?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Soils with continuous application of fertilizer phosphorus (P) may present high accumulations of P and may be at high risk of P loss. In this study, we investigated the effects of different fertilization strategies on soil P dynamics in three typical cropland soils over broad spatiotemporal scales in China. The soil P speciation (characterized both by Hedley fractionation and 31P nuclear magnetic resonance (31P NMR)) and soil P sorption characteristics were examined under five fertilization treatments: unfertilized control (CK); chemical nitrogen (N) and potassium (K) (NK); N, P and K (NPK); NPK plus straw (NPKS); and NPK plus manure (NPKM). We found that with an increase of 100 kg ha−1 in the cumulative P budget, the increases in Olsen-P and CaCl2-P contents ranged in 1.98–7.35 mg kg−1 and 0.46–1.46 mg kg−1, respectively, among the three soils. The recovery rates of P in Hedley fractionation (87–111%) were much higher than those of 31P NMR spectroscopy (40–91%). More percentage of organic P was detected in Hedley fractionation than 31P-NMR in acidic soil, while more percentage of organic P was detected in 31P-NMR than Hedley fractionation in neutral and alkaline soils. Chemical fertilizer combined with manure or straw can decrease the bond strength (k) values compared to chemical fertilizer alone, while change trends in the maximum P sorption capacity (Qm) were dependent on the soil types. Moreover, the degree of P saturation (DPS) was significantly correlated with the cumulative P budget in each site. A change point in the relation between DPS and soil organic carbon (SOC) was found. After the change point, the DPS increased by 0.63–1.06% as the SOC increased by 0.1 g kg−1. Agricultural measures that increased the contents of SOC and available P could increase DPS values effectively, especially manure amendment, which should be carefully considered to reduce P loss risk.
1. Introduction
Phosphorus (P) is essential for crop growth; however, it is the most frequently deficient nutrient in many agricultural regions due to its low availability and poor recovery (Zhang et al. Citation2021). Fertilizer P is easily adsorbed by the soil and converted from highly soluble P to sparingly soluble amorphous or crystalline P, which leads to a low use efficiency (10–25%) of applied P for crops (Balemi and Negisho Citation2012). To meet increasing food demand, an abundant supply of fertilizer is needed. However, the overuse of P fertilizer leads to excessive P accumulation in soil and causes finite high-quality P rock resource waste or even water body eutrophication (Li et al. Citation2019; Rowe et al. Citation2015). Meanwhile, excessive P accumulation in soil can also alter the amounts and forms of P and the characteristics of P sorption–desorption, which in turn has led to changes in the retention and loss risk of soil P (Withers et al. Citation2016).
The total stock of P is distributed among several fractions that have different bioavailabilities and degrees of incorporation with mineral or organic constituents in the soil. The forms of inorganic P (Pi) and organic P (Po) are largely dependent on the intrinsic properties of the soil type and can also be affected by agricultural practices and microbial activity (Ma et al. Citation2021). However, many more studies focus on the forms and transformation of Pi than Po due to its direct bioavailability for plants (Weihrauch and Opp Citation2018). In fact, organic P compounds are also necessary to maintain vital activities. Moreover, Po can be converted to Pi through mineralization by microbial and root-released phosphatases (Shen et al. Citation2011). Thus, both Pi and Po should be considered to comprehensively understand the cycling of soil P. Sequential fractionation schemes have long been adopted to quantify P forms, which have provided valuable information on soil P dynamics under different conditions, and the Hedley fractionation procedure has been widely used (Condron and Newman Citation2011). In addition, nuclear magnetic resonance spectroscopy (31P-NMR) is a powerful tool to identify organic P species, and it can complement the information collected by chemical sequential P fractionation (Turner, Mahieu, and Condron Citation2003). In agricultural soil, the forms of P can be quite different after fertilization management (Yan et al. Citation2017). Therefore, investigation of the dynamic changes in P speciation both by sequential fractionation and 31P-NMR following long-term fertilization in contrasting soils is instructive for rational P management.
The P sorption characteristics are useful for soil P retention capacity and environmental P risk assessments. Soil P isotherms are used to understand the mechanisms of soil P retention. The Langmuir equation is suitable for numerous soil types and can determine the maximum P sorption capacity and bonding strength (Wang and Liang Citation2014; Daly et al. Citation2015). The degree of P saturation (DPS) is generally estimated as the proportion of available P to the maximum P sorption capacity and has been increasingly viewed as an environmental indicator of the potential loss of P in soil (Kleinman and Peter Citation2017; Blombäck et al. Citation2021). A more direct way to estimate the risk for P loss is by measuring the dissolved P extracted by deionized water or CaCl2 solution. However, these indicators are soil specific and cannot provide information on the additional capacity of soils to retain P. The DPS considers both the available P concentration and the maximum P sorption capacity of soil. Several studies have reported that DPS was strongly positively correlated with soil water-soluble P and labile P (Gatiboni et al. Citation2021, Fu et al.). In addition, DPS can be changed by land use and fertilization management (Abdala et al. Citation2012; Fu et al. Citation2021). For instance, Yan et al. (Citation2017) reported that DPS was significantly higher in manure-treated soils than in soils treated with chemical fertilizers alone or with straw retention. Thus, the control of DPS in agricultural activities is important for ecosystem health, particularly in regions under long-term fertilization.
In the present study, three typical croplands of China are investigated: black soil (Luvic Phaeozem according to the Food and Agriculture Organization of the United Nations (FAO) classification), fluvo-aquic soil (Calcaric Cambisol in FAO) and red soil (Eutric Cambisol in FAO). These soils are found across highly diverse climatic zones, soil types and management practices and represent important agricultural regions and the most nutrient-additive areas of China. The changes in P speciation were determined by chemical fractionation and 31P-NMR in these three typical croplands, and the variations in soil P sorption characteristics and physicochemical properties were also considered. We hypothesized that under five long-term (1990–2015) fertilization treatments, i.e., unfertilized control (CK); chemical nitrogen (N) and potassium (K) (NK); N, P and K (NPK); NPK plus straw (NPKS); and NPK plus manure (NPKM), (1) the contents of Pi and Po would decrease in no-P treatments but increase in P-applied treatments owing to different crop P uptake and fertilizer P input; (2) the maximum P sorption capacity and bonding strength would be higher in no-P treatments than P-applied treatments; and (3) the DPS would increase in P-applied treatments but decrease in no-P treatments, which may both affected by P behaviors and soil properties.
1. Materials and methods
1.1. Site description and experimental design
Three long-term field fertilization sites were selected: Gongzhuling, black soil (GZL, 43°30′, 125°48′, located in the middle temperate zone), Jilin, northeastern China; Zhengzhou, fluvo-aquic soil (ZZ, 34°47′, 113°40′, warm temperate region), Henan, central China; and Qiyang, red soil (QY, 26°45′, 111°52′, subtropical region), Hunan, southern China. The cropping systems included monomaize cropping at GZL and wheat-maize double cropping at both ZZ and QY. No irrigation was provided to crops at GZL and QY, while the wheat crop was irrigated two or three times and the maize crop was irrigated once at ZZ. Information on the climatic conditions and initial soil physicochemical properties for the three sites is briefly shown in .
Table 1. Initial surface soil properties (0–20 cm in 1990) and climate conditions (data are the means from 1990–2015) at three long-term experimental sites (Gongzhuling, Zhengzhou, and Qiyang)
A randomized complete block design was used in the experiments, and detailed information regarding the long-term experiments is given by He et al. (Citation2015). The following five treatments were assessed in this study: (1) CK, (2) NK, (3) NPK, (4) NPKM, and (5) NPKS. N, P and K were applied as urea, calcium triple superphosphate, and potassium sulfate. The annual fertilization rates are provided in . For each site, the total N applied (inorganic plus organic) was the same for the NK, NPK, NPKS and NPKM treatments except the NPKS treatment at QY, which received extra N from straw; inorganic P fertilizer was the same for the NPK, NPKM and NPKS treatments; and inorganic K fertilizer was the same for the NK, NPK, NPKM, and NPKS treatments.
Table 2. Annual rates of fertilizer N, P and K application in inorganic and organic forms at the three long-term (1990–2015) fertilization sites
Weeds were controlled by hand weeding, and pesticides were applied when needed. Crops were harvested manually with sickles close to (approximately 0.02 m) the ground, and all of the harvested biomass was removed from the plots with little crop residue remaining (except in the NPKS).
1.2. Soil sampling and analysis
1.2.1. Soil characterization
For each treatment, soil samples were collected annually at a depth of 0–0.2 m immediately after the maize harvest. A soil auger (10 cm internal diameter) was used to collect the soil samples from three points in each plot, which were thoroughly mixed and treated as one composite sample. All of the soil samples were air-dried, sieved (2 mm) and stored for analysis. In this study, soils from 1990, 2000 (GZL and QY)/2002 (ZZ), and 2015 were obtained for analysis; these soils were archived samples that had been air-dried prior to long-term storage.
The soil organic C (SOC) was measured by sulfuric acid–K dichromate oxidation (Walkley and Black Citation1934); the soil pH was measured using a glass electrode (soil: water = 1:2.5). Total P was extracted with H2SO4-HClO4, soil Olsen-P was extracted using 0.5 mol L−1 NaHCO3 (Olsen et al. Citation1954), CaCl2-P was extracted using 0.01 mol L−1 CaCl2 (soil:solution = 1:5, shaken for 15 min at 25°C), and all P contents were determined using the spectrophotometric method (Murphy and Riley Citation1962). The contents of CaCO3, free Fe2O3 and Al2O3 were determined following the methods of Lu (Citation2000). In addition, the soil specific surface area (SSA) was measured using the Brunauer–Emmet–Teller method using a Gemini VII 2390 surface area analyzer (Micromeritics Instrument Corp., USA).
1.2.2. Sequential P fractionation and solution 31P-NMR spectroscopy
To determine the soil P speciation, the sequential extraction procedure of Hedley, Stewart, and Chauhan (Citation1982), as modified by Tiessen and Moir (Citation1993), was used. Briefly, triplicate subsamples of each soil (1 g) were sequentially extracted as follows: shaking in 30 mL of deionized water containing anion exchange resin, followed by extraction with 0.5 mol L−1 NaHCO3 (pH = 8.5), 0.1 mol L−1 NaOH, 1.0 mol L−1 HCl (dHCl) and hot concentrated HCl (cHCl), after which the soil residue was mineralized with concentrated H2SO4-H2O2 (300 µL 30 mg−1 soil) at 350°C for 3 h (rate of 4°C min−1). Between each consecutive step, the soil extract was shaken end-over-end in 50 ml centrifuge tubes for 16 h and then centrifuged for 10 min at 25,000 × g at 4°C. The supernatant was decanted for analysis. Both inorganic and organic P (calculated as the difference between the total P content after persulfate digestion and the inorganic P content) levels were determined in 0.5 mol L−1 NaHCO3, 0.1 mol L−1 NaOH and 12 mol L−1 HCl. All P fractions were detected using the spectrophotometric method (Murphy and Riley, Citation1962). In consideration of plant availability, P fractions were divided into labile P, moderately labile P, and nonlabile P (Rodrigues et al. Citation2016). Labile P includes Resin-P, NaHCO3-Pi, and NaHCO3-Po. Moderately labile P refers to NaOH-Pi, NaOH-Po and dHCl-P. Nonlabile P includes cHCl-Pi, cHCl-Po and residual-P.
Phosphorus (P-NaOH-EDTA) used in the 31P-NMR procedure was extracted by shaking 5 g of air-dried soil with 0.25 mol L−1 NaOH + 0.05 mol L−1 Na2EDTA solution (soil: solution = 1:20) by shaking on a reciprocal shaker for 16 h at 20°C, and the centrifuged supernatant solution was passed through a 0.45 μm membrane filter. The extract was frozen at −80°C and lyophilized for 31P-NMR analysis. Freeze-dried NaOH-EDTA extracts were redissolved in 1 ml of 1 mol L−1 NaOH and 0.1 ml of D2O and transferred to 5 mm NMR tubes. Solution 31P NMR spectra were obtained using a Bruker AVANCE spectrometer (Bruker Ltd., Germany) operating at 243 MHz with a 7 μs pulse (45°), a delay time of 2.0 s, and an acquisition time of 0.25 s. The P compounds were identified by their chemical shifts (ppm) in accordance with Turner, Mahieu, and Condron (Citation2003). The classes of P compounds include inorganic orthophosphate (~5.9 ppm), orthophosphate monoesters (3.0 ~ 5.8 ppm), orthophosphate diesters (−0.5 ~ 2.0 ppm), and pyrophosphate (~-4.5 ppm). The P extracted by NaOH-EDTA (P-NaOH-EDTA) was determined in diluted extracts (1:25) to prevent interference from EDTA. The soil contents of specific P compounds were calculated using the corresponding peak area portions and the P-NaOH-EDTA contents.
1.2.3. P sorption
In the sorption study, solutions with various concentrations of P that were used to define the sorption isotherms were prepared in a 0.01 mol L−1 KCl solution (pH = 7.0) using KH2PO4. Soil samples of 1.0 g in triplicate were added to a 50-ml centrifuge tube followed by the addition of 25 mL of 0.01 mol L−1 KCl solution containing P concentrations of 0, 10, 20, 40, 60, 80, and 160 mg L−1. Two drops of chloroform were added to inhibit microbial activity. The suspensions were shaken on an end-over-end mechanical shaker at 25°C for 1 h. The mixture was then equilibrated for 24 h at a constant controlled temperature (25°C). After centrifugation at 5000 × g for 10 min, the supernatant was analyzed for P using the molybdenum blue colourimetric method. The maximum P sorption capacity (Qm, mg kg−1) was calculated using the linearized form of the Langmuir equation:
where C is the P concentration in the equilibrium solution (mg L−1); k is a constant related to bond strength (L mg−1); q = q0+ q1, q is the total amount of P absorbed (mg kg−1); q0 is the original sorbed P (mg kg−1) determined by the P release at 0 initial P concentration; and q1 is the amount of added P sorbed by the soil and was calculated from the difference between initial and final solution P concentrations (mg kg−1).
The DPS was obtained for all of the samples by the following equation:
where DPS is the degree of P saturation, Olsen-P corresponds to the available P extract by 0.5 mol L−1 NaHCO3 (mg kg−1), and Qm is the maximum P sorption capacity calculated from the Langmuir equation (mg kg−1).
1.3. Cumulative P budget
Maize and wheat were harvested each year, and the grain yields and straw biomass were determined from the entire plot area. Crop samples were air-dried and then oven-dried at 65°C to a uniform moisture level and weighed. Plant samples were ground (<0.15 mm) and digested with H2SO4–H2O2, and the P concentration of the extracts was measured using the molybdenum blue colourimetric method (Page, Millar, and Keeney Citation1982). P uptake at each harvest was calculated by multiplying the grain and straw P concentrations by the grain yield and straw biomass. The annual P budget (from 1990 to 2015) was calculated by the fertilizer P input minus the annual crop P uptake (sum of grain and straw P uptake). The cumulative P budget was the sum of the annual P budgets.
1.4. Statistical analyses
All of the statistical analyses were conducted using SPSS 20.0. The data presented for all the chemical and physical analyses were the mean values of three replicates. A Pearson correlation analysis was used to explore the relationships between the cumulative P budget, P speciation, DPS and soil properties.
2. Results
2.1. The cumulative P budget, soil Olsen-P and CaCl2-P
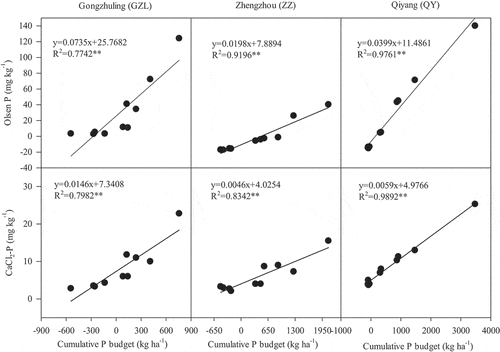
The cumulative P budget and Olsen-P and CaCl2-P contents in the three soils after different long-term fertilization treatments are presented in . The cumulative P budgets were −543〜756, −490〜2100 and −94〜3477 kg P ha−1 among GZL, ZZ and QY, respectively. In the P-applied treatments, the cumulative P budgets were positive (i.e., P input>P output) and ordered as NPKM>NPKS>NPK for all three sites. For the treatments without P application (CK and NK), the cumulative P budgets were negative, with much smaller deficiency values at QY than at the other two sites.
Table 3. The cumulative P budget and the contents of Olsen-P and CaCl2-P in each fertilization treatment at the three long-term experimental sites
The Olsen-P and CaCl2-P concentrations among all fertilization treatments and soil types were in the ranges of 2.1–141.3 mg kg−1 and 2.1–25.3 mg kg−1 and much higher in the P-applied treatments than in the no-P treatments, especially in the NPKM treatment. Both the contents of Olsen-P and CaCl2-P were significantly positively correlated with the cumulative P budgets among the three sites (P < 0.001) (). With an increase of 100 kg P ha−1 of the cumulative P budget, Olsen-P and CaCl2-P increased by 1.98–7.35 mg kg−1 and 0.46–1.46 mg kg−1 in the order of GZL>QY>ZZ.
2.2. Soil P speciation determined by sequential fractionation and 31P NMR spectroscopy
Soil P speciation can be affected by both the soil type and fertilization treatment, as shown in and . The Hedley procedure recovered 88–109% (average of 98%), 92–103% (average of 97%) and 87–111% (average of 100%) of the total P (extracted by H2SO4-HClO4 and independent of sequential fractionations) in GZL, ZZ and QY, respectively. The proportions of labile P were smallest in the initial soil samples and were 11%, 5% and 5% at GZL, ZZ and QY, respectively; the proportions of nonlabile P were largest for both GZL (51%) and QY (65%), while the moderately labile P was largest in ZZ (66%). Compared to that in the initial soils, the proportion of nonlabile P increased in the no-P treatment at all three sites, while the proportion of labile P (except for NK at QY) and moderately labile P decreased (). In contrast, the proportion of nonlabile P decreased in the P-applied treatment at all three sites, while the proportion of labile P showed an increasing trend. Furthermore, Pit (sum of all Pi fractions and residual P) was dominant in all treatments and soils, ranging from 73 to 93%, whereas Pot (sum of all Po fractions) was less than 27% in all samples. The average proportion of Pot among all fertilization treatments and years was slightly higher in GZL (18%) than in ZZ (10%) and QY (11%).
Table 4. The recovery rate and the ratio of Pi to Po obtained from the Hedley procedure and 31P NMR spectroscopy at the three long-term experimental sites
Figure 2. The P speciation obtained from Hedley fractionation and 31P NMR spectroscopy under different soil types and fertilization treatments. Initial: soil samples from 1990 at three sites; subscript 10: soils from 2000 for GZL and QY and 2002 for ZZ; subscript 25: soils from 2015 at three sites.
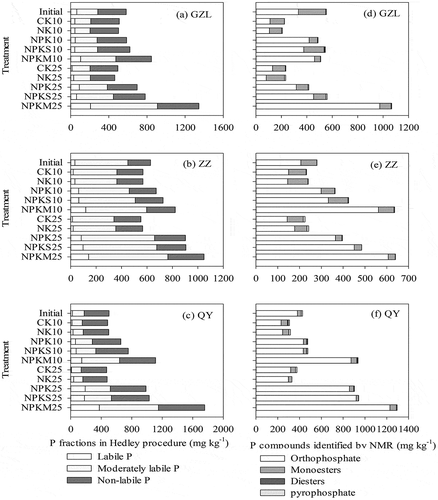
The content and proportions of P speciation following procedures of solution 31P-NMR analysis are shown in and . The recovery of P extracted by NaOH-EDTA (P-NaOH-EDTA) ranged from 40% to 91% of the total P (extracted by H2SO4-HClO4) among all of the soil samples. Inorganic orthophosphate concentrations were in the ranges of 81–974, 143–607 and 231–1229 mg kg−1 in GZL, ZZ and QY, respectively, which occupied more than 50% of the P-NaOH-EDTA in almost all samples. In contrast, the inorganic pyrophosphates were less than 4% of P-NaOH-EDTA and were not found in NPKS at ZZ in 2015. Orthophosphate monoesters dominated the Po pool and ranged from 5% to 50% of P-NaOH-EDTA in most of the samples. Orthophosphate diesters were mostly found in the acidic soil of QY but were not detected in the alkaline soil of ZZ. The proportions of orthophosphates in the P-applied treatments were higher than no P treatments, while the proportions of pyrophosphates and monoesters were higher in no P treatments than P-applied treatments in each site. Moreover, Pit (sum of orthophosphate and pyrophosphates) ranged from 38% to 98%, and Pot (sum of orthophosphate monoesters and diesters) ranged from 2% to 62% in all samples. The average proportion of Pot among all treatments and years was in the order of GZL (31%)>ZZ (20%)>QY (9%).
2.3. P sorption characteristics
The P sorption isotherm parameters based on the Langmuir model are presented in . All of the P sorption data fit the model well, and the coefficient of determination values (R2) were greater than 0.949. The values of maximum P sorption capacity (Qm) and binding energy (k) differed among treatments and soils. The average value of Qm was 608.5 mg kg−1 among all treatments and years (ranging in 263.2–833.3 mg kg−1) in QY, which was higher than the average values of GZL (377.2 mg kg−1, ranging in 208.3–625.0 mg kg−1) and ZZ (298.0 mg kg−1, ranging in 212.8–434.8 mg kg−1). The k values were in the order of QY>GZL>ZZ. In general, the Qm and k values were lowest under NPKM at each site; the highest k values were found in the NK treatment in 2015, but the highest Qm values were found in different treatments among the three sites.
Table 5. The P sorption indices and DPS for each fertilization treatment and soil type at the three long-term experimental sites
The DPS values were in the ranges of 0.5–59.6%, 0.9–24.9% and 0.6–42.4% in the GZL, ZZ and QY soils, respectively (). Compared with the initial soils, the DPS values gradually decreased over time in the no-P treatments but markedly increased in the P-applied treatments, especially under NPKM. In general, the DPS values were >24.9% under NPKM at each site after a 25-year continual combination of chemical fertilizer and manure, while the DPS values were <9.5% in the remaining treatments.
2.4. Correlations between the cumulative P budget, P speciation, DPS and selected soil properties
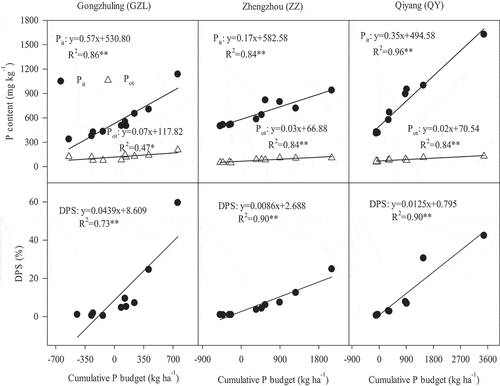
Summarizing the P fractions obtained by the Hedley procedure into organic and inorganic forms, it can be seen that there was a linear and significant increase in Pit (sum of all Pi fractions and residual P) with increasing cumulative P budget (P < 0.01), and the change rates (slopes of the linear equation obtained from the Pit response to cumulative P budget) were in the order of GZL (0.57)>QY (0.35)>ZZ (0.17). Additionally, the Pot (sum of all Po fractions) increased with increasing cumulative P budget, but the changing rates were much lower than the Pit at each site, which was in the range of 0.0086–0.0439 (). Furthermore, a positive linear relationship can be observed between the cumulative P budgets and DPS. For an increase of 100 kg ha−1 of the cumulative P budget, the DPS increased by 4.39%, 1.25% and 0.86% in the order of GZL>QY>ZZ.
Figure 3. Relationship between the Pit (sum of all Pi fractions and residual P in Hedley procedure), Pot (sum of all Po fractions in Hedley procedure) and degree of P saturation (DPS) with the cumulative P budget.
Of the selected soil properties, the highest pH was observed at ZZ, followed by GZL and QY (). Specifically, the pH decreased slightly over time for each treatment at GZL and ZZ but obviously decreased under NK, NPK, and NPKS over time at QY. The average SOC contents were ordered as GZL>QY>ZZ, and the SOC contents in the P-applied treatments were higher than those in the no-P treatments at all three sites. In addition, the concentration of CaCO3 was highest at ZZ, while the Fe2O3 and Al2O3 concentrations were highest at QY. CaCO3, Fe2O3 and Al2O3 concentrations differed slightly among treatments and years. The SSA values were higher at QY than at GZL and ZZ. The SSA values were similar among treatments within the same year; however, after 25 years of fertilization, they strongly decreased in GZL and ZZ but not in QY.
Table 6. Selected soil properties in all fertilization treatments and years at the three long-term experimental sites
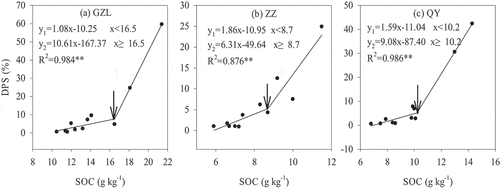
This study focused on the relationships between soil properties and DPS at each site because DPS can reflect both the P concentration and P sorption characteristics. The Pearson correlation analysis showed that DPS was significantly positively correlated with the SOC at all three sites (P < 0.001) (). Additionally, the DPS was negatively correlated with pH in ZZ (P < 0.05) and negatively correlated with Fe2O3 in QY (P < 0.05). Furthermore, the response of the relationship between DPS and SOC was well fitted by the linear-linear model (P < 0.01), with changes of 16.5, 8.7 and 10.2 g kg−1 in SOC content at GZL, ZZ and QY, respectively (). After the change point, the DPS increased by 0.63%-1.06% as the SOC content increased every 0.1 g kg−1 in the order of GZL>QY>ZZ. The coefficients of the equation after the change point were 9.8-, 3.4- and 5.7-fold those of the previous equation at GZL, ZZ and QY, respectively.
Table 7. Pearson’s correlation coefficients of the degree of P saturation (DPS) and selected soil properties
3. Discussion
3.1. Effects of long-term fertilization on soil P content and speciation
In heavily fertilized soils, the P level usually exceeds the agronomic optimum required for crops, resulting in large P accumulation and changes in P behaviors (Shi et al. Citation2013; Yan et al. Citation2017). In this study, the cumulative P budget differed greatly among fertilization treatments and soil types, which may be related to the large variation in the fertilizer P input and crop P uptake. Variation in the P contents can be largely explained by the differences in the P budget in agricultural soil (Rowe et al. Citation2015). The change in Olsen-P was 1.98–7.35 mg kg−1 with each increase of 100 kg ha−1 of the cumulative P budget across our study sites, which was similar to the results of 1.44–5.74 mg kg−1 around China (Cao et al. Citation2012). In addition, the increasing rates in both Olsen-P and CaCl2-P by each 100 kg ha−1 cumulative P budget differed greatly and were ordered as GZL>QY>ZZ. This large difference among the three sites can be explained by the soil physical-chemical properties, texture and temperature (Aulakh, Garg, and Kabba Citation2010; Cao et al. Citation2012). The increase values were higher in GZL and QY than in ZZ, which may be due to the high SOC and clay content usually maintaining a high P content (Zhu et al. Citation2013). In many countries and regions, the dissolved P extracted by 0.01 mol L−1 CaCl2 (CaCl2-P) is often used as a direct indicator of the proneness to P leaching (Blombäck et al. Citation2021). Thus, establishing the relationship between Olsen-P, CaCl2-P and the cumulative P budget is helpful for soil P management for both agricultural production and environmental protection.
The sequential P fractionation approach developed by Hedley, Stewart, and Chauhan (Citation1982) has been widely used to differentiate labile and stable P fractions in soils, expanding our knowledge of the transformation of inorganic and organic P under different land uses and management practices (Negassa and Leinweber Citation2010). However, this method can only describe the distribution of soil P over ‘operationally defined’ fractions (Gatiboni et al. Citation2021). Solution 31P NMR spectroscopy enables the identification and speciation of Po classes (Hashimoto and Watanabe Citation2014). However, this technique is unsuitable for the identification of inorganic P (Pi) species. In summary, there are limitations in providing information about the biogeochemical behavior of P by one method alone. In our study, Hedley fractionation and solution 31P NMR spectroscopy were combined to substantially characterize P transformation in typical agricultural soils after long-term fertilization. Generally, the average recovery of all fertilization treatments and years at each site was above 98%, indicating that the Hedley method is suitable for a wide range of soil types (Negassa and Leinweber Citation2010). In contrast, the average recovery of P extracted by NaOH-EDTA was only 76% in QY, followed by GZL at 65% and ZZ at 50%, which may imply that solution 31P NMR spectroscopy is more suitable in acidic soil than alkaline soil (Peng, Fang, and Zhan et al. Citation2009). Moreover, there were great discrepancies among the contents and percentages of Pit/Pot determined by these two methods. The average Pit/Pot was higher in Hedley fractionation than 31P-NMR in GZL and ZZ, and the trend was opposite in QY. It can be concluded that more percentage of organic P was detected by Hedley fractionation than 31P-NMR at QY, while more percentage of organic P was detected by the 31P-NMR procedure than Hedley fractionation at GZL and ZZ. This result could be partly because alkaline extractants (i.e., NaHCO3 and NaOH at a pH of 8.5) are more efficient at extracting Po in acidic soils of QY than in neutral soil of GZL and alkaline soil of ZZ (Turner et al. Citation2005). On the other hand, if the NaHCO3, NaOH or hot cHCl Pi extracts contain significant concentrations of poly and pyrophosphates that are not detected by the molybdenum blue method and thus are included in the Po estimation by subtraction, the overestimation of Po fractions is possible, whereas if the acid reagents induce Po hydrolysis, underestimation of Po fractions can occur (Turner et al. Citation2005).
In the present study, the black soil in GZL, fluvo-aquic soil in ZZ and red earth in QY were derived from contrasting parent materials and had different initial physicochemical properties. Moreover, different long-term fertilization treatments also strongly changed selected soil properties, as shown in . These soil properties can affect the contents and fractions of P directly or indirectly (Wang and Liang Citation2014; Weihrauch and Opp Citation2018). The organic P was highest at GZL, which may be due to its high SOC content in the black soil; dHCl-Pi was highest at ZZ, which may be related to the high CaCO3 content in the alkaline fluvo-aquic soil, and NaOH-P was highest at QY, which may be due to the high Fe2O3 and Al2O3 levels in the acidic red soil. Previous work has reported that organic P is associated with SOC, the dHCl-Pi fraction is largely bound to Ca, and the NaOH-Pi fraction is bound to the exterior of Al and Fe oxides (Tiessen and Moir Citation1993; De Schrijver et al. Citation2012).
3.2. Effects of long-term fertilization on soil P sorption characteristics
Fertilizer P application can influence soil P sorption behavior (Pizzeghello et al. Citation2011; Abdala et al. Citation2012; Yan et al. Citation2017). Our results indicated that chemical fertilizer combined with manure or straw can decrease the constant k values compared to chemical fertilizer alone, while changes in Qm were dependent on the soil type. Similar results in paddy soil have been reported by Lin, Wang, and Lin et al. (Citation2011). In contrast, Yan et al. (Citation2017) found that swine manure and rice straw increased the Qm compared to chemical fertilizer, while there were no significant differences in the constant k. These inconsistent results may be attributed to large variations in the type and rate of fertilizer P and the diverse soil properties and cropping systems among these study areas. Both Qm and k can be affected by soil properties and management systems (Daly et al. Citation2015; Fink et al. Citation2016; Jalali and Jalali Citation2016). It has been found that Qm was significantly correlated with the content of iron oxides in subtropical soils (Fink et al. Citation2016). Daly et al. (Citation2015) reported that binding energies were strongly negatively correlated with soil pH, while positively correlated with extractable aluminum. In addition, organic amendments significantly increased P sorption capacity compared to chemical fertilizer treatment, which may be due to the increased amount of amorphous Fe and Al in organic treatments (Yan al. Citation2017).
Among the three soils, the mean values of Qm and k among the different fertilization treatments and years were ordered as QY>GZL>ZZ, and the mean pH values were in the order of ZZ>GZL>QY, which may indicate that Qm and k decreased with increasing pH. Quintero, Boschetti, and Benavidez (Citation1999) also found that the P retention and maximum buffering capacities (calculated as Qm×k) in soils decreased as the pH increased from 3 to 7. When the pH value is low, a large number of variable positive charges occur; thus, the adsorption of phosphate radicals is strong. Increasing pH can inhibit P adsorption and decrease the adsorption energies (Abdala et al. Citation2012; Hou et al. Citation2018). In addition, the Fe2O3 and Al2O3 contents were highest in QY and lowest in ZZ, while the CaCO3 content was the opposite. Wang et al. (Citation2014) found that the P adsorption capacity tends to be relatively strong when there are a large number of iron and aluminium oxides. It has also been found that adsorption energies (k) were small in limed soils (Broggi et al. Citation2011). Furtherly, soils containing large quantities of clay can fix more P than soils with low clay (Arai and Sparks Citation2007). Soil organic matter can also increase P fixation by directly increasing the number of adsorption sites or indirectly inhibiting the crystallization of Al, which in turn can increase P sorption (Borggaard et al. Citation1990). A comparison of the black soil with the fluvo-aquic soil shows that the black soil contains more clay and SOC contents, which may explain why the capacity of P sorption is stronger in GZL than in ZZ.
4.3. Relationships between cumulative P budget, soil properties and DPS and implications for P management
The DPS has been used in many countries to evaluate the risk of P loss from agricultural soils. However, the DPS is determined by indirect methods in most studies, and the P sorption isotherms are not measured because it is laborious and time-consuming (Pizzeghello et al. Citation2011; Blombäck et al. Citation2021). In this study, we directly calculated the DPS as the ratio of Olsen-P in the soils to the Qm derived from the P sorption isotherms. Our results showed that the DPS ranged widely from 0.5% to 59.6% among fertilization treatments and soil types, which might be attributed to huge differences in cumulative P budgets and soil properties after different long-term fertilization practices. The DPS was significantly positively correlated with the cumulative P budget (P < 0.01), and the increasing rates were GZL>QY>ZZ. The increases were 17–57 mg kg−1 for Pit (sum of all Pi fractions and residual P) and 0.86–4.39 mg kg−1 for Pot (sum of all Po fractions) across the study sites with each increase of 100 kg ha−1 of the cumulative P budget. These results indicate that the accumulation of P in inorganic P fractions is more obvious than organic P in soils after a history of fertilization, which is consistent with other previous reports (Gatiboni et al. Citation2021). Moreover, our results also showed that the DPS values were significantly correlated with several soil properties, such as SOC, pH and Fe2O3. SOC is generally considered to be an important factor regulating soil DPS values, but this is largely based on theoretical considerations (Zou, Fu, and Cao Citation2011). Our linear-linear model demonstrated that there is a change point in the response relationship between DPS and SOC, providing evidence of a remarkable effect of SOC on the DPS in agricultural soils with high organic matter content. In addition, the positive relationship between the DPS and SOC indicates that the potential of P release may increase with increasing organic carbon content. This may be attributed to that SOC can provide organic acid anions competing for the binding sites with PO43-, which may decrease the soil retention of P (Weihrauch and Opp Citation2018).
Overall, all the CaCl2-P and Olsen-P contents and the DPS values were significantly positively correlated with the cumulative P budget among the three soils with contrasting physicochemical properties. Generally, the environmental risk of P loss from agricultural land can be estimated considering two aspects: the dissolved P content in runoff and the threshold of available P content or DPS in the soil (Davis et al. Citation2005). The soil Olsen-P contents in several fertilization treatments in 2015 were higher than 40 mg kg−1 in our study, above which many soils in China are at risk of P leaching (Zhao et al. Citation2007). The DPS values in all treatments were much lower than 25% among the three soils in 2015, except for the MPKM. A DPS value of 25% is usually established as a critical value, above which soils tend to have a higher desorbable P available for runoff or leaching (Pothig et al. Citation2010). The soil CaCl2-P and Olsen-P contents and DPS values were relatively high under NPKM after 25 years of continual fertilization, which indicates that chemical fertilizer combined with manure amendment may cause P loss in these three soil types. In the N-based fertilization plans, only total N applied (inorganic plus organic) was kept at the same level, and the extra P and K brought by manure and straw were ignored. Thus, to prevent the risk of P loss, the amount of manure added should be carefully considered (Pizzeghello et al. Citation2011). Moreover, more effort should be made to better utilize the large amount of cumulative P in soil for finite P resources and water security.
4. Conclusions
This study found that the contents of Olsen-P and CaCl2-P and the DPS values were significantly positively correlated with the cumulative P budgets (P < 0.001), indicating that a large accumulation of P in soil can improve P availability but also increase the risk of P loss from agricultural systems. The soil P speciation, P sorption characteristics and DPS values differed greatly among fertilization treatments and soil types. There were great discrepancies in the contents and percentages of soil Pi and Po characterized by Hedley fractionation and 31P NMR, which confirmed the need for the combined use of different methods to determine P speciation for the best management of soil P. Chemical fertilizer combined with manure or straw can decrease the constant k values compared to chemical fertilizer alone, while changes in Qm were dependent on the soil type. There is a change point in the response relationship between DPS and SOC. After the change point, the coefficients of the linear equation were 3.4–9.8-fold that before the change point. Agricultural measures that increased the contents of SOC and available P could effectively increase DPS values. In particular, N-based fertilization plans are widely used in China, and manure amendments or straw return should be carefully evaluated because they can add abundant amounts of extra P.
Acknowledgments
We acknowledge all staff for their valuable work associated with these long-term Monitoring Network of Soil Fertility and Fertilizer Effects in China.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abdala, D. B., A. K. Ghosh, I. Silva, R. Novais, and V. Venegas. 2012. “Phosphorus Saturation of a Tropical Soil and Related P Leaching Caused by Poultry Litter Addition.” Agriculture Ecosystems and Environment 162: 15–23. doi:https://doi.org/10.1016/j.agee.2012.08.004.
- Arai, Y., and D. L. Sparks. 2007. “Phosphate Reaction Dynamics in Soils and Soil Components: A Multiscale Approach.” Advances in Agronomy 94: 135–179.
- Aulakh, M. S., A. K. Garg, and B. S. Kabba. 2010. “Phosphorus Accumulation, Leaching and Residual Effects on Crop Yields from Long‐term Applications in the Subtropics.” Soil Use and Management 23: 417–427. doi:https://doi.org/10.1111/j.1475-2743.2007.00124.x.
- Balemi, T., and K. Negisho. 2012. “Management of Soil Phosphorus and Plant Adaptation Mechanisms to Phosphorus Stress for Sustainable Crop Production: A Review.” Journal of Soil Science and Plant Nutrition 12: 547–562.
- Blombäck, K., C. H. Bolster, A. Lindsj, K. Hess, and M. M. Parvage. 2021. “Comparing Measures for Determination of Phosphorus Saturation as a Method to Estimate Dissolved P in Soil Solution.” Geoderma 383: 117708. doi:https://doi.org/10.1016/j.geoderma.2020.114708.
- Borggaard, O. K., S. S. Jdrgensen, J. P. Moberg, and B. Raben-Lange. 1990. “Influence of Organic Matter on Phosphate Adsorption by Aluminium and Iron Oxides in Sandy Soils.” European Journal of Soil Science 41: 443–449. doi:https://doi.org/10.1111/j.1365-2389.1990.tb00078.x.
- Broggi, F., A. Oliveira, F. J. Freire, M. Freire, and C. Nascimento. 2011. “Fator capacidade de fósforo em solos de pernambuco mineralogicamente diferentes e influência do pH na capacidade máxima de adsorção.” Ciência E Agrotecnologia 35: 77–83. doi:https://doi.org/10.1590/S1413-70542011000100009.
- Cao, N., X. Chen, Z. Cui, and F. Zhang. 2012. “Change in Soil Available Phosphorus in Relation to the Phosphorus Budget in China.” Nutrient Cycling in Agroecosystems 94: 161–170. doi:https://doi.org/10.1007/s10705-012-9530-0.
- Condron, L. M., and S. Newman. 2011. “Revisiting the Fundamentals of Phosphorus Fractionation of Sediments and Soils.” Journal of Soils and Sediments 11 11 (5): 830–840. doi:https://doi.org/10.1007/s11368-011-0363-2.
- Daly, K., D. Styles, S. Lalor, and D. P. Wall. 2015. “Phosphorus Sorption, Supply Potential and Availability in Soils with Contrasting Parent Material and Soil Chemical Properties.” European Journal of Soil Science 66: 792–801. doi:https://doi.org/10.1111/ejss.12260.
- Davis, R. L., H. Zhang, J. L. Schroder, J. J. Wang, and A. Zazulak. 2005. “Soil Characteristics and Phosphorus Level Effect on Phosphorus Loss in Runoff.” Journal of Environmental Quality 34: 1640–1650. doi:https://doi.org/10.2134/jeq2004.0480.
- De Schrijver, V., L. Vesterdal, K. Hansen, P. De Frenne, L. Augusto, D. L. Achat, J. Staelens, et al. 2012. “Four Decades of Post-agricultural Forest Development Have Caused Major Redistributions of Soil Phosphorus Fractions.” Oecologia 169 (1): 221–234. doi:https://doi.org/10.1007/s00442-011-2185-8.
- Fink, J. R., A. V. Inda, J. Bavaresco, V. Barrón, J. Torrent, and C. Bayer. 2016. “Phosphorus Adsorption and Desorption in Undisturbed Samples from Subtropical Soils under Conventional Tillage or No‐tillage.” Journal of Plant Nutrition and Soil Science 179: 198–205. doi:https://doi.org/10.1002/jpln.201500017.
- Fu, D., Z. Xu, X. Wu, L. Zhao, A. Zhu, C. Duan, D. R. Chadwick, et al. 2021. “Land Use Effects on Soil Phosphorus Behavior Characteristics in the Eutrophic Aquatic-terrestrial Ecotone of Dianchi Lake, China”. Soil and Tillage Research 205: 104793. doi:https://doi.org/10.1016/j.still.2020.104793.
- Gatiboni, L. C., A. A. Junior, D. T. Orsolett, G. L. Mumbach, S. B. Kulesza, D. B. Abdala . 2021. “Phosphorus Speciation in Soils with Low to High Degree of Saturation Due to Swine Slurry Application”. Journal of Environmental Management 282: 111553. doi:https://doi.org/10.1016/j.jenvman.2020.111553.
- Hashimoto, Y., and Y. Watanabe. 2014. “Combined Applications of Chemical Fractionation, Solution 31P-NMR and P K-edge XANES to Determine Phosphorus Speciation in Soils Formed on Serpentine Landscapes.” Geoderma 230–231: 143–150.
- He, Y., W. Zhang, M. Xu, X. Tong, F. Sun, J. Wang et al, 2015. “Long-term Combined Chemical and Manure Fertilizations Increase Soil Organic Carbon and Total Nitrogen in Aggregate Fractions at Three Typical Cropland Soils in China.” Science of the Total Environment 532: 635–644. doi https://doi.org/10.1016/j.scitotenv.2015.06.011.
- Hedley, M. J., J. W. B. Stewart, and B. S. Chauhan. 1982. “Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations1.” Soil Science Society of America Journal 46: 970–976. doi:https://doi.org/10.2136/sssaj1982.03615995004600050017x.
- Hou, E. Q., Wen, D. Wen, Y. Kuang, J. Cong, C. Chen, X. He, et al. 2018. “Soil pH Predominantly Controls the Forms of Organic Phosphorus in Topsoils under Natural Broadleaved Forests along a 2500 Km Latitudinal Gradient”. Geoderma 315: 65–74. doi:https://doi.org/10.1016/j.geoderma.2017.11.041.
- Jalali, M., and M. Jalali. 2016. “Relation between Various Soil Phosphorus Extraction Methods and Sorption Parameters in Calcareous Soils with Different Texture.” Science of the Total Environment 566-567: 1080–1093. doi:https://doi.org/10.1016/j.scitotenv.2016.05.133.
- Kleinman, and J. A. Peter. 2017. “The Persistent Environmental Relevance of Soil Phosphorus Sorption Saturation.” Current Pollution Reports 3: 141–150. doi:https://doi.org/10.1007/s40726-017-0058-4.
- Li, Z., R. Zhang, C. Liu, R. Zhang, and Y. Liu. 2019. “Phosphorus Spatial Distribution and Pollution Risk Assessment in Agricultural Soil around the Danjiangkou Reservoir, China.” Science of the Total Environment 699: 134417. doi:https://doi.org/10.1016/j.scitotenv.2019.134417.
- Lin, C., F. Wang, X. Lin, Li, Q., He, C., Li, Y. 2011. “The Effection of Phosphorus Adsorption and Desorption of Long-term Fertilization on South Yellow Clayey Soil.” Fujian Journal of Agricultural Sciences 26:1034–1038.
- Lu, R. 2000. Analytical Methods of Soil Agricultural Chemistry (In Chinese), 173–180. Beijing, China: China Agricultural Science and Technology Press.
- Ma, J., Y. Ma, R. D. Wei, Y. Chen, L. Weng, X. Ouyang, Y. Li, et al. 2021. “Phosphorus Transport in Different Soil Types and the Contribution of Control Factors to Phosphorus Retardation”. Chemosphere 276: 130012. doi:https://doi.org/10.1016/j.chemosphere.2021.130012.
- Murphy, J., Riley, and J. P. Riley. 1962. “A Modified Single Solution for Determination of Phosphate in Nature Waters.” Analytica Chimica Acta 27: 31–36. doi:https://doi.org/10.1016/S0003-2670(00)88444-5.
- Negassa, W., and P. Leinweber. 2010. “How Does the Hedley Sequential Phosphorus Fractionation Reflect Impacts of Land Use and Management on Soil Phosphorus: A Review.” Journal of Plant Nutrition and Soil Science 172: 305–325. doi:https://doi.org/10.1002/jpln.200800223.
- Olsen, S. R., C. V. Cole, F. S. Watanabe, and L. A. Dean. 1954. “Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate.”USDA circular 939, 1–19.
- Page, A. L., R. H. Millar, and D. R. Keeney. 1982. Methods of Soil Analysis. Part 2.Chemical and Microbiological Properties, 71–76. Madison, WI, USA: American Society of Agronomy/Soil Science Society of America.
- Peng, X., H. Fang, X. Zhan, Hao, G., Lv, Z., Ma, G., Gu, B. 2009. “Transformation of Phosphorus Forms in Soils with Application of Sewage Sludge by Using Phosphorus-31-nuclear Magnetic Resonance Spectroscopy.” Journal of Agro-Environment Science 28 (10): 2104–2110.
- Pizzeghello, D., A. Berti, S. Nardi, and F. Morari. 2011. “Phosphorus Forms and P-sorption Properties in Three Alkaline Soils after Long-term Mineral and Manure Applications in North-eastern Italy.” Agriculture Ecosystems and Environment 141: 58–66. doi:https://doi.org/10.1016/j.agee.2011.02.011.
- Pothig, R., H. Behrendt, D. Opitz, and G. Furrer. 2010. “A Universal Method to Assess the Potential of Phosphorus Loss from Soil to Aquatic Ecosystems.” Environmental Science and Pollution Research International 17: 497–504. doi:https://doi.org/10.1007/s11356-009-0230-5.
- Quintero, C., G. Boschetti, and R. Benavidez. 1999. “Phosphorus Retention in Some Soils of the Argentinean Mesopotamia.” Communications in Soil Science and Plant Analysis 30: 1449–1461. doi:https://doi.org/10.1080/00103629909370299.
- Rodrigues, M., P. S. Pavinato, P. Withers, A. Teles, and W. Herrera. 2016. “Legacy Phosphorus and No Tillage Agriculture in Tropical Oxisols of the Brazilian Savanna.” Science of the Total Environment 542: 1050–1061. doi:https://doi.org/10.1016/j.scitotenv.2015.08.118.
- Rowe, H., P. A. Withers, P. Baas, N. I. Chan, and M. Weintraub. 2015. “Integrating Legacy Soil Phosphorus into Sustainable Nutrient Management Strategies for Future Food, Bioenergy and Water Security.” Nutrient Cycling in Agroecosystems 104: 1–20.
- Shen, J., L. Yuan, J. Zhang, H. Li, Z. Bai, X. Chen, W. Zhang, et al. 2011. “Phosphorus Dynamics: From Soil to Plant”. Plant Physiology 156: 997–1005. doi:https://doi.org/10.1104/pp.111.175232.
- Shi, Y., N. Ziadi, A. J. Messiga, R. Lalande, and Z. Hu. 2013. “Changes in Soil Phosphorus Fractions for a Long-term Corn-soybean Rotation with Tillage and Phosphorus Fertilization.” Soil Science Society of America Journal 77: 1402–1412. doi:https://doi.org/10.2136/sssaj2012.0427.
- Tiessen, H., and J. O. Moir. 1993. “Characterization of Available P by Sequential Extraction. In: Carter, M.R. (Ed.), Soil Sampling and Methods of Analysis.” Canadian Society of Soil Science 7:75–86.
- Turner, B. L., B. J. Cade-Menun, L. M. Condron, and S. Newman. 2005. “Extraction of Soil Organic Phosphorus.” Talanta 66: 294–306. doi:https://doi.org/10.1016/j.talanta.2004.11.012.
- Turner, B. L., N. Mahieu, and L. M. Condron. 2003. “Phosphorus-31 Nuclear Magnetic Resonance Spectral Assignments of Phosphorus Compounds in Soil NaOH–EDTA Extracts.” Soil Science Society of America Journal 67: 497–510. doi:https://doi.org/10.2136/sssaj2003.4970.
- Walkley, A. J., and I. A. Black. 1934. “An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method.” Soil Science 37: 29–38. doi:https://doi.org/10.1097/00010694-193401000-00003.
- Wang, L., and T. Liang. 2014. “Effects of Exogenous Rare Earth Elements on Phosphorus Adsorption and Desorption in Different Types of Soils.” Chemosphere 103: 148–155. doi:https://doi.org/10.1016/j.chemosphere.2013.11.050.
- Wang, Y., J. Tang, H. Zhang, J. L. Schroder, and Y. He. 2014. “Phosphorus Availability and Sorption as Affected by Long-term Fertilization.” Agronomy Journal 106: 1583–1591. doi:https://doi.org/10.2134/agronj14.0059.
- Weihrauch, C., and C. Opp. 2018. “Ecologically Relevant Phosphorus Pools in Soils and Their Dynamics: The Story so Far.” Geoderma 325: 183–194. doi:https://doi.org/10.1016/j.geoderma.2018.02.047.
- Withers, P., N. J. Flynn, G. P. Warren, M. Taylor, and B. J. Chambers. 2016. “Sustainable Management of Biosolid Phosphorus: A Field Study.” Soil Use and Management 32: 54–63. doi:https://doi.org/10.1111/sum.12235.
- Yan, X., Z. Wei, Q. Hong, Z. Lu, J. Wu . 2017. “Phosphorus Fractions and Sorption Characteristics in a Subtropical Paddy Soil as Influenced by Fertilizer Sources”. Geoderma 295: 80–85. doi:https://doi.org/10.1016/j.geoderma.2017.02.012.
- Zhang, Y., Y. Li, S. Wang, D. Umbreen, and C. Zhou. 2021. “Soil Phosphorus Fractionation and Its Association with Soil Phosphate-solubilizing Bacteria in a Chronosequence of Vegetation Restoration.” Ecological Engineering 164: 106208. doi:https://doi.org/10.1016/j.ecoleng.2021.106208.
- Zhao, X., X. Zhong, H. Bao, H. Li, G. Li, D. Tuo, Q. Lin, et al. 2007. “Relating Soil P Concentrations at Which P Movement Occurs to Soil Properties in Chinese Agricultural Soils.” Geoderma 142 (3–4): 237–244. DOI:https://doi.org/10.1016/j.geoderma.2007.07.012.
- Zhu, Y., R. Zhang, F. Wu, X. Qu, F. Xie, Z. Fu . 2013. “Phosphorus Fractions and Bioavailability in Relation to Particle Size Characteristics in Sediments from Lake Hongfeng, Southwest China.” Environmental Earth Sciences. Environmental Earth Science 68 (4): 1041–1052. DOI:https://doi.org/10.1007/s12665-012-1806-9.
- Zou, P., J. Fu, and Z. Cao. 2011. “Chronosequence of Paddy Soils and Phosphorus Sorption–desorption Properties.” Journal of Soils and Sediments 11: 249–259. doi:https://doi.org/10.1007/s11368-010-0301-8.