation2014). Therefore, these are not the cause of heavy metal-induced restriction of Fe transport in the xylem.
7.2. Mechanisms of loading and unloading of Fe into xylem and phloem
Understanding the speciation of metal ions in specific intracellular and intravascular compartments is crucial to understanding how the metals are kept soluble, possibly for their membrane transport and for delivery to their final target (Grillet et al. Citation2014).
Regarding the radial transport of Fe to the xylem via parenchyma cells in roots (), the mechanism of Fe-NA loading into xylem after the symplastic diffusion via plasmodesmata is not clear, including in regard to whether YSL transporters are involved. Although the mechanisms are not known, the symplastic connection between phloem and mesophyll cells in the growing leaves via the plasmodesmata might function as a series of molecular gates, instead of metal transporters, to take up the metal-containing compounds including Fe-containing peptides (Nishiyama et al. Citation2012) in a manner similar to the loading and unloading of photosynthates (Madore, Oross, and Lucas Citation1986).
Previously, light-dependent translocation of 59Fe-epihydroxymugineic acid from leaf veins to mesophyll cells in intact barley plants and also its influx into the chloroplasts isolated from barley leaves was demonstrated (Bughio et al. Citation1997). However, information on the in vivo mechanisms of Fe transport in the chloroplasts is still scarce (Balk and Schaedler Citation2014), as is information on the mechanisms of the trafficking of Fe to the synthesis site of [Fe-S] clusters and heme cofactors of photosystems and redox-involved proteins such as ferredoxins and nitrite reductase in developing chloroplasts and thylakoids.
7.3. Shoot-to-root signaling of iron-deficiency
Sensing of low-Fe-availability signals produced locally and conveyed by long-distance phloem transport induces the expression of transcription factors (IDEF1/IRO2, IDEF2) specifically at lateral-root parenchyma cells and apical root vascular cells in roots, and at mesophyll cells and vascular cells in the mature leaves (Kobayashi et al. Citation2010). The speciation of such signaling substances functioning in the root parenchyma and leaf mesophyll cells and in the phloem is not definitely determined, although Fe-NA in the cell cytosols and Fe-MAs in the phloem solute might be such candidates in graminaceous plants (Yoneyama and Ariga Citation2016). The genes encoding IRON MAN (IMA) peptides, which are able to bind Fe2+ and other divalent metals, were recently suggested to be important players in shoot-derived, phloem mobile signals in Arabidopsis (Grillet et al. Citation2018). IMA homologues were recently confirmed to play such a role in rice (Kobayashi, Nagano, and Nishizawa Citation2021), although the involved peptides in plant tissues and specifically in phloem saps have not been chemically identified.
Acknowledgments
The author thanks Professors Sei-ichi Takagi and Shigenao Kawai for their input regarding the interaction between iron availability by mugineic acids and chlorosis in graminaceous plants and his colleagues for their contribution to the speciation of metals in phloem saps of rice and castor bean, as summarized in . Finally, the author thanks Dr. Takeshi Shimizu for his courteous provision of the photos in .
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Agarwala, S. C., S. S. Bisht, and C. P. Sharma. 1977. “Relative Effectiveness of Certain Heavy Metals in Producing Toxicity and Symptoms of Iron Deficiency in Barley.” Canadian Journal of Botany 55 (10): 1299–1307. doi:10.1139/b77-151.
- Alcaraz, C. F., E. Hellín, F. Sevilla, and F. Martinez-Sánchez. 1985. “Influence of the Leaf Iron Contents on the Ferredoxin Levels in Citrus Plants.” Journal of Plant Nutrition 8 (7): 603–611. doi:10.1080/01904168509363371.
- Álvarez-Fernández, A., P. Díaz-Benito, A. Abadia, L.-M. A-f, and J. Abadia. 2014. “Metal Species Involved in Long Distance Metal Transport in Plants.” Frontiers in Plant Science 5 (Article): 105. doi:10.3389/fpls.2014.00105.
- Ando, Y., S. Nagata, S. Yanagisawa, and T. Yoneyama. 2013. “Copper in Xylem and Phloem Saps from Rice (Oryza Sativa): The Effect of Moderate Copper Concentrations in the Growth Medium on the Accumulation of Five Essential Metals and a Speciation Analysis of Copper-containing Compounds.” Functional Plant Biology 40 (1): 89–100. doi:10.1071/FP12158.
- Aoyama, T., T. Kobayashi, M. Takahashi, S. Nagasaka, K. Usuda, Y. Kakei, Y. Ishimaru, H. Nakanishi, S. Mori, and N. K. Nishizawa. 2009. “OsYSL18 Is a Rice iron(III)-deoxymugineic Acid Transporter Specifically Expressed in Reproductive Organs and Phloem of Lamina Joints.” Plant Molecular Biology 70 (6): 681–692. doi:10.1007/s11103-009-9500-3.
- Ariga, T., K. Hazama, S. Yanagisawa, and T. Yoneyama. 2014. “Chemical Forms of Iron in Xylem Sap from Graminaceous and Non-graminaceous Plants.” Soil Science and Plant Nutrition 60 (4): 460–469. doi:10.1080/00380768.2014.922406.
- Balk, J., and T. A. Schaedler. 2014. “Iron Cofactor Assembly in Plants.” Annual Review of Plant Biology 65 (1): 125–153. doi:10.1146/annurev-arplant-050213-035759.
- Barberon, M., and N. Geldner. 2014. “Radial Transport of Nutrients: The Plant Root as a Polarized Epithelium.” Plant Physiology 166 (2): 528–537. doi:10.1104/pp.114.246124.
- Bashir, K., Y. Ishimaru, H. Shimo, S. Nagasaka, M. Fujimoto, H. Takahashi, N. Tsutsumi, G. An, H. Nakanishi, and N. K. Nishizawa. 2011. “The Mitochondrial Iron Transporter Is Essential for Plant Growth.” Nature Communications 2 (1): 322. doi:10.1038/ncomms1326.
- Bollivar, D. W. 2006. “Recent Advances in Chlorophyll Biosynthesis.” Photosynthesis Research 89: 1–22.
- Briat, J. F. 2008. “Iron Dynamics in Plants.” Advances in Botanical Research 46: 137–180.
- Briat, J.-F., C. Duc, K. Ravet, and F. Gaymard. 2010. “Ferritins and Iron Storage in Plants.” . Biochimica Et Biophysica Acta (BBA) - General Subjects 1800 (8): 806–814. doi:10.1016/j.bbagen.2009.12.003.
- Brown, J. C., R. S. Holmes, R. E. Shapiro, and A. W. Specht. 1953. “Effects of Phosphorus and Copper Salts on Iron Chlorosis of Rice in Flooded and Nonflooded Soil and the Associated Enzymatic Activity.” Soil Science 79 (5): 363–372. doi:10.1097/00010694-195505000-00005.
- Brown, J. C., and L. O. Tiffin. 1960. “Iron Chlorosis in Soybeans as Related to the Genotype of Root Stock. 2. A Relationship between Susceptibility to Chlorosis & Capacity to Absorb Iron from Iron Chelate.” Soil Science 89 (1): 8–15. doi:10.1097/00010694-196001000-00003.
- Brüggemann, W., K. Maas-Kantel, and P. R. Moog. 1993. “Iron Uptake by Leaf Mesophyll Cells: The Role of the Plasma Membrane-bound Ferric-chelate Reductase.” Planta 190 (2): 151–155. doi:10.1007/BF00196606.
- Bughio, N., M. Takahashi, E. Yoshimura, N. N-k, and S. Mori. 1997. “Light-dependent Iron Transport into Isolated Barley Chloroplasts.” Plant and Cell Physiology 38 (1): 101–105. doi:10.1093/oxfordjournals.pcp.a029079.
- Bughio, N., H. Yamaguchi, N. K. Nishizawa, H. Nakanishi, and S. Mori. 2002. “Cloning and Iron-regulated Metal Transporter from Rice.” Journal of Experimental Botany 53 (374): 1677–1682. doi:10.1093/jxb/erf004.
- Cesco, S., M. Nikolic, V. Römheld, Z. Varanini, and R. Pinton. 2002. “Uptake of 59Fe from 59Fe-humate Complexes by Cucumber and Barley Plants.” Plant and Soil 241 (1): 121–128. doi:10.1023/A:1016061003397.
- Chino, M. 1967. “Studies on the Heavy Metal Toxicities in Plants ‒ the Mechanism of the Occurrence of Heavy Metal Induced Iron Chlorosis.” Bull. Sci. Rep. Facul. Agric. Ibaraki Uni 15: 105–164.
- Curie, C., Z. Panaviene, C. Loulergue, S. L. Dellaporta, J. F. Briat, and E. L. Walker. 2001. “Maize Yellow stripe-Like2 (YSL2) Encodes a Membrane Protein Directly Involved in Fe(III) Uptake.” Nature 409 (6818): 346–349. doi:10.1038/35053080.
- Dekock, P. C. 1956. “Heavy Metal Toxicity and Iron Chlorosis.” Annals of Botany 20 (1): 133–141. doi:10.1093/oxfordjournals.aob.a083508.
- Duy, D., R. Stübe, G. Wanner, and K. Philippar. 2011. “The Chloroplast Permease PIC1 Regulates Plant Growth and Development by Directing Homeostasis and Transport of Iron.” Plant Physiology 155 (4): 1709–1722. doi:10.1104/pp.110.170233.
- Feng, H., F. An, S. Zhang, Z. Ji, H. Ling, and J. Zuo. 2006. “Light-Regulated, Tissue-Specific, and Cell Differentiation-Specific Expression of the Arabidopsis Fe(III)-Chelate Reductase Gene AtFRO6.” Plant Physiology 140 (4): 1345–1354. doi:10.1104/pp.105.074138.
- Furukawa, J., N. Yamaji, H. Wang, N. Mitani, Y. Murata, K. Sato, M. Katsuhara, K. Takeda, and J. F. Ma. 2007. “An Aluminum-activated Citrate Transporter in Barley.” Plant and Cell Physiology 48 (8): 1081–1091. doi:10.1093/pcp/pcm091.
- Gile, P. L., and J. O. Carrero. 1920. “Cause of Lime-induced Chlorosis and Availability of Iron in the Soil.” Journal of Agricultural Research 20: 33–62.
- Grillet, L., P. Lan, W. Li, G. Mokkapati, and W. Schmidt. 2018. “IRON MAN Is a Ubiquitous Family of Peptides that Control IRON Transport in Plants.” Nature Plants 4 (11): 953–963. doi:10.1038/s41477-018-0266-y.
- Grillet, L., L. Ouerdane, P. Flis, M. T. T. Hoang, M.-P. Isaure, R. Lobinski, C. Curie, and S. Mari. 2014. “Ascorbate Efflux as a New Strategy for Iron Reduction and Transport in Plants.” Journal of Biological Chemistry 289 (5): 2515–2525. doi:10.1074/jbc.M113.514828.
- Grillet, L., and W. Schmidt. 2017. “The Multiple Facets of Root Iron Reduction.” Journal of Experimental Botany 68 (18): 5021–5027. doi:10.1093/jxb/erx320.
- Gross, J., R. J. Stein, A. G. Fett-Neto, and J. Fett. 2003. “Iron Homeostasis Related Genes in Rice.” Genetics and Molecular Biology 26 (4): 477–497. doi:10.1590/S1415-47572003000400012.
- Guelke, M., and F. von Blanckenburg. 2007. “Fractionation of Stable Iron Isotopes in Higher Plants.” Environmental Science & Technology 42 (6): 1896–1901. doi:10.1021/es062288j.
- Hall, S. M., and D. A. Baker. 1972. “The Chemical Composition of Ricinus Phloem Exudate.” Planta 106 (2): 131–140. doi:10.1007/BF00383992.
- Hantzis, L. J., G. E. Kroh, C. Jahn, M. Cantrell, G. Peers, M. Pilon, and K. Ravet. 2018. “A Program for Iron Economy during Deficiency Targets Specific Fe Proteins.” Plant Physiology 176 (1): 596–610. doi:10.1104/pp.17.01497.
- Hazama, K., S. Nagata, T. Fujimori, S. Yanagisawa, and T. Yoneyama. 2015. “Concentrations of Metals and Potential Metal-binding Compounds and Speciation of Cd, Zn and Cu in Phloem and Xylem Saps from Castor Bean Plants (Ricinus Communis) Treated with Four Levels of Cadmium.” Physiologia Plantarum 154 (2): 243–255. doi:10.1111/ppl.12309.
- Hell, R., and U. W. Stephan. 2003. “Iron Uptake, Trafficking and Homeostasis in Plants.” Planta 216 (4): 541–551. doi:10.1007/s00425-002-0920-4.
- Hewitt, E. J. 1948. “Relation of Manganese and Some Other Metals to the Iron Status of Plants.” Nature 161 (4091): 489–490. doi:10.1038/161489a0.
- Hewitt, E. J. 1951. “The Role of the Mineral Elements in Plant Nutrition.” Annual Review of Plant Physiology 2 (1): 25–52. doi:10.1146/annurev.pp.02.060151.000325.
- Higuchi, K., K. Suzuki, H. Nakanishi, H. Yamaguchi, N. K. Nishizawa, and S. Mori. 1999. “Cloning of Nicotianamine Synthase Genes, Novel Genes Involved in the Biosynthesis of Phytosiderophores.” Plant Physiology 119 (2): 471–479. doi:10.1104/pp.119.2.471.
- Higuchi, K., S. Watanabe, M. Takahashi, S. Kawasaki, H. Nakanishi, N. K. Nishizawa, and S. Mori. 2001. “Nicotianamine Synthase Gene Expression Differs in Barley and Rice under Fe-deficient Conditions.” The Plant Journal 25: 159–167.
- Hirai, M., K. Higuchi, H. Sasaki, T. Suzuki, T. Maruyama, M. Yoshiba, and T. Tadano. 2007. “Contribution of Iron Associated with High-molecular-weight Substances to the Maintenance of the SPAD Value of Young Leaves of Barley under Iron-deficient Conditions.” Soil Science and Plant Nutrition 53 (5): 612–620. doi:10.1111/j.1747-0765.2007.00190.x.
- Hunter, J. G., and O. Vergnano. 1953. “Trace-element Toxicities in Oat Plants.” Annals of Applied Biology 40 (4): 761–777. doi:10.1111/j.1744-7348.1953.tb01113.x.
- Inoue, H., T. Kobayashi, T. Nozoye, M. Takahashi, Y. Kakei, K. Suzuki, M. Nakazono, H. Nakanishi, S. Mori, and N. K. Nishizawa. 2009. “Rice OsYSL15 Is an Iron-regulated iron(III)-deoxymugineic Acid Transporter Expressed in the Roots and Is Essential for Iron Uptake in Early Growth of the Seedlings.” Journal of Biological Chemistry 284 (6): 3470–3479. doi:10.1074/jbc.M806042200.
- Inoue, H., D. Mizuno, M. Takahashi, H. Nakanishi, S. Mori, and N. K. Nishizawa. 2004. “A Rice FRD3-like (Osfrdl1) Gene Is Expressed in the Cells Involved in Long-distance Transport.” Soil Science and Plant Nutrition 50 (7): 1133–1140. doi:10.1080/00380768.2004.10408586.
- Irving, H., and R. J. P. Williams. 1953. The Stability of Transition-Metal Complexes, Jounal of Chemical Society October 3192–3210.
- Ishimaru, Y., H. Masuda, K. Bashir, H. Inoue, T. Tsukamoto, M. Takahashi, H. Nakanishi, et al. 2010. “Rice Metal-nicotianamine Transporter, OsYSL2, Is Required for the Long-distance Transport of Iron and Manganese.” The Plant Journal 62 (3): 379–390. DOI:10.1111/j.1365-313X.2010.04158.x.
- Ishimaru, Y., M. Suzuki, T. Tsukamoto, K. Suzuki, M. Nakazono, T. Kobayashi, Y. Wada, et al. 2006. “Rice Plants Take up Iron as an Fe3+-phytosiderophore and as Fe2+.” The Plant Journal 45 (3): 335–346. DOI:10.1111/j.1365-313X.2005.02624.x.
- Ishimaru, Y., R. Takahashi, K. Bashir, H. Shimo, T. Senoura, K. Sugimoto, K. Ono, et al. 2012. “Characterizing the Role of Rice NRAMP5 in Manganese, Iron and Cadmium Transport.” Scientific Reports 2 (1): 286. DOI:10.1038/srep00286.
- Kato, M., S. Ishikawa, K. Inagaki, K. Chiba, H. Hayashi, S. Yanagisawa, and T. Yoneyama. 2010. “Possible Chemical Forms of Cadmium and Varietal Differences in Cadmium Concentrations in the Phloem Sap of Rice Plants (Oryza sativaL.).” Soil Science and Plant Nutrition 56 (6): 839–847. doi:10.1111/j.1747-0765.2010.00514.x.
- Kawabe, S., T. Fukumorita, and M. Chino. 1980. “Collection of Rice Phloem Sap from Stylets of Homopterous Insects Severed by Yag Laser.” Plant and Cell Physiology 21 (8): 1319–1327. doi:10.1093/oxfordjournals.pcp.a076130.
- Kawai, S., S. Takagi, and Y. Sato. 1988. “Mugineic Acid-family Phytosiderophores in Root-secretions of Barley, Corn and Sorghum Varieties.” Journal of Plant Nutrition 11 (6–11): 633–642. doi:10.1080/01904168809363829.
- Kobayashi, T., A. J. Nagano, and N. K. Nishizawa. 2021. “Iron Deficiency-inducible Peptide-coding Genes OsIMA1 and OsIMA2 Positively Regulate a Major Pathway of Iron Uptake and Translocation in Rice.” . Journal of Experimental Botany 72 (6): 2196–2211. doi:10.1093/jxb/eraa546.
- Kobayashi, T., Y. Ogo, M. S. Aung, T. Nozoye, R. N. Itai, H. Nakanishi, T. Yamakawa, and N. K. Nishizawa. 2010. “The Special Expression and Regulation of Transcription Factors IDEF1 and IDEF2.” Annals of Botany 105 (7): 1109–1117. doi:10.1093/aob/mcq002.
- Koike, S., H. Inoue, D. Mizuno, M. Takahashi, H. Nakanishi, S. Mori, and N. K. Nishizawa. 2004. “OsYSL2 Is a Rice Metal-nicotianamine Transporter that Is Regulated by Iron and Expressed in the Phloem.” The Plant Journal 39 (3): 415–424. doi:10.1111/j.1365-313X.2004.02146.x.
- Kroh, G. E., and M. Pilon. 2020. “Regulation of Iron Homeostasis and Use in Chloroplasts.” International Journal of Molecular Sciences 21 (9): 3395. doi:10.3390/ijms21093395.
- Krüger, C., O. Berkowitz, U. W. Stephan, and R. Hell. 2002. “A Metal-binding Member of the Late Embryogenesis Abundant Protein Family Transports Iron in the Phloem ofRicinus Communis L.” Journal of Biological Chemistry 277 (28): 25062–25069. doi:10.1074/jbc.M201896200.
- Lanquar, V., F. Leliève, S. Bolte, C. Hames, C. Alcon, D. Neumann, G. Vansuyt, et al. 2005. “Mobilization of Vacuolar Iron by AtNRAMP3 and AtNRAMP4 Is Essential for Seed Germination on Low Iron.” The EMBO Journal 24 (23): 4041–4051. DOI:10.1038/sj.emboj.7600864.
- Lee J-Y, F. M. 2018. “Plasmodesmata in Phloem: Different Gateways for Different Cargoes.” Current Opinion in Plant Biology 43: 119–124. doi:10.1016/j.pbi.2018.04.014.
- Lee, S., J. C. Chiecko, S. A. Kim, E. L. Walker, Y. Lee, M. L. Guerinot, and G. An. 2009. “Disruption of OsYSL15 Leads to Iron Inefficiency in Rice Plants.” Plant Physiology 150 (2): 786–800. doi:10.1104/pp.109.135418.
- Liang, X., L. Qin, P. Liu, M. Wang, and H. Ye. 2014. “Genes for Iron-sulphur Cluster Assembly are Targets of Abiotic Stress in Rice, Oryza Sativa.” Plant, Cell & Environment 37 (3): 780–794. doi:10.1111/pce.12198.
- Ma, J. F., and K. Nomoto. 1996. “Effective Regulation of Iron Acquisition in Graminaceous Plants. The Role of Mugineic Acids as Phytosiderophores.” Physiologia Plantarum 97 (3): 609–617. doi:10.1111/j.1399-3054.1996.tb00522.x.
- Machold, O. 1971. “Lamellar Proteins of Green and Chlorotic Chloroplasts as Affected by Iron Deficiency and Antibiotics.” Biochimica Et Biophysica Acta (BBA) - Nucleic Acids and Protein Synthesis 238 (2): 324–331. doi:10.1016/0005-2787(71)90099-2.
- Madore, M. A., J. W. Oross, and W. J. Lucas. 1986. “Ymplastic Transport in Ipomea Tricolor Source Leaves.” Plant Physiology 82 (2): 432–442. doi:10.1104/pp.82.2.432.
- Markwell, J. P., J. P. Thornber, and R. T. Boggs. 1979. “Higher Plant Chloroplasts: Evidence that All the Chlorophyll Exists as Chlorophyll-protein Complexes.” Proceedings of the National Academy of Sciences 76 (3): 1233–1235. doi:10.1073/pnas.76.3.1233.
- Marschner, H., V. Römheld, and M. Kissel. 1987. “Localization of Phytosiderophore Release and of Iron Uptake along Intact Barley Roots.” Physiologia Plantarum 71 (2): 157–162. doi:10.1111/j.1399-3054.1987.tb02861.x.
- Marsh, H. V., H. J. Evans, and G. Matrone. 1963. “Investigations of the Role of Iron in Chlorophyll Metabolism. I. Effect of Iron Deficiency on Chlorophyll and Heme Content and on the Activities of Certain Enzymes in Leaves.” Plant Physiology 38 (6): 632–638. doi:10.1104/pp.38.6.632.
- Maruyama, T., K. Higuchi, M. Yoshida, and T. Tadano. 2005. “Comparison of Iron Availability in Leaves of Barley and Rice.” Soil Science and Plant Nutrition 51 (7): 1035–1042. doi:10.1111/j.1747-0765.2005.tb00142.x.
- Mellor, D. P., and L. Maley. 1948. “Order of Stability of Metal Complexes.” Nature 161 (4090): 436–437. doi:10.1038/161436b0.
- Mikami, Y., A. Saito, E. Miwa, and K. Higuchi. 2011. “Allocation of Fe and Ferric Chlate Reductase Activities in Mesophyll Cells of Barley and Sorghum under Fe-deficient Conditions.” Plant Physiology and Biochemistry 49 (5): 513–519. doi:10.1016/j.plaphy.2011.01.009.
- Mino, Y., T. Ishida, N. Ota, M. Inoue, K. Nomoto, T. Takemoto, H. Tanaka, and Y. Sugiura. 1983. “Mugineic acid-iron(III) Complex and Its Structurally Analogous cobalt(III) Complex: Characterization and Implication for Absorption and Transport of Iron in Gramineous Plants.” Journal of the American Chemical Society 105 (14): 4671–4676. doi:10.1021/ja00352a024.
- Mitsui, S., K. Tensho, K. Kumazawa, T. Fujita, and J. Yazaki. 1958. “Field Investigation of Chlorosis in Upland Rice Grown in the Fields near Copper Mining Factories.” Jap. Journal of Soil Science and Plant Nutrition 28: 505–507.
- Mukherjee, I., N. H. Campbell, J. S. Ash, and E. L. Connolly. 2006. “Expression Profiling of the Arabidopsis Ferric Chelate Reductase (FRO) Gene Family Reveals Differential Regulation by Iron and Copper.” Planta 223 (6): 1178–1190. doi:10.1007/s00425-005-0165-0.
- Murakami, T., K. Ise, M. Hayakawa, S. Kamei, and S. Takagi. 1989. “Stabilities of Metal Complexes of Mugineic Acids and Their Specific Affinities for iron(III).” Chemistry Letters, 12: 2137–2140.
- Murata, Y., J. F. Ma, N. Yamaji, D. Ueno, K. Nomoto, and T. Iwashita. 2006. “A Specific Transporter for iron(III)-phytosiderophore in Barley Roots.” The Plant Journal 46 (4): 563–572. doi:10.1111/j.1365-313X.2006.02714.x.
- Nelson, C. J., R. Alexova, R. P. Jacoby, and A. H. Millar. 2014. “Proteins with High Turnover Rate in Barley Leaves Estimated by Proteome Analysis Combined with in Planta Isotope Labeling.” Plant Physiology 2 (1): 91–108. doi:10.1104/pp.114.243014.
- Nevo, R., D. Charuvi, O. Tsabari, and Z. Reich. 2012. “Composition, Architecture and Dynamics of the Photosynthetic Apparatus in Higher Plants.” The Plant Journal 70 (1): 157–176. doi:10.1111/j.1365-313X.2011.04876.x.
- Nishio, J. N., S. E. Taylor, and N. Terry. 1985. “Changes in Thylakoid Galactolipids and Proteins during Iron Nutrition-mediated Development.” Plant Physiology 77 (3): 705–711. doi:10.1104/pp.77.3.705.
- Nishiyama, R., M. Kato, S. Nagata, S. Yanagisawa, and T. Yoneyama. 2012. “Identification of Zn-nicotianamine and Fe-2ʹ⸍deoxymugineic Acid in the Phloem Sap from Rice Plants (Oryza Sativa L.).” Plant and Cell Physiology 53 (2): 381–390. doi:10.1093/pcp/pcr188.
- Nozoye, T., S. Nagasaka, T. Kobayashi, M. Takahashi, Y. Sato, Y. Sato, N. Uozumi, H. Nakanishi, and N. K. Nishizawa. 2011. “Phytosiderophore Efflux Transporters are Crucial for Iron Acquisition in Graminaceous Plants.” Journal of Biological Chemistry 286 (7): 5446–5454. doi:10.1074/jbc.M110.180026.
- Nozoye, T., S. Nagasaka, T. Kobayashi, M. Takahashi, Y. Sato, N. Uozumi, H. Nakanishi, and N. K. Nishizawa. 2015. “The Phytosiderophore Efflux Transporter TOM2 Is Involved in Metal Transport in Rice.” Journal of Biological Chemistry 290 (46): 27688–27699. doi:10.1074/jbc.M114.635193.
- Nozoye, T., N. von Wirén, Y. Sato, T. Higashiyama, H. Nakanishi, and N. K. Nishizawa. 2019. “Characterization of the Nicotianamine Exporter ENA1 in Rice.” Frontiers in Plant Science 10: 502. doi:10.3389/fpls.2019.00502.
- Oertli, J. J., and L. Jacobson. 1960. “Some Quantitative Considerations in Iron Nutrition of Higher Plants.” Plant Physiology 35 (5): 683–688. doi:10.1104/pp.35.5.683.
- Oparka, K. J., and S. Santa Cruz. 2000. “THE GREATE SCAPE: Phloem Transport and Unloading of Macromolecules.” . Annual Review of Plant Physiology and Plant Molecular Biology 51 (1): 323–347. doi:10.1146/annurev.arplant.51.1.323.
- Pottier, M., C. Masclaux-Daubresse, K. Yoshimoto, and S. Thomine. 2014. “Autophagy as a Possible Mechanism for Micronutrient Remobilization from Leaves to Seeds.” Frontiers in Plant Science 5 (Article): 11. doi:10.3389/fpls.2014.00011.
- Raven, J. A., M. C. W. Evans, and R. E. Korb. 1999. “The Role of Trace Metals in Photosynthetic Electron Transport in O2-evolving Organisms.” Photosynthesis Research 60 (2/3): 111–149. doi:10.1023/A:1006282714942.
- Rhodes, M. J. C., and E. W. Yemm. 1966. “The Development of Chloroplasts and Photosynthetic Activities in Young Barley Leaves.” New Phytologist 65 (3): 331–342. doi:10.1111/j.1469-8137.1966.tb06369.x.
- Rodríguez-Celma, J., I. C. Pan, W. Li, P. Lan, T. J. Buckhout, and W. Schmidt. 2013. “The Transcriptional Response of Arabidopsis Leaves to Fe Deficiency.” Frontiers in Plant Science 4 (Article): 276. doi:10.3389/fpls.2013.00276.
- Saito, A., T. Iino, K. Sonoike, E. Miwa, and K. Higuchi. 2010. “Remodeling of the Major Light-harvesting Antenna Protein of PSII Protects the Young Leaves of Barley (Hordeum Vulgare L.) From Photoinhibition under Prolonged Iron Deficiency.” Plant and Cell Physiology 51 (12): 2013–2030. doi:10.1093/pcp/pcq160.
- Saito, A., M. Shimizu, H. Nakamura, S. Maeno, R. Katase, E. Miwa, K. Higuchi, and K. Sonoike. 2014. “Fe Deficiency Induces Phosphorylation and Translocation of Lhcb1 in Barley Thylakoid Membranes.” FEBS Letters 585 (12): 2042–2048. doi:10.1016/j.febslet.2014.04.031.
- Sasaki, A., N. Yamaji, K. Yokosho, and J. F. Ma. 2012. “Nramp5 Is a Major Transporter Responsible for Manganese and Cadmium Uptake in Rice.” The Plant Cell 24 (5): 2155–2167. doi:10.1105/tpc.112.096925.
- Schuler, M., R. Rellán-Álvarez, C. Fink-Straube, J. Abadía, and P. Bauer. 2012. “Nicotianamine Functions in the Phloem-Based Transport of Iron to Sink Organs, in Pollen Development and Pollen Tube Growth in Arabidopsis.” The Plant Cell 24 (6): 2380–2400. doi:10.1105/tpc.112.099077.
- Shi, R., G. Weber, J. Köster, M. Reza-Hajirezaei, C. Zou, F. Zhang, and N. von Wirén. 2012. “Senescence-induced Iron Mobilization in Source Leaves of Barley (Hordeum Vulgare) Plants.” New Phytologist 195 (2): 372–383. doi:10.1111/j.1469-8137.2012.04165.x.
- Shionoya, S., M. Kobayashi, S. Tsunoda, C. Funato, and M. Tadaki. 1959. “Studies on the Manganese and Iron Deficiency in Upland Crops.” Spec. Res. Rep. Gunma Pref. Agric. Sta., Japan 2: 1–74.
- Spiller, S., and N. Terry. 1980. “Limiting Factors in Photosynthesis. II. Iron Stress Diminishes Photochemical Capacity by Reducing the Number of Photosynthetic Units.” Plant Physiology 65 (1): 121–125. doi:10.1104/pp.65.1.121.
- Stacey, M. G., A. Patel, W. E. McClain, M. Mathieu, M. Remley, E. E. Rogers, W. Gassmann, D. G. Blevins, and G. Stacey. 2008. “The Arabidopsis AtOPT3 Protein Functions in Metal Homeostasis and Movement of Iron to Developing Seeds.” Plant Physiology 146 (2): 589–601. doi:10.1104/pp.107.108183.
- Stephan, U. W., I. Schmidke, V. W. Stephan, and G. Scholz. 1996. “The Nicotianamine Molecule Is Made-to-measure for Complexation of Metal Micronutrients in Plants.” BioMetals 9 (1): 84–90. doi:10.1007/BF00188095.
- Sugiura, Y., H. Tanaka, Y. Mino, T. Ishida, N. Ota, M. Inoue, K. Nomoto, H. Yoshioka, and T. Takemoto. 1981. “Structure, Properties, and Transport Mechanism of iron(III) Complex of Mugineic Acid, a Possible Phytosiderophore.” Journal of the American Chemical Society 103 (23): 6979–6982. doi:10.1021/ja00413a043.
- Takagi, S. 1966. “Studies on the Physiological Significance of Flooded Soil Condition in Rice Plant Growth ‒ with Special Reference to Flooding-induced Chlorosis of Rice Seedlings ‒.” Bull. Inst. Agr. Res. Tohoku Univ 18: 1–158.
- Takagi, S. 1976. “Naturally Occurring Iron-chelating Compounds in Oat- and Rice-root Washings I. Activity Measurement and Preliminary Characterization.” Soil Science and Plant Nutrition 22 (4): 423–433. doi:10.1080/00380768.1976.10433004.
- Takagi, S., S. Kamei, and M.-H. Yu. 1988. “Efficiency Iron Extraction from Soil by Mugineic Acid Family Phytosiderophores.” Journal of Plant Nutrition 11 (6–11): 643–651. doi:10.1080/01904168809363830.
- Takagi, S., K. Nomoto, and T. Takemoto. 1984. “Physiological Aspect of Mugineic Acid, a Possible Phytosiderophore of Graminaceous Plants.” Journal of Plant Nutrition 7 (1–5): 469–477. doi:10.1080/01904168409363213.
- Takemoto, T., K. Nomoto, S. Fushiya, R. Ouchi, G. Kusaka, H. Hikino, S. Takagi, Y. Matsuura, and M. Kakudo. 1978. “Structure of Mugineic Acid, a New Amino Acid Possessing an Iron-chelating Activity from Roots Washings of Water-cultured Hordeum Vulgare L.” Proceedings of the Japan Academy, Series B 54 (B): 469–478. doi:10.2183/pjab.54.469.
- Tegeder, M. 2014. “Transporters Involved in Source and Sink Partitioning of Amino Acids and Ureides: Opportunities for Crop Improvement.” Journal of Experimental Botany 65 (7): 1865–1878. doi:10.1093/jxb/eru012.
- Terry, N., and J. Abadía. 1986. “Function of Iron in Chloroplasts.” Journal of Plant Nutrition 9 (3): 609–646. doi:10.1080/01904168609363470.
- Tsukamoto, T., H. Nakanishi, H. Uchida, S. Watanabe, S. Matsuhashi, S. Mori, and N. K. Nishizawa. 2009. “52Fe Translocation in Barley as Monitored by a Positron-emitting Tracer Imaging System (PETIS): Evidence for the Direct Translocation of Fe from Roots to Young Leaves via Phloem.” Plant and Cell Physiology 50 (1): 48–57. doi:10.1093/pcp/pcn192.
- Van Bel, A. J. E. 2003. “The Phloem, a Miracle of Ingenuity.” Plant, Cell & Environment 26 (1): 125–149. doi:10.1046/j.1365-3040.2003.00963.x.
- von Wettstein, D., S. Gough, and C. G. Kannangara. 1995. “Chlorophyll Biosynthesis.” The Plant Cell 7 (7): 1039–1057. doi:10.2307/3870056.
- von Wirén, N., S. Mori, H. Marshner, and V. Römheld. 1994. “Iron Inefficiency in Maize Mutant Ys1 (Zea Mays L. Cv Yellow-Stripe) Is Caused by a Defect in Uptake of Iron Phytosiderophores.” Plant Physiology 106 (1): 71–77. doi:10.1104/pp.106.1.71.
- Wada, S., Y. Hayashida, M. Izumi, T. Kurusu, S. Hanamata, K. Kanno, S. Kojima, et al. 2015. “Autophagy Supports Biomass Production and Nitrogen Use Efficiency at the Vegetative Stage in Rice.” Plant Physiology 168 (1): 60–73. DOI:10.1104/pp.15.00242.
- Weber, G., N. von Wirén, and H. Hayen. 2008. “Investigation of Ascorbate-mediated Iron Release from Ferric Phytosiderophores in the Presence of Nicotianamine.” Biometals 21 (5): 503–513. doi:10.1007/s10534-008-9137-8.
- Willis, L. G., and J. O. Carrero. 1923. “Influence of Some Nitrogenous Fertilizers on the Development of Chlorosis in Rice.” Journal of Agricultural Research 24: 643–640.
- Yehuda, Z., M. Shenker, H. Römheld, H. Marschner, Y. Hadar, and Y. Chen. 1996. “The Role of Ligand Exchange in the Uptake of Iron from Microbial Siderophores by Gramineous Plants.” Plant Physiology 112 (3): 1273–1280. doi:10.1104/pp.112.3.1273.
- Yokosho, K., N. Yamaji, D. Ueno, N. Mitani, and J. F. Ma. 2009. “OsFRDL1 Is a Citrate Transporter Required for Efficient Translocation of Iron in Rice.” Plant Physiology 149 (1): 297–305. doi:10.1104/pp.108.128132.
- Yoneyama, T., and T. Ariga 2016: “Chemical Forms of Cadmium, Zinc, and Iron in the Phloem Saps from Rice (Oryza Sativa L.) And Castor Bean (Ricinus Communis L.).” 18th International Symposium on Iron Nutrition and Interaction in Plants. p. S4-OR-02. Madrid, Spain.
- Yoneyama, T., S. Ishikawa, and S. Fujimaki. 2015. “Route and Regulation of Zinc, Cadmium, and Iron Transport in Rice Plants (Oryza Sativa L.) During Vegetative Growth and Grain Filling: Metal Transporters, Metal Speciation, Grain Cd Reduction and Zn and Fe Biofortification.” International Journal of Molecular Sciences 16 (8): 19111–19129. doi:10.3390/ijms160819111.
- Yoneyama, T., F. Tanno, J. Tatsumi, and T. Mae. 2016. “Whole-plant Dynamic System of Nitrogen Use for Vegetative Growth and Grain Filling in Rice Plants (Oryza Sativa L.) As Revealed through the Production of 350 Grains from A Germinated Seed over 150 Days: A Review and Synthesis.” Frontiers in Plant Science 7: 1151. doi:10.3389/fpls.2016.01151.
- Yoshida, T., S. Kawai, and S. Takagi. 2004. “Detection of the Regions of Phytosiderophore Release from Barley Roots.” Soil Science and Plant Nutrition 50 (7): 1111–1114. doi:10.1080/00380768.2004.10408582.
- Zhai, Z., S. R. Gayomba, H. Jung, N. K. Vimalakumari, M. Piñeros, E. Craft, M. A. Rutzke, et al. 2014. “OPT3 Is a Phloem-Specific Iron Transporter that Is Essential for Systemic Iron Signaling and Redistribution of Iron and Cadmium in Arabidopsis.” The Plant Cell 26 (5): 2249–2264. DOI:10.1105/tpc.114.123737.
- Zhang, Y., Y. H. Xu, H. Y. Yi, and J. M. Gong. 2012. “Vacuolar Membrane Transporters OsVIT1 and OsVIT2 Modulate Iron Translocation between Flag Leaves and Seeds in Rice.” The Plant Journal 72 (3): 400–410. doi:10.1111/j.1365-313X.2012.05088.x.
