 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
It has been demonstrated that inoculation of Welsh onions (Allium fistulosum) with arbuscular mycorrhizal (AM) fungi during the nursery period followed by transplantation was effective in improving growth and reducing phosphorus fertilizer application. However, what range of soil phosphorus fertility is suitable for AM fungal inoculation remains unclear. To elucidate the optimal level of soil phosphorus fertility in a non-allophanic Andosol, we conducted a series of Welsh onion inoculation experiments under field conditions. Experiments were conducted in 2009–2010 and 2015–2016 in experimental plots at Tohoku University, northern Japan. Soil phosphorus fertility levels varied as follows: very high, high, medium, and low at 750–1,000, 150–250, 70–150, and 30–70 mg P2O5 kg soil−1, respectively (Truog method). Welsh onions were inoculated with a commercial Rhizophagus sp. R10 inoculum and grown for 7–9 weeks in a greenhouse nursery bed. The seedlings were transplanted into plots under the application of one, two or three levels of phosphorus fertilization. In the medium and high plots, inoculation increased marketable yields of Welsh onions irrespective of phosphorus fertilization, while no effect was found in the very high plot. In the low plot, inoculation did not clearly improve the yield, which was low irrespective of the treatments. These results indicate that AM fungal inoculation of Welsh onions in this soil was effective when available phosphorus ranged from 70 to 200 mg P2O5 kg soil−1.
1. Introduction
Arbuscular mycorrhizal (AM) fungi, which form symbiotic associations with plant roots, have been the focus of sustainable agricultural systems. AM fungi are expected to facilitate phosphorus (P) uptake by agricultural crops and reduce the input of P fertilizer. The AM fungal inoculum has been commercialized, and field inoculation trials have been extensively studied (e.g., Ortas Citation2012; Tawaraya, Hirose, and Wagatsuma Citation2012; Ceballos et al. Citation2013; Solaiman, Abbott, and Varma. Citation2014; Hijiri Citation2016; Niwa et al. Citation2018; Bender et al. Citation2019). However, AM fungal inoculation has not been widely applied in agriculture. One of the reasons for this is the lack of clarity regarding the soil conditions in which inoculation improves crop performance (Vosátka et al. Citation2012).
Welsh onions (Allium fistulosum L., ‘Negi’ in Japanese) are a major vegetable cultivated in East Asia. In Japan, Welsh onion production ranked third highest among vegetables in terms of value. Welsh onions have relatively sparse root systems, so their ability to acquire P from soil is weak. Therefore, a high dose of P fertilizer (100 − 450 kg P2O5 ha−1) is applied, although Welsh onions uptake a relatively small amount of P of approximately 20–40 kg P2O5 ha−1 (Nishio Citation2003; Tanaka and Oyamada Citation2000; Sano, Araki, and Washio Citation2011; Mishima, Endo, and Kohyama Citation2010). Furthermore, Welsh onions are widely cultivated in the Andosol area of Japan. Andosol has good physical characteristics and a deep plowable layer, but it also shows a strong P retention capacity, and the application rate of P fertilizer tends to be high. Such a heavy application of P fertilizer resulted in the accumulation of P in Andosols (Obara and Nakai Citation2004). Therefore, inoculation of Welsh onions with AM fungi may be a promising technology to reduce the excess application of P fertilizer. In fact, Tawaraya, Hirose, and Wagatsuma (Citation2012) reported that inoculation of Welsh onions with AM fungi at a nursery and transplantation of seedlings colonized with AM fungi into the field was highly effective in reducing P fertilization.
We conducted a series of field experiments in non-allophanic Andosol, one of major soil groups of Andosol, to explore the potential of AM fungal inoculation in Welsh onions. Some of the results have been published elsewhere (Suzuki et al. Citation2015; Akyol et al. Citation2019). In the present work, we re-analyzed these data in terms of available soil P and examined the optimum range of available P in soil that achieved a reasonable marketable yield of Welsh onions inoculated with AM fungi.
2. Materials and methods
2.1. Experimental site and design
Field experiments were conducted at the Field Science Center of the Graduate School of Agricultural Science, Tohoku University, Osaki, Miyagi, in northeastern Japan (38°44ʹ54.2”N, 140°45ʹ09.1”E). The soil type was Melanic Aluandic Andosol. The average soil characteristics in the plow layer (the top 15 cm) were as follows: texture, silt loam; CEC, 37 cmol(+) kg−1; total C, 93 g C kg−1; total N, 5.5 g N kg−1; pH (H2O), 5.5; Phosphate absorption coefficient, 18 g P2O5 kg−1. The experiments were conducted in 2009, 2010, 2015, and 2016. Experimental plots were set at five sites that were located adjacently within a 1 ha area showing different levels of phosphorus fertility due to differences in cropping history and management. The sites were designated as very high 1 (VH1), very high 2 (VH2), high (H), medium (M), and low (L) in terms of available phosphorus levels. Welsh onions were cultivated in these sites each year under this treatment by combining AM fungal inoculation with the application level of phosphorus fertilizer, as summarized in . Design of field experiments and outline of cultivation are shown in supplemental information (Table S1)
Table 1. Effect of arbuscular mycorrhizal fungal inoculation on marketable yield of Welsh onions at different phosphorus fertility and fertilization levels
2.2. Plant, inoculum, and nursing seedlings
Welsh onions (Allium fistulosum L. cv. Motokura) were sown in a nursery bed using a NITTEN paper pot (Nippon Beet Sugar Manufacturing Co., Japan) and grown for 7–9 weeks in a greenhouse. The nursery medium was based on Tawaraya, Hirose, and Wagatsuma (Citation2012), with some minor modifications as follows. A mixture of soil collected from a non-fertilized site in the field and ‘Akadama-tsuchi’ (a commercial horticulture medium prepared from volcanic subsoil, Komeri Co.) was used as the nursery medium after autoclaving (120°C, 1.2 atm.,20 min). Nitrogen, phosphorus, and potassium were added to the medium at a ratio of 1:1:1 (N: P2O5: K2O) g kg−1 dry medium. For the control treatment, the amount of added phosphorus was increased by 20% (100% in 2015) to ensure that the growth of seedlings was equal to that of inoculated seedlings.
For AM fungal inoculation, a commercial inoculum containing Rhizophagus sp. R10, which was previously called Glomus sp. R10 by the manufacturer, Idemitsu Kosan Co., Japan (Niwa et al. Citation2018), was added to the medium at 10% (w/w) in 2009 and 2010, and 5% (w/w) in 2015 and 2016. For the control treatment, autoclaved inoculum was added in the same manner. The seedlings were transplanted to the plots at a density of 10.5 seedlings/m2 in VH1 (2010) or 12.5 seedlings/m2 in other sites.
2.3. Field preparation, fertilization, and harvest
Liming was performed by applying 1 t/ha of dolomite limestone powder to the field during preparation. Fertilizers were broadcasted to the plot before transplanting and mixed with a rotary cultivator. P fertilizer was applied, as shown in . In 2009 and 2010, coated urea (200 kg N ha−1) and potassium sulfate (200 kg K2O ha−1) were applied as the base layer just before transplanting. In 2015 and 2016, ammonium sulfate (100 kg N ha−1) and potassium sulfate (100 kg K2O) were applied as the base layer. During cultivation, 5 g N m−2 of urea nitrogen was additionally applied once or twice, and earth-up was conducted 3–4 times to whiten and elongate the base of the shoot (edible part). Other cultivation practices were conducted in a conventional manner. Outline of Welsh onion cultivation is presented in Table S1 (supplemental information). Soil was collected from the plow layer 3–4 weeks after transplanting, and some chemical properties, including available phosphorus (Truog method), were analyzed.
Approximately 4–5 months after transplanting, 20–40 plants per a plot were harvested. Six to 10 plants of average size were selected for further examination. The selected shoots were cut 60 cm from the base by removing the top part of the leaves. This cut shoot was classified as marketable if the length of its white sheath was more than 30 cm and its sheath diameter was more than 1 cm. Yield was expressed as the total fresh weight of the marketable harvest per area.
Inoculation responsiveness was calculated using the following formula.
M: marketable yield with inoculation treatment
C: marketable yield with control treatment
2.4. Statistical analysis
Statistical analyses were performed using R (https://cran.r-project.org/).
3. Results
The addition of AM fungal inoculum to the nursery medium successfully grew well-colonized Welsh onion seedlings. Upon transplanting, the colonization rates reached 70%–90%. The growth of inoculated seedlings was comparable to that of the non-inoculated seedlings. The colonization rates in field after transplanting were shown in supplemental information (Fig. S1, S2 and S3). After transplanting, inoculation increased shoot growth throughout the cultivation period in H, M, and L, whereas it showed no effect in VH1 and VH2 (Suzuki et al. Citation2015). The marketable yield of Welsh onions is summarized in . The yield tended to be higher at experimental sites with higher available soil P levels compared to sites with lower levels. Inoculation increased the yield at sites H and M, but not VH and L. At site L, early growth was increased by inoculation, but no significant effect of inoculation on marketable yield was found. The level of P fertilizer did not affect the yields at sites L, M, and H. At sites VH1 and VH2 and inoculated plots at site H, yields were more than 3,000 g m2, which is the target yield of the local agricultural extension service (Miyagi prefecture Citation2006).
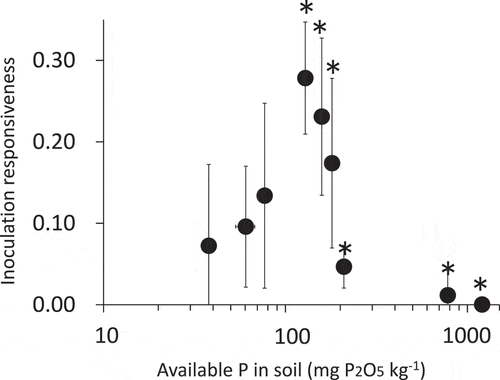
shows the relationship between the inoculation responsiveness of marketable yields and available soil P. This indicates that more than 10% of inoculation responsiveness was found within a range of 70–200 mg P2O5 kg soil−1. As indicated by asterisks in , the yields were greater than the target yield of 3,000 g/m2 according to the local agricultural extension service (Miyagi prefecture Citation2006).
Figure 1. The relationship between mycorrhizal inoculation responsiveness and available soil phosphate. Bars indicate standard errors. Standard error was calculated by replicate data of inoculated treatment (M) versus average data of the control (C). Asterisks indicate the plots with yields of 3,000 g m−2 or higher relative to the target yield
4. Discussion
The present study clearly showed that inoculation of Welsh onions with AM fungi was not effective in soils that were very rich or very poor in available P (). Since the main pool of P in soil absorbed by AM fungi is almost the same as that absorbed by plant roots (Bolan et al. Citation1984), the scarcity of available soil P reduces the efficacy of AM fungal inoculation on plant growth. To ensure that AM fungal inoculation is fully effective in Welsh onion cultivation, it is important that a moderate level of available P, i.e., 70–200 mg P2O5 kg soil−1 (Truog method), is present.
The application of P fertilizer usually exceeds the demand for P by crops or vegetables. Such continuous heavy application of P results in its accumulation in arable fields. In particular, this trend is remarkable in vegetable farms (Mishima, Endo, and Kohyama Citation2010). As a result, the average available soil P in vegetable farms in Japan surpassed 1000 mg P2O5 kg−1 (Truog method) (Obara and Nakai Citation2004), which is the same level present in VH1 and VH2 in this experiment. indicates that AM fungal inoculation might not be effective in such vegetable fields. However, such P accumulation in soil increases the risk of environmental pollution and is not desirable for the effective use of P as a limited natural resource (Kleinman et al. Citation2011). Therefore, it is recommended to reduce the excessive use of P fertilizer. According to the basic guidelines of soil fertility enforcement law in Japan (Ministry of Agriculture, Forestry and Fisheries Citation2008), the appropriate available P in soil for Andosols should range from 100 to 1,000 mg P2O5 kg−1. At site H, the available P in soil was at the low end of the recommended range. The AM fungal inoculation in these soils was so effective that good yields were achieved, which exceeded the target yield (3,000 g m−2) of the local agricultural extension service (Miyagi prefecture Citation2006). These results confirmed the findings of Tawaraya, Hirose, and Wagatsuma (Citation2012) and indicated that AM fungal inoculation technology is effective in both achieving good yields of Welsh onions and reducing excess use of P fertilizer. In the present experiment, the increase in P fertilizer application did not affect the yields even in site L and M (). The reason was not clear, but a similar trend that increase in P fertilization did not affect growth of Welsh onion in a light-colored Andosol was reported (Murayama and Miyazawa Citation2013). Welsh onion might be more adapted to lower level of soil available P than previously thought. Under the present experimental conditions. phosphate fertilization may not be so effective as AM fungal inoculation.
In the present experiments, the level of N fertilization was comparable or a little lower than those recommended by local agricultural service (Miyagi prefecture Citation2006). Since it is reported higher N/P ratio in nutrient solution caused higher mycorrhizal colonization and effectiveness (Hepper Citation1983), more increased N fertilization might increase effectiveness of AMF inoculation.
For AM fungi, the success of inoculation depends upon the compatibility of fungal species with host crops and the establishment of the AM fungal inoculum (Verbruggen et al. Citation2013). The inoculum of Rhizophagus sp. R10 was selected to promote the growth of various crops by the manufacturer and has been well recognized as a compatible species with Welsh onions in various field tests (Tawaraya, Hirose, and Wagatsuma Citation2012; Suzuki et al. Citation2015; Sato, Cheng, and Tawaraya Citation2018; Akyol et al. Citation2019). Furthermore, Rhizophagus sp. R10 has been recognized to colonize plant roots under high phosphorus fertility conditions (Suzuki et al. Citation2015). The establishment of inoculum fungi was ascribed to competition between the indigenous AM fungi and the inoculum. In the fields investigated here, the colonization potential of indigenous AM fungi was high. At VH1 and VH2 with high P fertility, the colonization rate in the non-inoculated plots was almost equal to that in the inoculated plots at 50–60 days after planting (Fig. S1). However, pre-inoculation during the nursery stage followed by transplantation of the well-colonized seedlings enabled the inoculated fungus to predominate over indigenous AM fungi. In fact, molecular analysis of microorganisms in roots at site H confirmed that the inoculated fungus Rhizophagus sp. R10 was well established (1 and 2 months after planting) (Akyol et al. Citation2019). In addition, higher rates of vesicle formation, which is characteristic of Rhizophagus sp., were found in the inoculated roots (Fig. S2, S3). Thus, the inoculated fungus Rhizophagus sp. R10 was adapted to high phosphorus fertility and was competitive to indigenous AM fungi by using pre-inoculation.
In conclusion, a series of field inoculation experiments of Welsh onions in a non-allophanic Andosol showed that inoculation of Welsh onions with AM fungi at the nursery stage followed by transplantation into the field was effective in achieving a reasonable marketable yield under moderate levels of soil P fertility. AM fungal inoculation may contribute to reducing P fertilizer input in the cultivation of Welsh onions.
Supplemental Material
Download MS Word (296.4 KB)Acknowledgments
We are grateful to the members of Environmental Crop Science Laboratory, Field Science Center, Tohoku University, for their technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Akyol, T. Y., R. Niwa, H. Hirakawa, H. Maruyama, T. Sato, T. Suzuki, A. Fukunaga, et al. 2019. “Impact of Introduction of Arbuscular Mycorrhizal Fungi on the Root Microbial Community in Agricultural Fields.” Microbes and Environments 34: 23–32. doi:10.1264/jsme2.ME18109.
- Bender, S. F., K. Schlaeppi, A. Held, and M. G. A. van der Heijden. 2019. “Establishment Success and Crop Growth Effects of an Arbuscular Mycorrhizal Fungus Inoculated into Swiss Corn Fields.” Agriculture, Ecosystems and Environment 273: 13–24. doi:10.1016/j.agee.2018.12.003.
- Bolan, N. S., A. D. Robson, N. J. Barrow, and L. A. G. Aylmore. 1984. “Specific Activity of Phosphorus in Mycorrhizal and Non-mycorrhizal Plants in Relation to the Availability of Phosphorus to Plants.” Soil Biology and Biochemistry 16: 299–304. doi:10.1016/0038-0717(84)90023-3.
- Ceballos, I., M. Ruiz, C. Fernández, R. Peña, A. Rodríguez, and I. R. Sanders. 2013. “The in Vitro Mass-Produced Model Mycorrhizal Fungus, Rhizophagus Irregularis, Significantly Increases Yields of the Globally Important Food Security Crop Cassava.” PLoS ONE 8 (8): e70633. doi:10.1371/journal.pone.0070633.
- Hepper, C. M. 1983. “The Effect of Nitrate and Phosphate on the Vesicular-arbuscular Mycorrhizal Infection of Lettuce.” New Phytologist 93: 389–399. doi:10.1111/j.1469-8137.1983.tb03439.x.
- Hijiri, M. 2016. “Analysis of a Large Dataset of Mycorrhiza Inoculation Field Trials on Potato Shows Highly Significant Increases in Yield.” Mycorrhiza 26: 209–214. doi:10.1007/s00572-015-0661-4.
- Kleinman, P. J. A., A. N. Sharpley, R. W. McDowell, D. N. Flaten, A. R. Buda, L. Tao, L. Bergstrom, and Q. Zhu. 2011. “Managing Agricultural Phosphorus for Water Quality Protection: Principles for Progress.” Plant and Soil 349: 169–182. doi:10.1007/s11104-011-0832-9.
- Ministry of Agriculture, Forestry and Fisheries. 2008. Soil Fertility Enforcement Law https://www.maff.go.jp/j/seisan/kankyo/hozen_type/h_dozyo/pdf/chi4.pdf
- Mishima, S., A. Endo, and K. Kohyama. 2010. “Nitrogen and Phosphate Balance on Crop Production in Japan on National and Prefectural Scales.” Nutrients Cycling in Agroecosystems 87: 159–173. doi:10.1007/s10705-009-9324-1.
- Miyagi prefecture. 2006. Guideline for Vegetable Cultivation in Miyagi Prefecture https://www.maff.go.jp/j/seisan/kankyo/hozen_type/h_sehi_kizyun/pdf/miyagi_yasai18_09.pdf
- Murayama, T., and K. Miyazawa. 2013. “Growth of Welsh Onion (Allium Fistulosum L.) After Pre-transplanting Phosphorus Application.” Japanese Journal of Soil Science and Plant Nutrition 84: 455–461. doi:10.20710/dojo.84.6_455
- Nishio, M. 2003. “Analysis of the Actual State of Phosphate Application in Arable Farming in Japan.” Japanese Journal of Soil Science and Plant Nutrition 74: 435–443. doi:10.20710/dojo.74.4_435.
- Niwa, R., T. Koyama, T. Sato, K. Adachi, K. Tawaraya, S. Sato, H. Hirakawa, S. Yoshida, and T. Ezawa. 2018. “Dissection of Niche Competition between Introduced and Indigenous Arbuscular Mycorrhizal Fungi with respect to Soybean Yield Responses.” Scientific Reports 8: 7419. doi:10.1038/s41598-018-25701-4.
- Obara, H., and M. Nakai. 2004. “Available Phosphate of Arable Lands in Japan. Changes of Soil Characteristics Jn Japanese Arable Lands (II).” Japanese Journal of Soil Science and Plant Nutrition 75: 59–67. doi: 10.20710/dojo.75.1_59
- Ortas, I. 2012. “The Effect of Mycorrhizal Fungal Inoculation on Plant Yield, Nutrient Uptake and Inoculation Effectiveness under Long-term Field Conditions.” Field Crops Research 125: 35–48. doi:10.1016/j.fcr.2011.08.005.
- Sano, O., A. Araki, and T. Washio. 2011. “Effects of the Application of a Liquid Fertilizer Containing Phosphorus to Seedlings on the Growth and the Nutrient Uptake in Autumn-harvesting Welsh Onion (Allium Fistulosum L.) In Andosols.” Bulletin of the Research Institute for Agriculture Okayama Prefectural Technology Center for Agriculture, Forestry, and Fisheries 2: 53–59.
- Sato, T., W. Cheng, and K. Tawaraya. 2018. “Effects of Indigenous and Introduced Arbuscular Mycorrhizal Fungi on the Growth of Allium Fistulosum under Field Conditions.” Soil Science and Plant Nutrition 64: 705–709. doi:10.1080/00380768.2018.1543534.
- Solaiman, Z. A., L. K. Abbott, and A. Varma., Ed. 2014. Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration. Berlin: Springer-Verlag.
- Suzuki, T., R. Tajima, S. Hara, T. Shimizu, T. Uno, T. Ito, and M. Saito. 2015. “Effect of Arbuscular Mycorrhizal Fungal Inoculation on the Growth of Welsh Onion in Soil Rich in Available Phosphate, and Characterization of Indigenous Arbuscular Mycorrhizal Fungi Isolated from the Soil.” Soil Microorganisms 69: 48–57. doi:10.18946/jssm.69.1_48.
- Tanaka, Y., and T. Oyamada. 2000. “Characteristics of Absorption of Manure in Autumn Winter Welsh Onions Using Plug Seedlings.” Bulletin of the Horticultural Institute, Ibaraki Agricultural Center 8: 13–18.
- Tawaraya, K., R. Hirose, and T. Wagatsuma. 2012. “Inoculation of Arbuscular Mycorrhizal Fungi Can Substantially Reduce Phosphate Fertilizer Application to Allium Fistulosum L. And Achieve Marketable Yield under Field Condition.” Biology and Fertility of Soils 48: 839–843. doi:10.1007/s00374-012-0669-2.
- Verbruggen, E., M. G. A. van der Heijden, M. C. Rillig, and E. T. Kiers. 2013. “Mycorrhizal Fungal Establishment in Agricultural Soils: Factors Determining Inoculation Success.” New Phytologist 197: 1104–1109. doi:10.1111/j.1469-8137.2012.04348.x.
- Vosátka, M., A. Látr, S. Gianinazzi, and J. Albrechtová. 2012. “Development of Arbuscular Mycorrhizal Biotechnology and Industry: Current Achievements and Bottlenecks.” Symbiosis 58: 29–37. doi:10.1007/s13199-012-0208-9.
