?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Sodification is a major soil problem that limits plant productivity in arid and semi-arid regions. For ameliorating sodic soils, the application of chemical amendments that can release calcium (Ca), which expels soil exchangeable sodium (Na), is the most widely accepted approach for solving this problem. However, an alternative more economical method would be desired in developing countries that have large areas of sodic soils. The goal of the present study was to examine the effectiveness of using compost to expel soil exchangeable Na. Composts made from cattle manure, rice straw, and rapeseed pomace were added to a sodic soil (Tongliao, Inner Mongolia, China) at mass ratios of 0 − 10%, and the soil was leached with water. Soil exchangeable cations in the forms of outer sphere and inner sphere complexes were then extracted successively and their concentrations determined. Changes in the soil dispersibility resulting from the addition of compost was also examined. The concentration of Na in both the outer sphere and inner sphere complexes decreased when rice straw or rapeseed pomace compost added, but this was not observed when cattle manure compost, which is abundant in water-soluble Na, was used. The amount of K in both forms increased with increasing rate of addition of each compost, suggesting that the K in the compost replaced both the Na that is adsorbed to soil by electrostatic attraction and by chemical interactions. The largest removal of Na, 73% for outer sphere complex and 93% for inner sphere complex, was attained in the case of rapeseed pomace compost, which had the largest water-soluble K/water-soluble Na ratio, at a mass ratio of 5%. The addition of compost also neutralized soil pH and reduced soil dispersion, the latter being effective in improving the physical properties of the soil.
1. Introduction
Dry land such as arid and semi-arid lands occupy 41% of the total surface of the Earth (Millennium Ecosystem Assessment Citation2005), where sodification is one of the most serious problems in soil degradation. Sodic or saline-sodic soils contain a high percentage of exchangeable sodium (Na), which is supplied through the weathering of parent rocks, rising of the groundwater table, or irrigation with water if the Na concentration is high (Chartres Citation1993). For example, in Tongliao, Inner Mongolia, China, the soil was originally derived from ingenious rock, which is rich in Na (Na2O, 20–60 g kg−1), and Na-bicarbonate and calcium (Ca)-bicarbonate are major components of the groundwater (Fan et al. Citation2009). In addition, it is generally thought that repeated channel evolution and the construction of irrigation systems result in an increase in the groundwater table, an increased frequency of flooding, and, consequently, accelerated sodification (Fan et al. Citation2009).
The most common method used for remediating sodic soils is the removal of Na by replacing it with Ca via the application of gypsum (Qadir, Qureshi, and Ahmad Citation1996; Gharaibeh, Eltaif, and Shra’ah Citation2010). However, gypsum is not an easily affordable material in developing countries, where a large area of sodic soils are distributed. The use of gypsum appears to be based on the order of the ease of cation exchange reactions: Na > potassium (K) > magnesium (Mg) > Ca. However, Robbins and Carter (Citation1983) reported that the cation selectivity preference order in eight salt-affected soils was K > Ca > Mg ≥ Na. Wada and Seki (Citation1994) also reported that K was more preferred to Ca and that it interfered with the replacement of Na by Ca in a marine sediment. These contradicting phenomena may be related to the difference in the affinity of each cation to pores in soil particles with different sizes where cations are typically located. According to Ferreira and Schulthes (Citation2011), Ca can replace Na more effectively than K in a large nano pore (>0.74 nm), while K is superior to or similar to Ca in its ability to replace Na in a medium (around 0.5 nm) and small (<0.35 nm) nano pores, respectively. When various basic cations are simultaneously present in a solution, Ca, Mg, and Na tend to be hydrated while K tends to be dehydrated (Collins Citation1997; Marañón Di Leo and Marañón Citation2005). Because of these differences, K may preferentially enter into smaller pores and replace the Na that is adsorbed there.
Both physical and chemical forces are involved in the adsorption of basic cations to soil. Physical forces include electrostatic attractions that includes van der Waals force and ion exchange, which are referred to as outer sphere complexation. Chemical forces include ligand exchange, covalent bonding, and specific adsorption, which is referred to as inner sphere complexation. Small cations, such as dehydrated K, in inner layers or at frayed edge sites of 2:1 type clay minerals, forms an inner sphere complex. Since the inner sphere complexation is much stronger than the outer sphere complexation (Sparks Citation2003), K would be expected to be retained stably and neutralize the negative charges of soils, and consequently suppresses the re-adsorption of Na. Thus, to confirm the type of adsorption of material K in soil, estimating the effectiveness of K materials as an efficient agent for removing Na from sodic soils is an important issue.
Tong and Watanabe (Citation2016) examined the effect of cattle manure as a K material on the desorption of exchangeable Na in sodic soils. The elution of Na increased while the amount of exchangeable K increased with increasing the mixing ratio of cattle manure. They also found that the addition of cattle manure neutralized soil pH and improved the hydraulic conductivity of the soil. Other studies reported that an increased level of organic matter in soils led to a greater preferential adsorption of K over Na and that organic matter had a rate-promoting effect on the adsorption of K to soil (Zia, Nawaz, and Murtaza Citation1999; Wang and Huang Citation2001). It therefore appears that compost is a potential K material that could be used to remove Na from sodic soils. Other positive effects of the application of organic matter on salt-affected soils, including an increase in water holding capacity, infiltration rate, and microbial population and activity (Liang et al. Citation2003; Bharadwaj et al. Citation2011; Hussain et al. Citation2011; Wang et al. Citation2014), along with an improvement in plant growth or crop yield (Cha-um and Kirdmanee Citation2011; Oo, Iwai, and Saenjan Citation2015) have also been reported. Enhancing the cation exchange capacity (CEC) by supplying cation adsorption sites could reduce the percentage of Na in total exchangeable cations (Walker and Bernal Citation2008).
The objectives of the present study were to evaluate the potential of composts as a remediating agent for treating sodic soils and to estimate their Na removing mechanism. For these purposes, the ability of three types of compost for removing Na were compared, in which the changes in the amounts of soil cations forming outer sphere and inner sphere complexes were estimated separately using a successive extraction procedure (Tucker Citation1985a, b). In this method, a chorine chloride ([HOCH2CH2N+(CH3)3]Cl-) and ammonium chloride (NH4Cl) solution are used as extractants. As chorine ion has a larger diameter and the interaction with negative charge is relatively weak, it extracts cations forming outer sphere complexes in large pores preferentially (Tucker Citation1985a). Cations forming inner sphere complexes such as those in small pores can be extracted with a NH4Cl solution. The effect of cation exchange between compost and soil on soil dispersibility was also examined.
2. Materials and methods
2.1. Soil and compost samples
Soil sample was collected at Hou Chaganhua, east of Tongliao City, Inner Mongolia, China (N44°01′50″, E 122°40′07″), where sodic and saline-sodic soils account for 6.0 × 103 km2 or 14% of the total agricultural lands (Fan et al. Citation2002). The mean annual rainfall is 384 mm, of which 85% occurs from May to October, and the mean annual evaporation is 1890 mm (Fan et al. Citation2009). Soil sample (Haplic Solonetz) was collected from the 0‒15 cm depth layer, air-dried, and passed through a 0.5-mm mesh sieve before use. The properties of the soil sample were as follows: pH (1:2.5), 10.1; electrical conductivity (EC) (1:5), 0.54 dS m−1; CEC, 78 mmolc kg−1; soil texture, sandy loam (sand, 80%; silt, 6.9%, and clay, 13.2%); and major clay minerals, Kaolinite and mica (mica species were not determined; Tong and Watanabe Citation2016). According to the Equationequation 1(1)
(1) (Rengasamy Citation2006), ECe of this soil was estimated to be 1.84:
CEC was determined by the method of Schollenberger and Simon (Citation1945).
A cattle manure compost (Nagoya University Farm, Togo, Aichi Prefecture, Japan), two rice straw composts with 2-month (rice straw compost A; Ibaraki Agricultural Research Institute, Mito, Ibaraki Prefecture, Japan), and 4-month (rice straw compost B; Anjo Agricultural Research Center, Anjo, Aichi Prefecture, Japan) composting, and a rapeseed pomace compost (fermented with rice bran added at 10:1 on weight basis; Nanohana-Kan, Higashiomi, Shiga Prefecture, Japan) were used as compost samples. Two rice straw composts were used because their structure and composition were expected to vary due to the difference in the composting period. The samples were air-dried and sieved (<0.5 mm), and the pH (H2O) (pH meter M-12, Horiba, Kyoto, Japan), EC (pH/COND meter D-24, Horiba), and water-soluble cation contents were measured. pH and EC were measured after shaking with ultrapure water mixed at a ratio of 20:1 (w/w) for 30 min (pH) or 1 h (EC). Water-soluble cations were extracted by shaking a 1 g sample suspended in 20 ml of ultra-pure water for 1 h at room temperature and were determined using inductively coupled plasma atomic emission spectrometry (ICP-AES; IRIS, Nippon Jarrel Ash, Tokyo, Japan). CEC was also determined according to Chapman (Citation1965).
2.2. Changes in contents of basic cations forming outer sphere and inner sphere complexes after an addition of compost to soil
A soil sample or quartz sand (1 g) was mixed with 0, 0.02, 0.05, 0.1 g of each of the compost samples (mass ratios of 0, 2, 5, and 10%, respectively) in triplicate. The samples were then allowed to stand in 5 ml of distilled water overnight at room temperature. The suspension was then shaken for 1 h at room temperature and centrifuged (8000 × g, 5 min). The pH and EC of the supernatant was measured, which was regarded as the soil pH and EC. Basic cations in the supernatant from the non-amended soil were also determined by atomic absorption spectrophotometry (Ca and Mg) or flame photometry (K and Na) using an atomic absorption spectrophotometer (A-2000, Hitachi, Tokyo, Japan). The precipitate was washed twice with 10 ml of 70% ethanol and twice with 10 ml of 10% ethylene glycol, by shaking for 30 min and centrifuging. The cations forming the outer sphere complex in the soil were extracted with 10 ml of 1 M choline chloride dissolved in 70% ethanol by shaking for 30 min at room temperature (Tucker Citation1985b) and centrifuging at 8000 × g for 15 min. The extraction procedures were repeated a total of 4 times and the extracts were combined. The cations forming the inner sphere complex were extracted from the residues with 10 ml of 1 M NH4Cl dissolved in 60% ethanol (pH 8.5) by shaking for 30 min at room temperature and centrifuging at 8000 × g for 5 min. The extract was collected in a flask in which 2 ml of 8 M HCl was added beforehand to prevent the precipitation of Ca and Mg as carbonate salts (Tucker Citation1985b). These extraction procedures were repeated a total of 4 times and the extracts were combined.
Basic cations in the extracts were determined using the atomic absorption spectrophotometer. The differences in the amount of cations extracted from the mixtures of quartz sand and each compost and that from the quartz sand were regarded as the exchangeable cations in the composts and were subtracted from the yield of cations from the mixtures of soil and each compost. Using the sum of the amounts of cations in inner sphere and outer sphere complexes (Xsum), the exchangeable Na percentage (ESP) and exchangeable K percentage (EPP) were calculated:
Where the values of CEC were calculated as the sum of the CEC of the Tongliao soil and that of each compost samples considering the addition rate.
2.3. Effect of addition of compost on dispersibility of soil
The Tongliao soil sample (10 g) with the cattle manure, rice straw A, or rapeseed pomace compost (>0.2 mm) mixed at mass ratios of 0%, 2%, 5%, or 10%, was placed in a 1-l glass jar bottle in triplicate. A 50 ml aliquot of distilled water was added to the bottles, and the suspension was allowed to stand overnight at 20°C. After adding 950 ml of distilled water, the bottles were capped with rubber stoppers, inverted upside down 20 times, and then allowed to stand until soil particles with diameters of <0.05 mm settled for 10 cm. The suspension was then collected from the 10 cm depth, passed through a 53-μm mesh sieve, dried at 105°C for 24 h, and weighed. The dispersibility (%) was obtained by dividing the amount of <0.05 mm fraction from each treatment by the amount of <0.05 mm fraction in 10 g of the non-amended soil, which was determined after removal of the organic matter by a hydrogen peroxide treatment according to the method reported by Day (Citation1965), and multiplying by 100.
3. Results
3.1. Basic cations adsorbed to soil forming the outer sphere complex
shows the estimated amounts of basic cations forming the outer sphere complex in the Tongliao soil after mixing with compost. The initial values for Tongliao soil are shown in . The amount of Naout after the cattle manure compost was added at rates of 2%, 5%, and 10% was smaller than, similar to, and higher than that in the initial soil, respectively (P < 0.05). The amount of Naout in the soil with the other composts added at 2‒10% was smaller (P < 0.05) than that in the initial soil. In the soil with rice straw compost A or B added, the maximum decrease in the amount of Naout, 47% and 62% of the initial, respectively, was observed at the additive rate of 5%. The addition of the rapeseed pomace compost showed the largest Naout decreasing effect, at 4.8‒5.4 mmolc kg−1 or 65‒73% of the initial.
Figure 1. Amount of outer sphere complexed cations in Tongliao soil with composts mixed at various ratios. Dotted line indicates the initial contents in the soil. Error bars indicate standard deviation (n = 3)
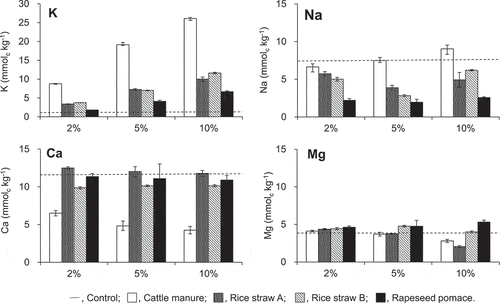
Table 1. Properties of the compost samples used
In all the compost treatments, the amount of Kout increased (P < 0.05) to a larger extent with the rate of additive. The effect was greater in the order: cattle manure compost, the two rice straw composts, and the rapeseed pomace compost, at all additive rates. The increase in the amount of Kout was proportional to the water-soluble K content in the four composts, i.e., cattle manure compost (696 mmolc kg−1): rice straw composts A and B (407 and 426 mmolc kg−1): rapeseed pomace compost (232 mmolc kg−1) = 3:2:1.
The amount of Caout decreased when the cattle manure compost or rice straw compost B was added, irrespective of the additive rate (P < 0.005), and the extent was larger when the cattle manure compost was used. The addition of rice straw compost A or rapeseed pomace compost generally had no significant effect on the amount of Caout. A significant change (decrease; P < 0.005) in the amount of Mgout was observed when the cattle manure compost or rice straw A was added at a rate of 5% or 10%.
3.2. Basic cations adsorbed to soil forming the inner sphere complex
shows the estimated amounts of basic cations that are present as the inner sphere complex in soil. The initial values in Tongliao soil are shown in . The proportion of exchangeable K that was present in complexed form in the inner sphere, 87%, was larger than the values for Na, 42%, and Ca and Mg, 32‒33%. The amount of Nain decreased in all the treatments (P < 0.01), although the relationship between the rate of decrease and the rate of added compost was unclear except for the treatments with the rapeseed pomace compost. A largest decrease was observed in case of the rapeseed pomace compost treatment, which reached 4.2‒5.0 mmolc kg−1, or by 78%, 89%, and 93% at the additive rate of 2%, 5%, and 10%, respectively. The decrease in the amount of Nain in the other treatments ranged from 1.4‒2.8 mmolc kg−1, equivalent to 26‒52% of the initial value. The sum of the decreases in exchangeable Na resulting from the addition of compost was the largest when the additive rate was 2% (cattle manure compost) or 5% (the others), and was 2.9 (cattle manure compost), 5.8 (rice straw compost A), 7.3 (rice straw compost B), and 10.1 mmolc kg−1 (rapeseed pomace compost), or 23%, 45%, 57%, and 80% of the initial amount, respectively.
Table 2. Contents of water-soluble basic cations and basic cations forming outer sphere and inner sphere complex in Tongliao soil
Figure 2. Amount of inner sphere complexed cations in the Tongliao soil with composts mixed at various ratios. Dotted line indicates the initial contents in the soil. Error bars indicate standard deviation (n = 3)
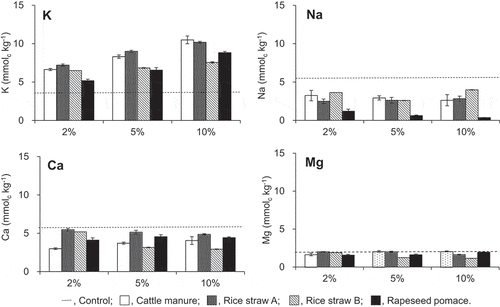
The Kin value generally increased after the addition of compost. However, the rate of increase (1.4‒2.9 times) was smaller compared to that for Kout (3.2‒47 times). The differences among the four compost treatments were also small, and the treatments with the rice straw compost A added in the 2‒10% range and those with the cattle manure compost added at 5% and 10% showed the largest increase. The sum of the increases in exchangeable K resulting from the addition of compost was 10‒32, 6‒16, and 3‒11 mmolc kg−1 for the cattle manure, rice straw (A and B), and rapeseed pomace compost treatments, respectively.
The addition of compost -also resulted in a decrease in the amount of Cain (P < 0.01) except for the case of the rice straw A treatment, added at 2%. A decrease in Mgin was observed in the treatments with the rice straw compost B added at 5% and 10% as well as those with the rapeseed pomace compost added at 2% and 5% (P < 0.005). The addition of the rice straw compost A resulted in a decrease in Mgin when the additive rate was 10% (P < 0.005).
3.3. Soil pH and dispersibility
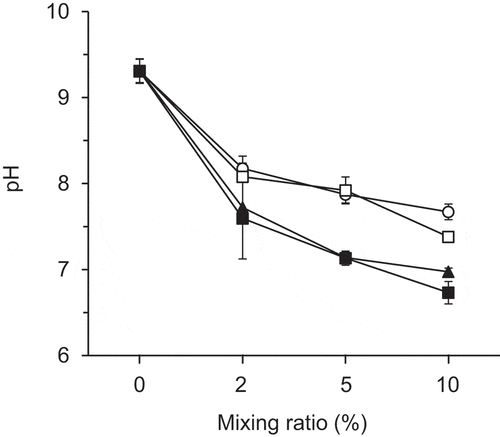
The addition of compost decreased soil pH () from 9.3 to 6.7 (rapeseed pomace compost), 7.0 (rice straw compost B), 7.4 (rice straw compost A), or 7.7 (cattle manure compost) with a larger extent at a higher additive rate. The order among the soils with different composts corresponded to the order of compost pH ().
Figure 3. Relationship between the mixing ratio of the compost and soil pH. Error bars indicate standard deviation (n = 3)
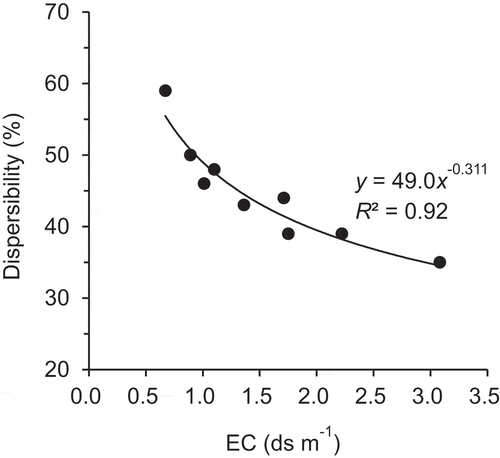
shows the dispersibility of the Tongliao soil for three kinds of compost added at 2‒10%. The dispersibility of the initial soil in distilled water was equivalent to 80% of that after the organic matter was decomposed with hydrogen peroxide. The value decreased to 35‒59% after the addition of any compost, which tended to be larger with higher additive rate. A significant negative correlation (r = 0.88; P < 0.005) was observed between EC and the dispersibility for all treatments and their relationship could be regressed to an exponential curve ().
Table 3. Dispersibility, electrical conductivity (EC), exchangeable potassium percentage (EPP), and exchangeable sodium percentage (ESP) of Tongliao soil with compost mixed at various ratios
4. Discussion
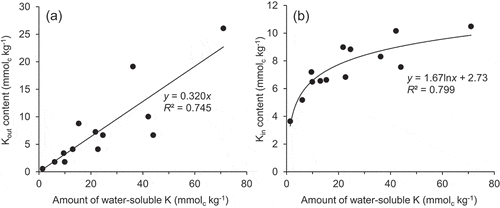
The differences in the decrease in Naout among the treatments with different composts added at the same rates may depend on the potential for each compost to supply K. In fact, there is a positive correlation between the Kout content after reaction and the amount of water-soluble K (), i.e., sum of the soluble K content in the Tongliao soil () and the amount of soluble K supplied from compost (). However, the apparent removal of Naout, as estimated from the differences in the amount of Na that was extracted with a 1 M choline chloride solution between the control and respective compost treatments, was correlated negatively with the amount of water-soluble compost K added to soil (r = −0.64; P < 0.05). This trend is probably related to the water-soluble Na content in the compost, which was higher in the compost with a higher water-soluble K content (). The replacement of Naout by K from the compost may become difficult if a large amount of Na is supplied simultaneously such as in the case of the treatment with cattle manure compost added at 5% and 10%. The water-soluble K/water-soluble Na ratio () was larger in the compost that showed the larger decrease in exchangeable Na ( and ), which could reflect the potential for compost to remove Na from the soil.
In the soil without added compost, the proportion of exchangeable K that was present as the inner sphere complex was high compared with the other three basic cations, reflecting the smaller diameter and the tendency to readily lose hydrated water molecules of this ion (Collins Citation1997; Marañón Di Leo and Marañón Citation2005). The addition of compost resulted in an increase in the amount of Kin, while the amounts of Cain and Mgin remained unchanged. These results suggest that the loss of the Nain was mainly due to being replaced by K. The higher selectivity of the inner sphere complexation to K rather than Na could be affected by the easier dehydration property of K. However, the removal of the Nain was not enhanced with increasing additive rate of composts except for the case of the rapeseed pomace compost. Although the Kin content was also correlated positively with the amount of water-soluble K (r = 0.81; P < 0.005), the increasing rate in the Kin content decreased with increasing water-soluble K (). Thus, there may be an upper limit in the number of the adsorption sites where K can replace other cations forming inner sphere complex.
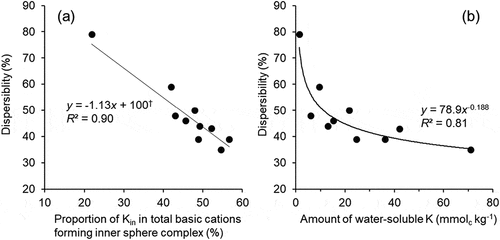
Figure 6. Relationship between relative abundance of Kin in total cations forming the inner sphere complex and dispersibility† (a) and that between amount of water-soluble K and dispersibility (b).
†Regression equation when the data from control soil (dispersibility, 79%) was excluded is: y = -1.13x +100 (R² = 0.67; P < 0.01)
In this study, the amounts of Ca and Mg in both the outer sphere and inner sphere complexes ( and ) generally decreased after the addition of compost. It therefore appears that Ca and Mg did not contribute to the removal of exchangeable Na. However, these results do not necessarily indicate that Ca and Mg cannot replace exchangeable Na because the water-soluble Ca and Mg contents in the composts were much smaller compared to the amounts of K and Na (). As a general property of compost, K is the major contributor to the removal of exchangeable Na. If the outer-sphere complex formation site is regarded as an adsorption site by cation exchange reaction, for example, the remarkable increase in the total amount of K, Na, Ca, and Mg in the outer sphere complex formation site by the addition of cattle manure may be due to the contribution of the negative charge of the compost. In addition, the reason why the total amount of K, Na, Ca, and Mg in the inner sphere complex site does not increase significantly in proportion to the amount of compost applied may be that this binding site is caused by clay minerals. Minor effect of the difference in chemical structure, such as the amount of weakly acidic functional groups, was also expected because the amounts of basic cations in the inner sphere complex differ slightly between the soils with rice straw composts A and B that have similar water-soluble cation contents.
The application of compost decreased soil pH (). A similar effect was also reported by Mahdy (Citation2011). The decrease in soil pH toward neutral could enhance the availability of P and micronutrients, in addition to their direct supply from compost (Walker and Bernal Citation2008; Lakhdar et al. Citation2009) and the enhancement of the activity of soil enzymes, including phosphatase (Liang et al. Citation2003).
One mechanism for improving the physical properties of sodic soils via the application of organic matter involves the promotion of soil aggregation (Wang et al. Citation2014). In the present study, since soil dispersion was determined 1 day after mixing the soil samples with the composts, the reduction in soil dispersion may be attributed to the loss of Na rather than the formation of aggregates. Laurenson et al. (Citation2010) reported that the soil dispersion occurs when the EC1:5 is <0.2 dS m−1 if EPP is in the range of 11‒32%, while it occurs only when the EC1:5 is <0.65 dS m−1 if EPP is in the range of 32‒46%. Such a threshold for ESP was 2‒8%, 8‒25%, and 25‒35% when the EC1:5 was < 0.2, <0.65, and <1.30 dS m−1, respectively. In the non-amended Tongliao soil, a combination of EPP, ESP, and EC (EC1:5; ) was on the border or within the range in which a soil dispersion was observed in Laurenson et al. (Citation2010), i.e., EPP 5%, ESP 17%, and EC 0.54 dS m−1. The addition of each compost increased EC1:5 to >0.65 dS m−1 while reducing the ESP to 3‒13%. These values also suggest a suppressive effect of compost on soil dispersion, although it should be noted that the above thresholds are probably not the same among soils with differences in the type and content of clay minerals.
To analyze the effect of pH, EC, ESP, and EPP on the soil physical properties, multivariate regression analysis was performed using the method of increasing and decreasing the variables. As a result, the following equation was obtained for the dispersibility (y, %) as objective variables:
EquationEquation 4(4)
(4) suggests that the combination of ESP and EPP is critical to control soil dispersion. A significant negative (r = −0.95; P < 0.005) correlation was also observed between the dispersibility and the relative abundance of Kin in total cations forming the inner sphere complex (), although similar relationship was not observed for Kout. These findings suggested the contribution of the composition of cations forming the inner sphere complex to soil physical properties. Thus, the removal of exchangeable Na by replacing it with compost K was beneficial for suppressing soil dispersion and maintaining water permeability.
Function of composts as K source on soil dispersion was evaluated by analyzing the relationship between the amount of soluble K and the dispersibility (). The results indicated an enhancement of the suppression of soil dispersion with increasing amount of soluble K. Since their relationship was regressed to an exponential curve, a large amount of K supply may not be necessary. The symmetry between and supported the contribution of Kin to soil dispersion.
In the present study, the behavior and function of the soil cations were considered by dividing them into those in the outer and inner sphere complexes. However, the relationship between the forms of complexes based on the extraction methods and the existing sites of cations in soil, such as those in terms of pore size, is still unclear. This should be investigated as well as the stability of Kin to support the usefulness of a K-rich compost for improving a sodic soil. Although the effect of K on soil dispersion is smaller than that of Na, its strength varies depending on the kind of clay minerals. Kaolinite, a major clay mineral in the Tongliao soil is known to be less affected by K (Levy and Van Der Watt Citation1990), whereas in the case of smectite group, which is often observed in sodic or saline-sodic soils, water permeability becomes lower with increasing the layer charge density (Shainberg et al. Citation1987). Thus, the availability of present results to other soils, e.g., those with different soil mineralogy, should also be examined. The effect of K on soil permeability induced by a single application of compost may be reduced if K is decreased due to washing out by rain or irrigation. In relation to this, repetitive application of compost is expected not only to maintain the K effect on soil permeability but also improve soil physical properties through developing soil aggregates.
5. Conclusions
The findings of the present study indicate that compost has potential for use as an amendment for remediating sodic and saline-sodic soils in terms of the acceleration of Na removal, soil neutralization, and the suppression of soil dispersion. The high content of water-soluble K, which has a strong ability to form inner sphere complexes, was a clear advantage of the compost because a considerable proportion of exchangeable Na in the sodic soil was in this form. The comparison of composts suggests that the contents and balance of water-soluble K and water-soluble Na can be used in the selection of the type and additive rate of compost. The increase in Kin can suppress the formation of the inner sphere complex of Na that come up from a lower layer due to vaporization. Since this function matches the purpose to keep the Na concentration in the surface soil low by irrigation, stability of Kin against a high concentration of Na is required to confirm.
Acknowledgments
The authors are grateful to Dr. Y. Mori, Kyushu University, for his kind analysis of soil clay minerals. We appreciate Aichi and Ibaraki Prefectural Agricultural Research Centers for allowing us to use rice straw compost. We also thank Dr. M. Ishiguro, Hokkaido University, for valuable suggestions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Bharadwaj, A., V. Khandelwal, P. Choudhary, and A. K. Bhatia. 2011. “Comparative Studies of Organic Enrichers in the Improvement of Physicochemical and Microbiological Characteristics of Saline/usar Soils.” Journal of Chemical Pharmaceutical Research 3 (6): 997–1003.
- Chapman, H. D. 1965. “Cation-Exchange Capacity.” In Methods of Soil Analysis. Part 2–Chemical and Microbiological Properties, edited by C. A. Black, D. D. Evans, L. E. Ensminger, J. L. White, and F. E. Clark, 891–901. Madison, Wisconsin, USA: American Society of Agronomy, Inc.
- Chartres, C. J. 1993. “Sodic Soils: An Introduction to Their Formation and Distribution in Australia.” Australian Journal of Soil Research 31 (6): 751–760. doi:10.1071/SR9930751.
- Cha-um, S., and C. Kirdmanee. 2011. “Remediation of Salt-affected Soil by the Addition of Organic matter—An Investigation into Improving Glutinous Rice Productivity.” Scientia Agricola 68 (4): 406–410. doi:10.1590/S0103-90162011000400003.
- Collins, K. D. 1997. “Charge Density-dependent Strength of Hydration and Biological Structure.” Biophysical Journal 72 (1): 65–76. doi:10.1016/S0006-3495(97)78647-8.
- Day, P. R. 1965. “Particle fractionation and particle-size analysis.” In Methods of Soil Analysis. Part 1–Physical and Mineralogical Properties, Including Statistics and Measurement and Sampling, edited by C. A. Black, D. D. Evans, L. E. Ensminger, J. L. White and F. E. Clark, 545–567, Madison, Wisconsin, USA: American Society of Agronomy, Inc.
- Fan, F., Q. G. Zhang, J. C. Tai, D. Z. Sun, Y. C. Yang, X. F. Song, and J. L. Ma. 2009. “The Formation and Classification of Saline-alkali Soil in Tongliao City.” Journal of Inner Mongolia University for Nationalities 24: 409–413. (in Chinese with English summary).
- Fan, F., Y. L. Zhang, Z., . L. Zhu, Q. G. Zhang, Y. Y. Dong, J. Wang, and Y. Cao. 2002. “Soil Features and Control Strategies for Development of Natural Grassland in Tongliao City.” Journal of Inner Mongolia University for Nationalities 17: 130–135. (in Chinese with English summary).
- Ferreira, D. R., and C. P. Schulthes. 2011. “The Nanopore Inner Sphere Enhancement Effect on Cation Adsorption: Sodium, Potassium, and Calcium.” Soil Science Society of America Journal 75 (2): 389–396. doi:10.2136/sssaj2010.0130nps.
- Gharaibeh, M. A., N. I. Eltaif, and S. H. Shra’ah. 2010. “Reclamation of a Calcareous Saline-sodic Soil Using Phosphoric Acid and By-product Gypsum.” Soil Use and Management 26 (2): 141–148. doi:10.1111/j.1475-2743.2010.00260.x.
- Hussain, N., G. Hassan, M. Arshadullah, and F. Mujeeb. 2011. “Evaluation of Amendments for the Improvement of Physical Properties of Sodic Soil.” International Journal of Agriculture & Biology 3 (3): 319–322.
- Lakhdar, A., M. Rabhi, T. Ghnaya, F. Montemurro, N. Jedidi, and C. Abdelly. 2009. “Effectiveness of Compost Use in Salt-affected Soil.” Journal of Hazardous Materials 171 (1–3): 29–37. doi:10.1016/j.jhazmat.2009.05.132.
- Laurenson, S., N. Bolan, E. Smith, and M. McCarthy. 2010. “Winery Wastewater Irrigation: Effects of Potassium and Sodium on Soil Structure.” In CRC CARE Technical Report Series, No. 19. CRC for Contamination Assessment and Remediation of the Environment, Adelaide, Australia.
- Levy, G. J., and H. V. H. Van Der Watt. 1990. “Effect of Exchangeable Potassium on the Hydraulic Conductivity and Infiltration Rate of Some South African Soils.” Soil Science 149 (2): 69–77. doi:10.1097/00010694-199002000-00002.
- Liang, Y. C., Y. F. Yang, C. G. Yang, Q. Q. Shen, J. M. Zhou, and L. Z. Yang. 2003. “Soil Enzymatic Activity and Growth of Rice and Barley as Influenced by Organic Matter in an Anthropogenic Soil.” Geoderma 115 (1–2): 149–160. doi:10.1016/S0016-7061(03)00084-3.
- Mahdy, A. M. 2011. “Comparative Effects of Different Soil Amendments on Amelioration of Saline-sodic Soils.” Soil and Water Research 6 (No. 4): 205–216. doi:10.17221/11/2011-SWR.
- Marañón Di Leo, J., and J. Marañón. 2005. “Hydration and Diffusion of Cations in Nanopores.” Journal of Molecular Structure: THEOCHEM 729 (1–2): 53–57. doi:10.1016/j.theochem.2005.02.070.
- Millennium Ecosystem Assessment. 2005. Ecosystems and Human Well-being: Synthesis. Washington DC, USA: Island Press.
- Oo, A. N., C. B. Iwai, and P. Saenjan. 2015. “Soil Properties and Maize Growth in Saline and Nonsaline Soils Using Cassava-industrial Waste Compost and Vermicompost with or without Earthworms.” Land Degradation & Development 26 (3): 300–310. doi:10.1002/ldr.2208.
- Qadir, M., R. H. Qureshi, and N. Ahmad. 1996. “Reclamation of a Saline-sodic Soil by Gypsum and Leptochloa Fusca.” Geoderma 74 (3–4): 207–217. doi:10.1016/S0016-7061(96)00061-4.
- Rengasamy, P. 2006. “Soil Salinity and Sodicity.” In Growing Crops with Reclaimed Wastewater, edited by D. Stevens, 125–138. Canberra, Australia: CSIRO.
- Robbins, C. W., and D. L. Carter. 1983. “Selectivity Coefficients for Calcium-magnesium- sodium-potassium Exchange in Eight Soils.” Irrigation Science 4: 95–102.
- Schollenberger, C. J., and R. H. Simon. 1945. “Determination of Exchange Capacity and Exchangeable Bases in Soil – Ammonium Acetate Method.” Soil Science 59 (1): 13–24. doi:10.1097/00010694-194501000-00004.
- Shainberg, I., R. Keren, N. Alperovitch, and D. Goldstein. 1987. “Effect of Exchangeable Potassium on Hydraulic Conductivity of Smectite-sand Mixture.” Clay & Clay Minerals 35 (4): 305–310. doi:10.1346/CCMN.1987.0350408.
- Sparks, D. L. 2003. “Sorption Phenomena on Soils.” In Environmental Soil Chemistry, 133–147. 2nd ed. London: Academic Press.
- Tong, L. M., and A. Watanabe. 2016. “Effect of Applying Cattle Manure as A Potassium Containing Material for the Reclamation of Saline-sodic Soils: A Model Experiment.” International Journal of Soil and Plant Science 13 (4): 1–12. doi:10.9734/IJPSS/2016/30159.
- Tucker, B. M. 1985a. “A Proposed New Reagent for the Measurement of Cation Exchange Properties of Carbonate Soils.” Australian Journal of Soil Research 23 (4): 633–642. doi:10.1071/SR9850633.
- Tucker, B. M. 1985b. Laboratory Procedures for Soluble Salts and Exchangeable Cations in Soils. Division of Soils Technical Paper 47. Canberra, Australia: CSIRO.
- Wada, S., and H. Seki. 1994. “Ca-K-Na Exchange Equilibria on a Smectitic Soil: Modeling the Variation of Selectivity Coefficient.” Soil Science and Plant Nutrition 40 (4): 629–636. doi:10.1080/00380768.1994.10414302.
- Walker, D. J., and M. P. Bernal. 2008. “The Effects of Olive Mill Waste Compost and Poultry Manure on the Availability and Plant Uptake of Nutrients in a Highly Saline Soil.” Bioresouce Technology 99 (2): 396–403. doi:10.1016/j.biortech.2006.12.006.
- Wang, F. L., and P. M. Huang. 2001. “Effects of Organic Matter on the Rate of Potassium Adsorption by Soils.” Canadian Journal of Soil Science 81 (3): 325–330. doi:10.4141/S00-069.
- Wang, L., X. Sun, S. Li, T. Zhang, W. Zhang, P. Zhai, and B. Bond-Lamberty. 2014. “Application of Organic Amendments to a Coastal Saline Soil in North China: Effects on Soil Physical and Chemical Properties and Tree Growth.” PLoS One 9 (2): e89185. doi:10.1371/journal.pone.0089185.
- Zia, K. M., H. Nawaz, and G. Murtaza. 1999. “Organic Matter and pH Effects on Base Exchange in Coarse Textured Soils.” International Journal of Agriculture & Biology 1: 36–38.