ABSTRACT
SummaryContinuous monocropping degrades soil physicochemical properties, leads to the accumulation of toxic compounds and changes soil microbial community composition. Pre-flooding the soil is a promising strategy to improve its productivity; however, little is known about the underlying mechanisms. Here, strawberry (Fragaria × ananassa) pot experiments were conducted with soil that had been used for continuous monocropping. The treatments were pre-flooding alone, with rice straw added and with rice straw plus a biofertilizer (mainly contained Bacillus velezensis) amendment, and the impacts of the treatments on soil physicochemical properties, bacterial diversity and composition and the integrated plant–soil–microbe responses were tracked. The strawberry biomass significantly increased after pre-flooding treatments. Pre-flooding increased the soil pH and the available P and K concentrations, while it decreased soil electrical conductivity. The bacterial diversity and abundance significantly increased after pre-flooding treatments. A Mantel test showed that soil pH and salinity were the two most important factors in shaping bacterial composition, but they exerted opposite effects. The increased strawberry biomass after pre-flooding was significantly correlated with bacterial composition and soil salinity in long-term continuous strawberry monocropping systems.
1. Introduction
Soil microbial community composition plays a vital function in plant nutrition, development and immunity (Castrillo et al. Citation2017; Fitzpatrick et al. Citation2018; Li et al. Citation2019). Greater microbial diversity promotes the production and complementary use of resources, and it promotes productivity and stability (Fan et al. Citation2021). However, agricultural practices, such as the application of fertilizers and pesticides, as well as and tillage methods, result in changes of the microbial community’s composition and decreases of its diversity (Griffiths et al. Citation2007; Li et al. Citation2019). Continuous monocropping also changes soil physicochemical properties, alters the microbial composition and decreases microbial diversity (Zuppinger-Dingley et al. Citation2014). For example, long-term strawberry monocropping increases the relative abundance levels of Fusarium, Humicola and Arthrobotrys spp. fungi and decreases the relative abundance levels Bacillus, Sphingomonas and Sphingopyxis spp. bacteria (Chen et al. Citation2020). Microbial taxa, such as Bacillus spp., enhance the plant-available forms of nutrients in rhizospheres, control disease-causing pathogenic microbial growth, and induce pest defense systems (García-Fraile, Menéndez, and Rivas Citation2015; Kang et al. Citation2015). In addition, long-term monocultures change soil properties (especially decreasing soil pH), and soil pH is significantly correlated with microbial diversity (Zhou et al. Citation2015; Wang et al. Citation2018). Furthermore, Fan et al (Citation2021) found that soil biodiversity of some phylotypes play key roles in maintaining plant productivity.
Strawberry (Fragaria × ananassa), in the family Rosaceae, is a major economic fruit planted in arable lands worldwide. Because of the plants characteristics, the limited available land and the lack of a practical planting system, strawberry is usually cultivated in continuous monocropping systems (Chen et al. Citation2020). Unfortunately, the cultivation of strawberry is severely threatened by problems associated with these systems. Long-term strawberry monocropping causes the enrichment of autotoxic substances and the deterioration of soil biological and physicochemical properties, which result in declines in crop yield, soil degradation and plant disease (Zhou and Wu Citation2012; Chen et al. Citation2020). For example, Chen et al. (Citation2020) found that continuous strawberry cropping led to changes in abiotic and biotic factors in the soil and that the accumulation of phenolic acids (i.e., p-hydroxybenzoic acid, ferulic acid and cinnamic acid) significantly inhibited crop growth.
Pre-flooding soil used in continuous monocropping is an ancient practice employed in China and other Asian countries that minimizes soilborne plant diseases and improves crop production (Stover Citation1962). This practice has rarely been applied to alleviate strawberry monocropping issues. The flooding treatment may create an anaerobic state and change soil properties and nutrient cycling. For example, Ye et al. (Citation2019) found that flooding had a major influence on soil pH and soil nutrient levels. Soil pH affects soil microbial community composition and diversity (Zhou et al. Citation2016; Wang et al. Citation2019). Rath et al. (Citation2019) found that soil salinity was also an important factor in shaping microbial community composition. Long-term monocropping was the major factor accelerating soil salinization, and soil salinity affects the majority of the dominant bacterial and archaeal taxa (Wei et al. Citation2020). A promising method to alleviate such problems is to pre-flood soils used in continuous monocropping systems (Huang et al. Citation2015), including strawberry cultivation.
Straw incorporation and biofertilizer applications also enhance plant production without increasing chemical fertilizer applications (Yang et al. Citation2019). Rice straw additions alleviate the soil degradation caused by intensive and continuous cultivation (Lou et al. Citation2011). Moreover, rice straw additions are beneficial to soil microbes; consequently, higher soil microbial biomass and activity levels are frequently observed under straw amendment conditions (Zhou et al. Citation2020b). Bio-fertilizers improve soil health by directly suppressing pathogens. Recently, Xiong et al. (Citation2017) found that biofertilizers also change soil microbial communities through soil pathogen inhibition. Consequently, previous studies have proposed that co-applications of straw and biofertilizer may alter soil physicochemical properties and microbial communities, as well as improve plant productivity (Lu et al. Citation2020). Thus, rice straw and biofertilizer applications in combination with pre-flooding should have synergistic effects on the sustainable productivity of strawberry in continuous monocultures, but limited trials have been performed.
In this study, soil used for long-term continuous strawberry monocropping was subjected to flooding and the impacts of pre-flooding treatments on strawberry biomass and soil biogeochemical properties were tracked. The main objectives were to investigate the effectiveness of, and mechanisms involved in, pre-flooding treatments on the continuous monocropping of strawberry. Disturbances change the soil bacterial composition and soil properties, and such changes may be directly or indirectly caused by an increased soil pH or decreased soil salinity level.
2. Experimental producedures
2.1. Experimental description and soil sampling
Soil for the greenhouse pot experiments was collected from a 10-year continuously monocropped field of strawberries at the Shanghai City Zhuanghang Experimental Station (30°53ʹ N, 121°23ʹ E). The soil at this site is classified as anthrosol. The region is characterized by subtropical climate, with an average annual temperature and precipitation of 16°C and 1,200 mm, respectively. In total, 20 cores (6 cm in diameter) were randomly collected from the plow-layer of soil (0–20 cm) with a drill and transported to the laboratory on ice. The soil was passed through a 2-mm sieve to remove plant roots, mixed thoroughly and then stored at 4°C until being used in the greenhouse pot experiments. The greenhouse pot experiments were performed using a randomized complete block design with three replicates for each treatment at the Shanghai Academy of Agricultural Sciences. There were four treatments in this study: soil that had been used for monocropping without pre-flooding (CK), the same soil with pre-flooding (CF), the same soil with pre-flooding and the addition of 0.5% rice straw (FS), the same soil with pre-flooding, the addition of 0.5% rice straw and a biofertilizer amendment (FSB). The flooded soil was treated with sterilized water for 30 days at room temperature (irrigated to saturation for 30 days). The soil was air dried at the room temperature for approximately 1 month after the water was removed. For FS and FSB treatments, the rice straw was shredded into < 1-cm pieces and mixed thoroughly with soils. The biofertilizer contained 1 × 109 CFU ml−1 of Bacillus velezensis K3, which was grown in liquid Luria-Bertani medium at 30°C for 5 d. The B. velezensis K3 had been isolated from strawberry rhizosphere soil, which promotes strawberry growth and inhibits plant pathogens (Zhou et al. Citation2020a). The rice straw application was 0.5% in weight, whereas the biofertilizer (containing 30×-diluted fermented liquid) application rate was 600 ml (12 kg soil)−1. The final number of B. velezensis K3 was approximately 1.6 × 109 CFU g−1 soil. After the treatments (no other inorganic fertilizers were applied), strawberries were planted in pots (50 × 12 × 12 cm) at a density of four plants per pot on 30 October and immediately transported to a greenhouse having an average temperature of 25°C and an average humidity of 60%.
2.2. Soil sample collection and chemical analyses
After two months, we collected six random soil cores adjacent to the plants (bulk soils), to a depth of approximately 5 cm, from each pot and pooled them to yield on composite sample. To minimize bias, we collected two composite samples per replicate plot, for a total of 24 composite samples. The soil samples were passed through a 2-mm sieve and divided into two subsamples. One subsample was air dried at room temperature for chemical analyses, and the other was stored at −80°C for molecular analyses. Soil pH was determined using a glass combination electrode with a 2.5:1 water: soil ratio (Li et al. Citation2013). Soil electrical conductivity (EC) was measured using a conductivity meter at 1:5 soil: water ratio. Soil organic matter (SOM) was analyzed using the potassium dichromate method (Strickland and Sollins Citation1987). Soil available P (AP) and available K (AK) were determined using the molybdenum blue procedure (Olsen Citation1954) and the flame photometer method (Warncke and Brown Citation1998), respectively. Alkaline N (AN) was analyzed using the alkaline hydrolysis diffusion method (Bao Citation2000).
2.3. Soil genomic DNA extraction, quantitative PCR (qPCR) and high-throughput sequencing
Soil genomic DNA was extracted from 0.25 g of soil using a Power Soil DNA Isolation Kit (MOBIO Laboratories Inc., Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. Six successive extractions of microbial DNA from a replicate soil were combined to minimize the extraction bias (Wang et al. Citation2019). The DNA was then purified using a DNeasy Tissue Kit (Qiagen, Valencia, CA, USA) and checked by electrophoresis at a 1.0% agarose gel.
The bacterial 16S rRNA gene copy numbers were quantified in duplicate using a Roche LightCycler 480 II Real-time PCR detection system (Roche Diagnostics, Indianapolis, IN, USA) with the primers 515 F/806 R (Wang et al. Citation2018). The program was as follows: 95°C for 1 min followed by 40 cycles of 94°C for 15 s, 55°C for 34 s and 72°C for 15 s. To estimate the 16S rRNA gene copy numbers, standard curves were developed using 10-fold serial dilutions of known copy numbers of plasmid DNAs possessing the correct genes (the R2 value of the standard curves was > 0.99). To minimize bias, the 16S rRNA gene copies of the standard curves and each soil sample were measured simultaneously in the same real-time system.
All the DNA samples were diluted to 1 ng μl−1 to produce amplicon libraries. The primers used were soil bacterial specific primers (515 F/806 R), which were designed specifically to reduce the amplification of plant plastid-related sequences (Peiffer et al. Citation2013). The PCR reactions were conducted using Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, USA) and the following amplification procedure: 2 min at 94°C, followed by 30 cycles of 30 s at 94°C, 30 s at 50°C and 1 min at 72°C, and then, a final extension for 6 min at 72°C. The PCR products were mixed with the same volume of 1× loading buffer (containing SYBR green) and then subjected to electrophoresis on a 2% agarose gel for quantification and qualification. The PCR products were subsequently mixed in equi-density ratios for purification using a Qiagen Gel Extraction Kit (Qiagen, Hilden, Germany). The amplicon libraries were generated using a TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA). The forward primer was the universal primer 515 F with an added construct on the 5′ end of the 5′ Illumina Adapter, (5′AATGATACGGCGACCACCGAGATCTACAC3′) + Forward Primer Pad (5′TATGGTAATT3′), and the universal bacterial reverse primer was 806 R with an added construct on the 5′ end of the reverse complement of 3′ Illumina adapter, (5′CAAGCAGAAGACGGCATACGAGATGCCGCATTCGAT3′) + Barcode (12 base pairs) (Marina et al. Citation2016). The amplicon libraries were qualified on a Qubit@ 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA) and an Agilent Bioanalyzer 2100 system. Finally, the 16S rRNA gene fragments were sequenced using an Illumina MiSeq 2500 platform.
2.4. Gene sequence analyses
Sequences were analyzed using QIIME 1.9.1 (Caporaso et al. Citation2010). The reads were assigned to samples on the basis of their unique barcodes. The primer sequences and barcodes were then cut off, after which the paired-end reads were merged using the FLASH analysis tool based on the read overlap from the opposite ends of the same DNA fragment (Mago and Salzberg Citation2011). Low-quality sequences were filtered using several specific criteria in accordance with Bokulich et al. (Citation2013). Briefly, (1) reads with a quality score < 20 over a 50-bp window size were removed; (2) only reads with a ≥ 10-bp overlap and less than 20% mismatches were combined to obtain longer sequences; and (3) sequences were assigned to different sample libraries on the basis of the unique barcodes and primers, with less than 0% and 2% mismatches, respectively. Chimeras were detected by comparing the sequences with those in the reference database using the UCHIME algorithm (Edgar et al. Citation2011) and then removed. The effective tag sequences were analyzed using Uparse software (Edgar Citation2013), and a similarity of ≥ 97% was considered to indicate the same operational taxonomic unit (OTU). One OTU was then selected from each group of the same OTUs as a representative sequence for annotation using the Silva database (ver. 138) (Christian et al. Citation2013) and the RDP classifier algorithm (Wang et al. Citation2007). Because the Silva database has some invalidated taxonomic names, especially at the Class level, we divided the Gammaproteobacteria into Betaproteobacteria and Gammaproteobacteria, in accordance with the traditional polyphasic taxonomy (Sanford et al. Citation2020). Next, the OTU abundance was normalized on the basis of the sample having the least number of sequences (29,118 sequences), and all the subsequent analyses were performed in accordance with the normalized data. The α-diversity indices, including OTUs, diversity, richness and coverage, were calculated using QIIME 1.9.1. The sequences obtained in this study can be found in the NCBI Sequence Read Archive database (accession number: PRJNA675707).
2.5. Statistical analyses
A one-way analysis of variance (ANOVA) was conducted to determine the effects of treatments on soil characteristics, α-diversity indices and the relative abundance levels of taxa, and significant differences among group means were compared using Tukey’s test at P < 0.05. Pearson’s correlation analyses were conducted to assess the relationships between soil characteristics and both the α-diversity indices and the relative abundance levels of taxa. The one-way ANOVA and Pearson’s correlation analyses were conducted using the SPSS BASE ver. 19.1 statistical software (SPSS, Chicago, IL, USA) (Ahn et al. Citation2012). The β-diversity was analyzed using the calculated weighted Fast UniFrac distance, and non-metric multidimensional scaling (NMDS) was determined using the distance measured. A partial Mantel test was performed to assess the influence of soil properties on bacterial communities. Structural equation modeling (SEM) was performed to gain a mechanistic understanding of how flooding treatments influenced soil properties, microbial compositions and strawberry biomass. The bacterial community composition was obtained using a principal components analysis, and its first scale (which explained 37.59% of the variation in bacterial communities among samples) was used in the subsequent SEM analysis. The SEM analysis was performed using the specifications of the conceptual model of hypothetical relationships (Figure S1), assuming that the flooding treatment increased strawberry biomass by altering soil properties and the microbial composition. The fitness of the model was indicated by a low χ2/df value (< 2), a non-significant χ2 test (P > 0.05) and a high goodness-of-fit index (> 0.9) (Zeng et al. Citation2016; Ning et al. Citation2019).
3. Results
3.1. Pre-flooding changed soil chemical properties and increased strawberry biomass
All the flooding treatments significantly (P < 0.05) changed the strawberry biomass () and soil properties, including soil pH, EC and soil concentrations of AP, AK, AN and SOM (). The ANOVA showed that soil EC after flooding treatments decreased significantly, with the highest decrease occurring after the FS treatment (EC 789). However, all the flooding treatments significantly increased pH, AP, AK, and strawberry biomass, compared with the CK. The concentrations of soil AN and SOM were not significantly different among CK, CF and FS, but the FSB treatment significantly increased soil AN and decreased SOM.
Table 1. Strawberry biomass and properties of soil subjected to continuous strawberry monocropping under different flooding conditions
3.2. Pre-flooding increased bacterial 16S rRNA gene copy numbers
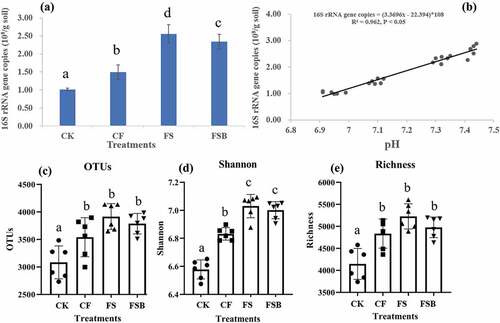
Pre-flooding the strawberry monocropping soil increased the size of the soil bacterial community as assessed by the qPCR analysis of the bacterial 16S rRNA gene ()). The numbers of bacterial 16S rRNA genes in 1.0 g soil of CK, CF, FS and FSB were 1.0 × 108, 1.5 × 108, 2.6 × 108 and 2.3 × 108, respectively. The bacterial 16S rRNA gene copy numbers in the CF, FS and FSB treatments were 1.47, 2.52 and 2.31 times that of the CK treatment, respectively. The rice straw addition (FS and FSB) increased the bacterial 16S rRNA gene copy numbers more than only flooding (CF). We also found that the biofertilizer amendment decreased the bacterial 16S rRNA gene copy numbers. The 16S rRNA gene copy numbers were positively correlated with soil pH (r = 0.981, P < 0.001; )), EC (r = −0.645, P = 0.001), AK (r = 0.784, P < 0.001) and AN (r = 0.610, P = 0.002) in all the soils, whereas there were no significant correlations with soil AP and SOM (Table S1).
Figure 2. The abundance and α-diversity indices of bacteria in soil subjected to continuous strawberry monocropping under different pre-flooding conditions (29,118 sequences). (a) Bacterial abundance (16S rRNA gene copies/g soil). (b) Linear functions were used to describe the relationships between bacterial copy numbers and pH, (c), operational taxonomic units (OTUs); (d), Diversity; (e), Richness. Different letters above columns denote significant difference (P < 0.05, Tukey’s test).
3.3. Pre-flooding increased bacterial α-diversity
After quality filters, 1,399,855 high-quality sequences (69% of the raw sequences) were obtained from the 24 samples. They had an average length of 256 bp, and there were approximately 58,327 sequences per sample (34,186–72,856 sequences). The number of OTUs ranged from 2,419 to 3,357 at a 97% similarity level, and the Good’s coverage values were greater than 0.96, indicating that the sequencing depth was sufficient to capture the bacterial fungal diversity. The flooding treatments significantly increased microbial diversity, richness and OTUs (). The Shannon indexes for the CF, FS and FSB treatments were 3.9%, 6.8% and 6.4% greater, respectively, than that of the CK, but there was no significant difference between the FS and FSB treatments. The highest microbial α-diversity value (Shannon index) was observed in the FS treatment, whereas the biofertilizer amendment slightly decreased the microbial OTUs, diversity and richness index.
3.4. Pre-flooding changed the soil bacterial composition
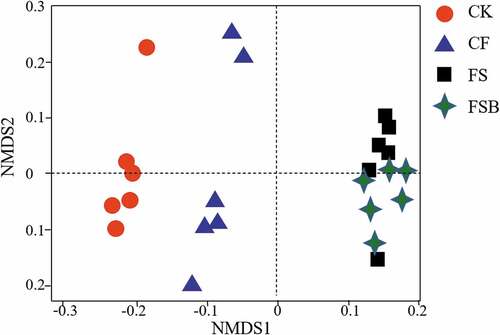
The bacterial composition was assessed using high-throughput sequencing data, and the CK was distinct from that of the CF. However, the bacteria in FS and FSB shared a similar bacterial community composition (; Table S2).
Figure 3. Non-metric multidimensional scaling analysis of the dissimilarities in bacterial communities in soil subjected to continuous strawberry monocropping under different flooding conditions (29,118 sequences).
The relative abundance levels of different phyla/classes under different treatment conditions are shown in ()). The relative abundance levels of Proteobacteria, Actinobacteria and Gemmatimonadetes were greater than 10%, while the relative abundance levels of Chloroflexi, Acidobacteria, Bacteroidetes, Planctomycetes, Firmicutes, Verrucomicrobia, Patescibacteria and Cyanobacteria ranged from 0.9% to 9.8%. There were no significant differences in the abundance levels of Alphaproteobacteria, Gammaproteobacteria, Chloroflexi, Acidobacteria, Planctomycetes and Cyanobacteria among the treatments, but there were significant difference in the other classes/phyla among treatments ()). Notably, the relative abundance of Deltaproteobacteria was significantly increased after the flooding treatments, and rice straw addition also increased its abundance. In the FSB treatment, the relative abundance level of Bacteroidetes significantly increased, while the level of Firmicutes decreased.
Figure 4. Relative abundance levels of the dominant microbial phyla/classes (a) and families (b) in soil subjected to continuous strawberry monocropping under different flooding conditions.
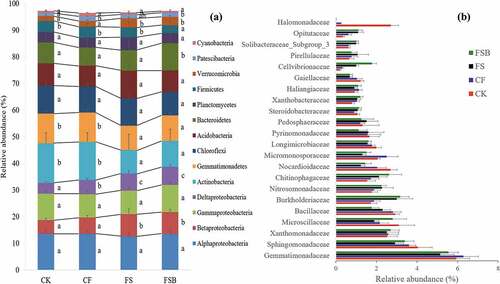
There were no significant (P > 0.05) differences in the abundance levels of the families Xanthomonadaceae, Nitrosomonadaceae, Pyrinomonadaceae, Pedosphaeraceae, Haliangiaceae and Pirellulaceae among the treatments, but there were significant (P < 0.05) differences in the average relative abundance levels (> 1% in at least one treatment) of other families among treatments, as shown in ()). Notably, the relative abundance levels of Sphingomonadaceae, Microscillaceae, Bacillaceae, Nocardioidaceae, Longimicrobiaceae, Gaiellaceae and Halomonadaceae decreased after the flooding treatment, whereas the relative abundance levels of Burkholderiaceae, Nitrosomonadaceae, Chitinophagaceae, Xanthobacteraceae, Solibacteraceae and Opitutaceae increased after the flooding treatment. Furthermore, for the FS and FSB treatments, the relative abundance levels of Burkholderiaceae, Gaiellaceae, and Cellvibrionaceae decreased to a greater extent than in the CF treatment, whereas Steroidobacteraceae, Solibacteraceae and Opitutaceae showed the opposite trend.
3.5. Linking strawberry biomass to soil properties and bacterial communities
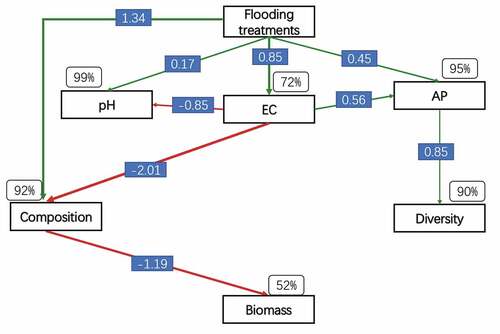
The SEM adequately fitted the data, assessing causal relationships among soil properties, bacterial communities and strawberry biomass under different flooding treatments (). The model explained 52% of the strawberry biomass, explained 90% and 92% of bacterial diversity and composition, respectively, and explained 99%, 72% and 95% of soil pH, EC and AP, respectively. Flooding treatments significantly changed soil pH, EC and AP. The bacterial composition was directly altered by soil EC and showed a negative directly influence on strawberry biomass. In addition, flooding treatments directly altered soil bacterial composition, which then influenced strawberry biomass. Alterations in bacterial diversity occurred in response to changes in soil AP, which were caused directly by flooding or soil EC. Although other relationships among variables were not significant, they clearly improved the model fit when incorporated together (Table S3).
Figure 5. Structural equation modeling of the relationships among soil properties (pH, EC and AP), plant and bacterial diversity and composition. The model produced in a good fit for the data, with χ2/df = 1.364, P = 0.256 and goodness-of-fit index = 0.969. Red and green arrows indicate positive and negative correlations, respectively (P < 0.05). The numbers on the lines are the correlation coefficients. Percentages close to variables represent the variance accounted for by the model (R2).
Pearson’s correlation coefficients were used to evaluate relationships between abundant families and soil properties (Figure S2; Table S4). Most of the abundant families were significantly correlated with certain environmental factors, but the families Gemmatimonadaceae, Xanthomonadaceae, Pyrinomonadaceae, Pedosphaeraceae, Steroidobacteraceae and Pirellulaceae were not significantly correlated with any of the soil factors (Figure S2; Table S4). The soil pH and EC exerted opposite influences on soil bacterial families under flooded conditions. For example, the relative abundance levels of Sphingomonadaceae, Microscillaceae, Bacillaceae, Nocardioidaceae, Micromonosporaceae, Longimicrobiaceae, Gaiellaceae and Halomonadaceae were significantly negatively correlated with soil pH, but significantly positively correlated with EC. The relative abundance levels of Burkholderiaceae, Nitrosomonadaceae, Chitinophagaceae, Xanthobacteraceae, Cellvibrionaceae and Opitutaceae were significantly positively correlated with soil pH, but significantly negatively correlated with EC. Similarly, at the phylum level, nearly half of the dominant bacterial phyla, such as Deltaproteobacteria and Actinobacteria, also showed significant positive or negative correlations with soil pH; however, those phyla showed contrasting correlations with EC (). Mantel tests showed that the variations in microbial community composition had significantly positive correlations with soil properties (). Based on the correlation coefficients of the Mantel test, soil pH, EC and AP were the top three factors that correlated with microbial community composition.
Table 2. Pearson’s correlation coefficients of the dominant microbial phyla/class and properties of soil subjected to continuous strawberry monocropping under different flooding conditions
Table 3. Correlations between properties of soil subjected to continuous strawberry monocropping and microbial community composition as determined by Mantel tests under different flooding conditions
4. Discussion
4.1. Pre-flooding treatments increased strawberry biomass and soil nutrient contents
The flooding of soils that had been used for monocropping increased strawberry biomass, with the highest increase being observed after the CF treatment. The flooding treatment may have increased the soil available nutrients (Wright et al. Citation2015) and changed the soil’s microbial composition (). However, the FBS treatment did not increase the strawberry biomass further. This may be due to rice straw having a high C/N ratio, and rice straw decomposition accompanied by N immobilization has an initial negative effect on crop yield (Zhou et al. Citation2020b).
However, in our study, the flooding treatments induced increases of soil available nutrient contents (i.e., AP and AK), but not the SOM level (). The flooding treatments may enhance strawberry growth and further increase root exudate releases into the soil (Wang et al. Citation2019). These results indicated that pre-flooding increases the soil nutrients available for plant growth. Flooding treatments significantly decreased the soil EC, but increased the soil pH, which was consistent with other studies (De-Campos, Huang, and Johnston Citation2011; Ye et al. Citation2019). The irrigation to a saturation level may dissolve soil salts, resulting in decreased soil salinity after the water was removed. In addition, the increase in soil pH may result from the flooding treatment diluting soil organic acids, such as phenolic acids, that accumulate with strawberry monocropping (Chen et al. Citation2020).
4.2. Pre-flooding treatments increased soil bacterial abundance and α-diversity
Long-term continuous cropping reduces soil microbial abundance (Zhou et al. Citation2014). However, we found that flooding the continuous cropping soil could increase microbial abundance ()). Thus, pre-flooding strawberry monocropping soils could recover the microbial abundance that had been reduced owing to long-term monocropping. We also found that the addition of rice straw and biofertilizer to flooded soil further increased the bacterial abundance ()). The rice straw addition may increase the soil pH (), which was significantly positively correlated with bacterial abundance ()) (Wang et al. Citation2018).
The underground microbial biodiversity level plays important roles in nutrient cycling, pathogen control, organic matter transformation and plant productivity in terrestrial ecosystems (Delgado-Baquerizo et al. Citation2020; Fan et al. Citation2021). According to OTUs, and Shannon and Chao1 indices, all the pre-flooding treatments increased the bacterial α-diversity level in the soil during long-term strawberry monocropping compared with the CK (). This may be because temporal flooding stimulated microbial activity that had been suppressed by air-drying treatments, thereby increasing the strict/facultative anaerobic organisms’ abundance.
4.3. Pre-flooding treatments changed the soil’s bacterial community composition
Flooding treatments significantly changed the bacterial community composition, and non-metric multidimensional scaling revealed that all four treatments resulted in structurally distinct bacterial communities (; Table S2). The soil bacterial composition after the CK treatment was distinctly different from compositions after the CF, FS and FSB treatments, which is in agreement with previous observations that water regiments result in distinct soil microbial community composition (Tian, Wu, and Zheng Citation2013). The FS and FSB treatments were grouped tightly together on the basis of unweighted UniFrac distances, and the distance between the FS or FSB and CK was greater than between the CF and CK (). Thus, the FS- and FSB-treated soils subjected to long-term strawberry monocropping appeared to select for particular microbial species. In the FSB treatment, we inoculated B. velezensis into soils and found that the bacterial composition was significantly different from that of the other treatment (; Table S2). The biofertilizer application may stimulate some taxa of microbes (Xiong et al. Citation2017). For example, Bacteroidetes was significant overrepresented in the FSB treatment ()). In addition, Xiong et al. (Citation2017) found that soil inoculated with Bacillus and Trichoderma spp. selected for some of the same soil microbes. Consistent with our study, the Bacillus application also enriched Bacteroidetes species.
Long-term monocropping results in soil depleted of Deltaproteobacteria and enriched with Gemmatimonadetes (Wu et al. Citation2019). However, in our study, the relative abundance level of Deltaproteobacteria significantly increased, while that of Gemmatimonadetes decreased after the flooding treatments. This result indicated that flooding treatments recovered microbial communities that had been lost in soils subjected to long-term strawberry monocropping. At the family level, Burkholderiaceae, Nitrosomonadaceae, Chitinophagaceae, Xanthobacteraceae, Solibacteraceae and Opitutaceae increased after flooding treatments, and Xanthobacteraceae was positively correlated with strawberry biomass (Figure S2; Table S4). The family Xanthobacteraceae, which enhances plant growth and reduces disease in monocropping systems (Callens et al. Citation2018; Wang et al. Citation2019), was present at significantly greater levels after the three flooding treatments compared with the CK. This family was clearly stimulated by the flooding, and it may play a key role in strawberry growth. Some taxa were significantly decreased by the pre-flooding treatments. The family Halomonadaceae, which is present in highly saline soils (Jorquera et al. Citation2016), was 10 to 524 times lower in flooded soil compared with the CK, and it was significantly negatively correlated with strawberry biomass. Thus, Halomonadaceae may play a key role as a monocropping obstacle and inhibit plant growth. Additionally, soil salinity may be an important factor resulting in plant production losses in monocropping systems.
4.4. Factors influencing bacterial community composition under flooding conditions
In our study, we found salinity (i.e., EC) was an important driver that shaped the soil microbial community and strawberry yield under flooding conditions (Table S4; Figure S2). The SEM further confirmed that salinity had a direct effect on microbial community composition (). This was expected because flooding treatments significantly decreased soil salinity from 2,020 to 789 μm cm−1(). Other studies have indicated that salinity is a major factor that shapes microbial community composition in various habitats, including agricultural, desert and natural ecosystems (Rath et al. Citation2019; Zhang et al. Citation2019; Wei et al. Citation2020). Our results corroborated that salinity is an important factor in shaping soil microbial communities.
In addition to salinity, other factors, such as AK and AP, were also significantly correlated with microbial community composition (). Although these factors have been widely confirmed to shape soil microbial communities, a wide range of other factors also influence soil microbial communities (Fierer Citation2017). For example, soil pH is the best predictor of microbial community composition in soils (Lauber et al. Citation2008; Wang et al. Citation2018). In the present study, soil pH values ranged from 6.94 to 7.43, while EC ranged from 2,020 to 789. Soil salinity and pH were the two most important factors impacting soil microbial communities after flooding, but they exerted opposite effects. This is because most microbes prefer relatively high pH (no more than 8) values and low salinity levels (Wei et al. Citation2020).
Here, EC significantly increased the abundance levels of Sphingomonadaceae, Microscillaceae and Bacillaceae, but decreased the levels of Burkholderiaceae, Nitrosomonadaceae and Chitinophagaceae. Recently, Zwetsloot et al. (Citation2020) showed that Burkholderiaceae degrades a range of toxic compounds (caused by long-term monocropping systems). Flooding soil that was subjected to monocropping decreased the salinity and increased the relative abundance of Burkholderiaceae. Those results indicated that Burkholderiaceae may promote the elimination of the toxic compounds. Therefore, in flooding-treated soil, the salinity directly regulated microbial community composition and indirectly affected the strawberry yield.
4.5. Pre-flooding treatments increased strawberry biomass
In our study, we found that pre-flooding strawberry monocropping soil increased soil pH and AP, but decreased EC, which was very important for plant growth. In addition, we found the microbial composition was significantly correlated with strawberry biomass as assessed by the SEM analysis (). This result indicated that microbial composition may have an important function in strawberry growth. This was evident by reduced plant productivity after long-term monocropping, which was associated with changes in the microbial composition, especially a decreasing abundance of Deltaproteobacteria and an increasing abundance of Gemmatimonadetes (Wu et al. Citation2019), while pre-flooding treatments increased strawberry biomass and the abundance of Deltaproteobacteria and decreasing the abundance of Gemmatimonadetes ()). We also found that pre-flooding directly and indirectly, by changing the soil EC, altered the soil’s microbial composition. These results indicate that flooding can be used to regulate soil microbial composition and ultimately promote plant growth, especially in soils used for long-term monocropping.
5. Conclusions
This study demonstrated that pre-flooding soil that has been used for long-term strawberry monocropping increased the soil pH, microbial diversity and strawberry biomass, and decreased soil salinity. The differences in microbial compositions between pre-flooding and non-flooding treatments were clear. Microbial composition was directly affected by flooding treatments and indirectly affected by salinity, which dramatically influenced the strawberry biomass in our study. Pre-flooding treatments increased the abundance of Burkholderiaceae, which may be beneficial for crop growth and the elimination of the toxic compounds caused by long-term monocropping. This study provides a possible method to improve soil quality problems that accompany the long-term monocropping of strawberry. In the future, we will attempt to isolate Burkholderiaceae strains from soils and further explore their mechanisms that affect soil health and crop growth.
Supplemental Material
Download MS Word (347.5 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Ahn, J. H., J. Song, B. Y. Kim, M. S. Kim, J. H. Joa, and H. Y. Weon. 2012. “Characterization of the Bacterial and Archaeal Communities in Rice Field Soils Subjected to Long-term Fertilization Practices.” Journal of Microbiology 50 (5): 754–765. doi:10.1007/s12275-012-2409-6.
- Bao, S. D. 2000. Soil and Agricultural Chemistry Analysis. Beijing: China Agricultural Press.
- Bokulich, N. A., S. Subramanian, J. J. Faith, D. Gevers, J. I. Gordon, R. Knight, D. A. Mills, and J. G. Caporaso. 2013. “Quality-filtering Vastly Improves Diversity Estimates from Illumina Amplicon Sequencing.” Nature Methods 10 (1): 57–59. doi:10.1038/nmeth.2276.
- Callens, M., H. Watanabe, Y. Kato, J. Miura, and E. Decaestecker. 2018. “Microbiota Inoculum Composition Affects Holobiont Assembly and Host Growth in Daphnia.” Microbiome 6 (1): 56. doi:10.1186/s40168-018-0444-1.
- Caporaso, J. G., J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, et al. 2010. “QIIME Allows Analysis of High-throughput Community Sequencing Data.” Nature Methods 7 (5): 335–336. doi:10.1038/nmeth.f.303.
- Castrillo, G., P. J. P. L. Teixeira, S. H. Paredes, T. F. Law, L. D. Lorenzo, M. E. Feltcher, O. M. Finkel, et al. 2017. “Root Microbiota Drive Direct Integration of Phosphate Stress and Immunity.” Nature 543 (7646): 513–518. doi:10.1038/nature21417.
- Chen, P., Y. Z. Wang, Q. Z. Liu, Y. T. Zhang, W. H. Li, H.-Q. Li, and W.-H. Li. 2020. “Phase Changes of Continuous Cropping Obstacles in Strawberry (Fragaria × Ananassa Duch.) Production.” Applied Soil Ecology 155: 103626. doi:10.1016/j.apsoil.2020.103626.
- Christian, Q., P. Elmar, Y. Pelin, G. Jan, S. Timmy, Y. Pablo, P. Jörg, and G. F. Oliver. 2013. “The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-based Tools.” Nucleic Acids Research 41: 590–596. doi:10.1093/nar/gks1219.
- De-Campos, A. B., C. H. Huang, and C. T. Johnston. 2011. “Biogeochemistry of Terrestrial Soils as Influenced by Short-term Flooding.” Biogeochemistry 111 (1–3): 239–252. doi:10.1007/s10533-011-9639-2.
- Delgado-Baquerizo, M., P. B. Reich, C. Trivedi, D. J. Eldridge, S. Abades, F. D. Alfaro, F. Bastida, et al. 2020. “Multiple Elements of Soil Biodiversity Drive Ecosystem Functions across Biomes.” Nature Ecology & Evolution 4 (2): 210–220. doi:10.1038/s41559-019-1084-y.
- Edgar, R. C., B. J. Haas, J. C. Clemente, C. Quince, and R. Knight. 2011. “UCHIME Improves Sensitivity and Speed of Chimera Detection.” Bioinformatics 27 (16): 2194–2200. doi:10.1093/aje/kwu026.
- Edgar, R. C. 2013. “UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads.” Nature Methods 10 (10): 996–998. doi:10.1038/NMETH.2604.
- Fan, K., M. Delgado-Baquerizo, X. Guo, D. Wang, Y. G. Zhu, and H. Chu. 2021. “Biodiversity of Key-stone Phylotypes Determines Crop Production in a 4-decade Fertilization Experiment.” The ISME Journal 15 (2): 550–561. doi:10.1038/s41396-020-00796-8.
- Fierer, N. 2017. “Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome.” Nature Reviews. Microbiology 15 (10): 579–590. doi:10.1038/nrmicro.2017.87.
- Fitzpatrick, C. R., J. Copeland, P. W. Wang,D, S. Guttman, P. M. Kotanen, and M. T. J. Johnson. 2018. “Assembly and Ecological Function of the Root Microbiome across Angiosperm Plant Species.” Proceedings of the National Academy of Sciences of the United States of America 115 (6): E1157–E1165. doi:10.1073/pnas.1717617115.
- García-Fraile, P., E. Menéndez, and R. Rivas. 2015. “Role of Bacterial Biofertilizers in Agriculture and Forestry.” AIMS Bioengineering 2 (3): 183–205. doi:10.3934/bioeng.2015.3.183.
- Griffiths, B. S., S. Caul, J. Thompson, A. N. E. Birch, J. Cortet, M. N. Andersen, and P. H. Krogh. 2007. “Microbial and Microfaunal Community Structure in Cropping Systems with Genetically Modified Plants.” Pedobiologia 51 (3): 195–206. doi:10.1016/j.pedobi.2007.04.002.
- Huang, X. Q., T. Wen, J. B. Zhang, L. Meng, T. B. Zhu, L. L. Liu, and Z. C. Cai. 2015. “Control of Soil-borne Pathogen Fusarium Oxysporum by Biological Soil Disinfestation with Incorporation of Various Organic Matters.” European Journal of Plant Pathology 143 (2): 223–235. doi:10.1007/s10658-015-0676-x.
- Jorquera, M. A., F. Maruyama, A. V. Ogram, O. U. Navarrete, L. M. Lagos, N. G. Inostroza, J. J. Acuña, J. I. Rilling, and M. D. L. L. Mora. 2016. “Rhizobacterial Community Structures Associated with Native Plants Grown in Chilean Extreme Environments.” Microbial Ecology 72 (3): 633–646. doi:10.1007/s00248-016-0813-x.
- Kang, S. M., A. L. Khan, M. Waqas, Y. H. You, M. Hamayun, G. J. Joo, R. Shanzad, K. S. Choi, and I. J. Lee. 2015. “Gibberellin-producing Serratia Nematodiphila PEJ1011 Ameliorates Low Temperature Stress in Capsicum Annuum L.” European Journal of Soil Biology 68: 85–93. doi:10.1016/j.ejsobi.2015.02.005.
- Lauber, C. L., M. S. Strickland, M. A. Bradford, and N. Fierer. 2008. “The Influence of Soil Properties on the Structure of Bacterial and Fungal Communities across Land-use Types.” Soil Biology & Biochemistry 40 (9): 2407–2415. doi:10.1016/j.soilbio.2008.05.021.
- Li, X., A. Jousset, W. D. Boer, V. C. J. Carrión, T. Zhang, X. Wang, and E. E. Kuramae. 2019. “Legacy of Land Use History Determines Reprogramming of Plant Physiology by Soil Microbiome.” The ISME Journal 13 (3): 738–751. doi:10.1038/s41396-018-0300-0.
- Li, X., Y. Deng, Q. Li, C. Lu, J. Wang, H. Zhang, J. Zhu, et al. 2013. “Shifts of Functional Gene Representation in Wheat Rhizosphere Microbial Communities under Elevated Ozone.” The ISME Journal 7 (3): 660. doi:10.1038/ismej.2012.120.
- Lou, Y., M. Xu, W. Wang, X. Sun, and K. Zhao. 2011. “Return Rate of Straw Residue Affects Soil Organic C Sequestration by Chemical Fertilization.” Soil and Tillage Research 113 (1): 70–73. doi:10.1016/j.still.2011.01.007.
- Lu, P., L. D. Bainard, B. Ma, and J. H. Liu. 2020. “Bio-fertilizer and Rotten Straw Amendments Alter the Rhizosphere Bacterial Community and Increase Oat Productivity in a Saline–alkaline Environment.” Scientific Reports 10 (1): 19896. doi:10.1038/s41598-020-76978-3.
- Mago, T., and S. L. Salzberg. 2011. “FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies.” Bioinformatics 27 (21): 2957–2963. doi:10.1093/bioinformatics/btr507.
- Marina, R. S., J. Chen, F. Multinu, A. Hokenstad, T. J. Distad, E. H. Cheek, G. L. Keeney, et al. 2016. “Potential Contribution of the Uterine Microbiome in the Development of Endometrial Cancer.” Genome Medicine 8 (1): 122. doi:10.1186/s13073-016-0368-y.
- Ning, Q., L. Chen, Z. Jia, C. Zhang, D. Ma, F. Li, J. Zhang, et al. 2019. “Multiple Long-term Observations Reveal a Strategy for Soil pH-dependent Fertilization and Fungal Communities in Support of Agricultural Production.” Agriculture, Ecosystems & Environment 293 :106837–106846. doi:10.1016/j.agee.2020.106837.
- Olsen, S. R. 1954. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate. Washington: United States Department Of Agriculture.
- Peiffer, J. A., A. Spor, O. Koren, Z. Jin, S. G. Tringe, J. L. Dangl, E. S. Buckler, et al. 2013. “Diversity and Heritability of the Maize Rhizosphere Microbiome under Field Conditions.” Proceedings of the National Academy of Sciences of the United States of America 110 (16): 6548–6553. doi:10.1073/pnas.1302837110.
- Rath, K. M., N. Fierer, D. V. Murphy, and J. Rousk. 2019. “Linking Bacterial Community Composition to Soil Salinity along Environmental Gradients.” The ISME Journal 13 (3): 836–846. doi:10.1038/s41396-018-0313-8.
- Sanford, R. A., K. G. Lloyd, K. T. Konstantinidis, and F. E. Löffler. 2020. “Microbial Taxonomy Run Amok.” Trends in Microbiology 29 (5): 394–404. doi:10.1016/j.tim.2020.12.010.
- Stover, R. H. 1962. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species. Kew: Commonwealth Mycological Institute.
- Strickland, T. C., and P. Sollins. 1987. “Improved Method for Separating Light- and Heavyfraction Organic Material from Soil.” Soil Science Society of America Journal 51 (5): 1390–1393. doi:10.2136/sssaj1987.03615995005100050056x.
- Tian, Y., B. Wu, and Y. Zheng. 2013. “Modeling the Surface Water-groundwater Interaction in Arid and Semi-arid Regions Impacted by Agricultural Activities.” Agu Fall Meeting, December 9-13 , San Francisco.
- Wang, Q., G. M. Garrity, J. M. Tiedje, and J. R. Cole. 2007. “Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy.” Applied and Environmental Microbiology 73 (16): 5261–5267. doi:10.1128/AEM.00062-07.
- Wang, Q., M. Ma, X. Jiang, B. Zhou, D. Guan, F. Cao, S. Chen, et al. 2019. “Long-term N Fertilization Altered 13 C-labeled Fungal Community Composition but Not Diversity in Wheat Rhizosphere of Chinese Black Soil.” Soil Biology & Biochemistry 135 :117–126. doi:10.1016/j.soilbio.2019.04.009.
- Wang, Q., X. Jiang, D. Guan, D. Wei, B. Zhao, M. Ma, S. Chen, et al. 2018. “Long-term Fertilization Changes Bacterial Diversity and Bacterial Communities in the Maize Rhizosphere of Chinese Mollisols.” Applied Soil Ecology 125 :88–96. doi:10.1016/j.apsoil.2017.12.007.
- Warncke, D., and J. R. Brown. 1998. “Potassium and Other Basic Cations.” Recommended Chemical Soil Test Procedures for the North Central Region 100: 31.
- Wei, G., M. Li, W. Shi, R. Tian, C. Chang, Z. Wang, N. Wang, et al. 2020. “Similar Drivers but Different Effects Lead to Distinct Ecological Patterns of Soil Bacterial and Archaeal Communities.” Soil Biology & Biochemistry 144 :107759. doi:10.1016/j.soilbio.2020.107759.
- Wright, M. L., H. Jonathan, A. J. Gordon, and L. Adam. 2015. “Upper Midwest Climate Variations: Farmer Responses to Excess Water Risks.” Journal of Environmental Quality 44 (3): 810–822. doi:10.2134/jeq2014.08.0352.
- Wu, A. L., X. Y. Jiao, F. F. Fan, J. S. Wang, J. Guo, E. W. Dong, L. G. Wang, et al. 2019. “Effect of Continuous Sorghum Cropping on the Rhizosphere Microbial Community and the Role of Bacillus Amyloliquefaciens in Altering the Microbial Composition.” Plant Growth Regulation 89 (3): 299–308. doi:10.1007/s10725-019-00533-y.
- Xiong, W., S. Guo, A. Jousset, Q. Zhao, H. Wu, R. Li, G. A. Kowalchuk, et al. 2017. “Bio-fertilizer Application Induces Soil Suppressiveness against Fusarium Wilt Disease by Reshaping the Soil Microbiome.” Soil Biology & Biochemistry 114 :238–247. doi:10.1016/j.soilbio.2017.07.016.
- Yang, L., X. Zhou, Y. Liao, Y. Lu, J. Nie, and W. Cao. 2019. “Co-incorporation of Rice Straw and Green Manure Benefits Rice Yield and Nutrient Uptake.” Crop Science 59 (2): 749–759. doi:10.2135/cropsci2018.07.0427.
- Ye, C., O. M. Butler, C. Chen, W. Liu, Q. Zhang, and Q. Zhang. 2019. “Shifts in Characteristics of the Plant-soil System Associated with Flooding and Revegetation in the Riparian Zone of Three Gorges Reservoir, China.” Geoderma 361: 114015. doi:10.1016/j.geoderma.2019.114015.
- Zeng, J., X. Liu, L. Song, X. Lin, H. Zhang, C. Shen, and H. Chu. 2016. “Nitrogen Fertilization Directly Affects Soil Bacterial Diversity and Indirectly Affects Bacterial Community Composition.” Soil Biology & Biochemistry 92: 41–49. doi:10.1016/j.soilbio.2015.09.018.
- Zhang, K., Y. Shi, X. Cui, P. Yue, K. Li, X. Liu, B. M. Tripathi, et al. 2019. “Salinity Is a Key Determinant for Soil Microbial Communities in a Desert Ecosystem.” mSystems 4 (1): e00225–18. doi:10.1128/mSystems.00225-18.
- Zhou, D., S. Wu, C. Chu, Z. Zhao, and Q. Wang. 2020a. “The Isolation of a Bacillus Velezensis and Its Application.” CN patant. CN202010175169.6, March 13.
- Zhou, G., S. Gao, Y. Lu, Y. Liao, J. Nie, and W. Cao. 2020b. “Co-incorporation of Green Manure and Rice Straw Improves Rice Production, Soil Chemical, Biochemical and Microbiological Properties in a Typical Paddy Field in Southern China.” Soil and Tillage Research 197: 104499. doi:10.1016/j.still.2019.104499.
- Zhou, J., D. Guan, B. Zhou, B. Zhao, M. Ma, J. Qin, and X. Jiang, et al 2015. “Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China.” Soil Biology and Biochemistry 90: 42–51. https://doi.org/10.1016/j.soilbio.2015.07.005
- Zhou, J., X. Jiang, B. Zhou, B. Zhao, M. Ma, D. Guan, J. Li, et al. 2016. “Thirty Four Years of Nitrogen Fertilization Decreases Fungal Diversity and Alters Fungal Community Composition in Black Soil in Northeast China.” Soil Biology & Biochemistry 95 :135–143. doi:10.1016/j.soilbio.2015.12.012.
- Zhou, X., D. Gao, J. Liu, P. Qiao, X. Zhou, H. Lu, X. Wu, et al. 2014. “Changes in Rhizosphere Soil Microbial Communities in a Continuously Monocropped Cucumber (Cucumis Sativus L.) System.” European Journal of Soil Biology 60 :1–8. doi:10.1016/j.ejsobi.2013.10.005.
- Zhou, X., and F. Wu. 2012. “Dynamics of the Diversity of Fungal and F Usarium Communities during Continuous Cropping of Cucumber in the Greenhouse.” FEMS Microbiology Ecology 80 (2): 469–478. doi:10.1111/j.1574-6941.2012.01312.x.
- Zuppinger-Dingley, D., B. Schmid, J. S. Petermann, V. Yadav, G. B. D. Deyn, and D. F. B. Flynn. 2014. “Selection for Niche Differentiation in Plant Communities Increases Biodiversity Effects.” Nature 515 (7525): 108–111. doi:10.1038/nature13869.
- Zwetsloot, M. J., J. M. Ucros, K. Wickings, R. C. Wilhelm, J. Sparks, D. H. Buckley, and T. L. Bauerle. 2020. “Prevalent Root-derived Phenolics Drive Shifts in Microbial Community Composition and Prime Decomposition in Forest Soil.” Soil Biology & Biochemistry 145: 107797. doi:10.1016/j.soilbio.2020.107797.

