?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Soil organic carbon and main nutrient stocks play key roles in maintaining soil fertility and yields of agricultural crops including cocoa (Theobroma cacao). The amount of nutrients removed from the soil by the cocoa trees could be influenced by its physiological growth stage. A study was carried out to examine the changes in some soil physical and chemical properties with main nutrients availabilities under cocoa plantations at the Cocoa Research Institute in the Eastern Region of Ghana. Cocoa plantations of different ages (5, 10, 14, 18 and 23 years) were chosen based on similar land history. Soils at the study sites are classified as Xanthic Ferralsol (WRB series). On each plantation, one acre (0.4ha) plot was demarcated and divided into three blocks for soil sampling. Five core samples were taken at 0–15 cm depth per block, bulked together and subsample taken to the laboratory for analyses. Particle size analyses showed that all soils were of sandy loam texture. All soils were acidic with pH below 5.5, relatively low exchangeable K and Ca. Bulk density values varied with ages at highest in 18 years. Soils under 23 years old plantations had relatively lower bulk density than those of the other ages. Total nitrogen content under the 5, 10, and 18 years of cocoa plantations was below the critical values considered adequate for good cocoa growth. Soil carbon stock and available phosphorus content of the soil generally tend to increase with age of the cocoa plantations. These differential variations in the soil nutrients, namely, total N, available P, exchangeable K, Ca, and Mg among five different cocoa plantation ages suggest that total nitrogen, available P, and exchangeable Mg were higher than the critical levels for good cocoa growth under 23 years of cocoa plantations only, but exchangeable K and Ca were lower the critical levels for all ages of cocoa plantations.
1. Introduction
Land-use and management changes have major influence on soil organic carbon (SOC) stock (Cheng Citation2020). The type of vegetation cover also influences the abundance of organic carbon (C) in the soil (Jobbagy and Jackson Citation2000). Globally, the amount of C stored in soils is estimated to be 1.5–3 times more than in vegetation (Dixon Citation1995). In tropical countries, the contribution of agriculture and forest to greenhouse gases (GHG), carbon dioxide (CO2), methane, and nitrous oxide emissions is very important (Asumadu-Sarkodie and Owusu Citation2016). Agriculture being a major contributing factor of climate change, contributing up to 29% of the annual anthropogenic GHG emissions, is also part of the solution as it offers opportunities for alleviation through SOC capture, plant biomass production, and improved land use management (Vermeulen, Campbell, and Ingram Citation2012). SOC is the balance between plant inputs and biologically mediated losses. The amount of SOC is large compared to anthropogenic CO2 fluxes in the atmosphere. Thus, small changes in the SOC pool could have a major impact on atmospheric CO2 concentration (Lal Citation2020).
With the inclusion of the cocoa sector in the national carbon emission accounting budgets of Ghana (Chagas et al. Citation2010), the need to quantify the C stored is urgent. Thus, since Ghana is to include the C sequestered in the cocoa (Theobroma cacao) sector in its proposal for developing a national C accounting strategy, as outlined in its Readiness Plan Proposal (Chagas et al. Citation2010), the C quantities stored and the soil characteristics of the cocoa ecosystems in Ghana must be included.
The success of cocoa production in Ghana has been based primarily on exploiting the fertility built up by virgin forest and through opening of new lands rather than cropping intensification to increase yield per unit area by the use of fertilizers (Appiah et al. Citation1997; Gockowski and Sonwa Citation2010). The continuous crop removal through harvested beans results in the loss of nutrients from the soils (Ahenkorah et al. Citation1987; Appiah et al. Citation1997) leading to nutrient imbalances (Stoorvogel and Smaling Citation1990). Low soil fertility has been identified as one of the major causes of decline in yield of cocoa (Hartemink Citation2005). The cocoa trees have very high nutrient requirement for growth and yield, increasing very rapidly in the first 5 years and then reaching a plateau after that with subsequent increases depending mainly on export of nutrients in increased yield (Ling Citation1984). Very high amounts of nutrients are recycled in the leaf litter particularly for N, P, K, Ca, and Mg after the sixth year. In addition, in mature cocoa plantations, nutrient recycling in the litter is significant. Overall nutrient requirements for mature cocoa may therefore not be high (Ling Citation1984).
Not only C storage but also the other soil characteristics, such as soil nutrients contents affected by land-use and management changes, need to be determined in cocoa ecosystems. Despite its usefulness in agroforestry and plantation systems (Dawoe Citation2009), the few studies on land use systems involving cocoa trees in sub-Saharan Africa and elsewhere have not explored comprehensively its impacts on soil characteristics. For example, Mohammed et al. (Citation2016) only measured soil C stocks of cocoa soils and litter in Western and Eastern Regions of Ghana with virtually few or no other direct studies of the tree on soil characteristics. The work of Monroe et al. (Citation2016) in Brazil only studied the soil C stock of a four years old cocoa. The different ages of cocoa plantations from former lands should affect soil characteristics too. To bridge this gap, our study sought to quantify soil C stock and main nutrient characteristics (total N, available P exchangeable K, Ca, and Mg) under varying cocoa plantations and to establish how C storage is influenced by soil factors and age of the plantation in a tropical climate.
2. Materials and methods
2.1. Study site
The study was carried out at the Cocoa Research Institute of Ghana (CRIG), New Tafo-Akim (latitude 6° 13ʹ N; longitude 0° 21ʹ W), which is approximately 222 m above the sea level, situated in the humid rainforest belt of the Eastern Region of Ghana. The mean annual rainfall is about 1500 mm, with the mean annual minimum and maximum temperatures ranging between 22 and 31°C (Adu and Asiamah Citation1992). The soils at the site belong to Xanthic Ferralsol (IUSS Working Group WRB Citation2014). They are mostly brown to yellowish red, well-drained, and developed in situ from weathered materials of hornblende granodiorite (Adu and Asiamah Citation1992).
2.2. Plot demarcation and soil sampling
Cocoa plantations of different ages (5, 10, 14, 18 and 23 years after plantations) were chosen based on similar land history. The plantations were established by clearing secondary forest. These plantations irrespective of their ages receive the same quantity of cocoa fertilizer applied at 375 kg ha−1 annually. The cocoa fertilizer applied to mature cocoa plantations (3 years and above) contains main nutrients such as P, K, Ca, and Mg. Their amounts were 82.5 P2O5 kg ha−1, 67.5 K2O kg ha−1, 33.8 CaO kg ha−1 and 22.5 MgO kg ha−1, respectively. On each plantation, a 0.4 ha plot was demarcated and divided into three subplots for soil sampling in 2016. Five core samples (measuring 5 cm in diameter and 15 cm in height) were collected per farm, bulked together, and sub-sample taken to the laboratory for analyses. The samples were air dried and sieved through a 2 mm mesh prior to analysis.
2.3. Soil analyses and calculation
Soil samples for bulk density, porosity, and moisture determination were taken with core samplers from the plots, then weighed, and dried at 105°C for 24 h. The soil pH was determined using Suntex pH/Temp (SP-701) meter in a soil: water ratio of 1:2.5. SOC was determined using the modified Walkley and Black wet oxidation method. Soil total N(TN) was determined using the Kjeldahl digestion and distillation method, while available P was determined by the Troug method. Exchangeable Ca, K, and Mg were extracted with 1 N ammonium acetate solution, and the concentrations were determined using the Atomic Absorption Spectrophotometer (Varian SpectrAA 220 FS). The hydrometer method was used to determine the relative proportions of sand, silt, and clay. All methods were referred Sparks et al. (Citation1996), and all measurements were carried out at the Soil Science Laboratory of the Cocoa Research Institute of Ghana.
Soil carbon stock (SCS, Mg ha−1) was estimated using the relation described by Kautsar et al. (Citation2020) below:
where SOC is the soil organic carbon concentration (g kg−1), ρ is the soil bulk density (g cm−3), D is the horizon thickness for sampling depth (cm). In this study, the depth was 15 cm.
2.4. Statistical analysis
Analysis of variance (ANOVA) was performed on all parameters separately using the GenStat statistical package (edition 12, Lawes Agricultural Trust, Rothamsted Experimental Station, http://www.vsni.co.uk)) to evaluate the effect of age. Least significant difference values (P < 0.05) were calculated for each parameter. Regression and correlation analyses were done to determine the relationship between some of the measured parameters and SCS.
3. Results
3.1. Soil physical properties
Soil bulk density was significantly (P < 0.05) lowest in the 23 years old plantation and highest in the 18 years old plantation. Total porosity was generally higher in the older plantations compared with the 5 years old plantation (). Similar observations were made for the gravimetric soil moisture contents. Soil particle sizes did not differ significantly among the different-age cocoa plantations. Sand content was over 70% in all the cocoa plantations. Silt content was less than 10% with clay content of the plantations being less than 15% ().
Table 1. Soil physical properties among different ages of cocoa plantation
3.2. Soil chemical properties and main nutrients availabilities
shows the soil chemical properties among different cocoa plantation ages. Soil pH did not differ significantly among the cocoa age stands. Soil pH ranged from 4.58 to 5.29 being generally higher in younger cocoa age stand. SOC was significantly (P < 0.05) influenced by the age of the cocoa plantation. SOC was highest (1.20%) in the 23 years old plantation and lowest in the 5 years old plantation (0.71%). Except 14 years old plantation, soil TN varied from 0.07% to 0.11%. The 5 years old plantation recorded the lowest and the 23 years old plantation the highest. Conversely, soil available P was about four times greater in the 23 years old plantation compared with the 10 years old plantation and about 3 times greater compared with the 5 years old plantation (). Exchangeable K ranged from 0.19 cmolc kg−1 in the 5 years old plantation to 0.24 cmolc kg−1 in the 23 years old plantation. Exchangeable Ca content ranged from 1.79 to 2.95 cmolc kg−1 being generally higher in the older plantations. Exchangeable Mg content was not significantly influenced by the age of the cocoa plantation.
Table 2. Soil chemical properties among different ages of cocoa plantation
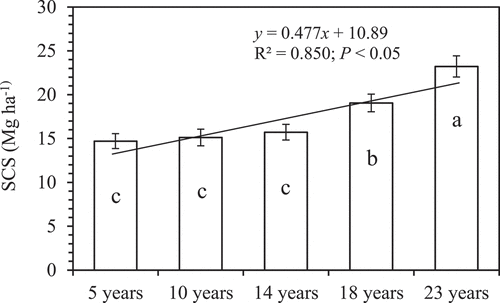
3.3. Soil carbon stock (SCS)
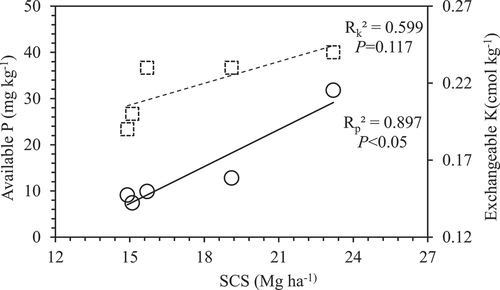
Mean value of SCS was highest in the 23 years old plantation and least in the 5 years old plantation (). As per results, SCS generally increased with the age of the plantation with a significant straight line (P < 0.05, in ). There was a significant relationship between SCS and available P (P < 0.05), and light position correlation between SCS and exchangeable K (P = 0.117), respectively ().
4. Discussions
4.1. Soil carbon stocks (SCS)
Mean SCS of the 23 years cocoa plantation was greater than that of the other age stands. SCS is the interplay between soil bulk density, sampling depth, and SOC content as equation (1). The relatively greater SOC content of the 23 years old plantation resulted in its greater SCS possibly due to leaf litter accumulation on the soil surface (Umrani and Jain Citation2010). As forests develop, input of carbon from litter increases and protects the soil from nutrient loss by leaching (Ofori-Frimpong, Afrifa, and Acquaye Citation2010). Tree litter is known to act as mulch, reduce nutrient loss by erosion and leaching, and increase SOC content (Asare Citation2016), which looked like different to long-term upland rice experiment in Uganda reported by Inubushi et al. (Citation2020) showing that the SOC contents did not affect by chemical and compost applications. Our results were consistent with Ahenkorah (Citation1981), who reported a better promise in its buildup under the old plantations than in the young ones. The young plantations as a result of less leaf litter cover and less canopy closure resulting in direct sun radiation and raindrop impact expose organic matter to microbial decomposition and loss through increased emissions and other loss pathways (Ahenkorah Citation1981).
Overall, the mean SCS of cocoa trees was different from that estimated for cocoa trees in a 30 years old cocoa plantation on a soil with 60–67% sand content in Cameroon (14.4 Mg C ha−1 at 20 cm depth) reported by Norgrove and Hauser (Citation2013). The present study estimated C stock in cocoa trees above those reported by Isaac, Timmer, and Quashie-Sam (Citation2007) as 10.3 Mg C ha−1 at 20 cm depth in an 8 years old cocoa plantation on Rhodic Ferralsol soil in Ghana. Isaac et al. (Citation2005) estimated the C storage of a 15 years old cocoa plantation in Ghana to be 16.8 and 15.9 Mg C ha−1 at 15 cm for a 25 years old system, both of which were at variance with the present finding. This gives credence to the fact that SCS is influenced by cocoa plantation ages.
4.2. Main soil nutrient characteristics among different ages of cocoa plantations
The cocoa tree takes up nutrients from the soil to produce the pods and eventually the yield. As this process continues, the nutrient levels decrease leading to low crop yield in the long run. Besides nutrients being mined from the soil, part is also exported through the harvested beans. Soil TN was significantly higher in the 23 years cocoa plantations compared with the others. The TN levels of 5, 10 and 18 years cocoa plantations were lower than the critical level of 0.09% soil N considered adequate for good cocoa growth (Egbe, Ayodele, and Obatolu Citation1989). The high TN contents at 0.11% under 23 years older plantations could be ascribed to the fact that these plantations have a lot of leaf litter on the floor that decomposes and releases the nutrients through recycling compared with the others. Soil available P was highest in the 23 years old plantation compared to the others (). Only the value of available P obtained from 23 years older plantations (31.8 mg kg−1) was above the critical level of 20 mg kg−1 suggested by Ahenkorah (Citation1981). The high available P content in the 23 years old plantation could be attributed to the phosphate adsorbs and accumulates by soil organic matter (as expressing as SOC) increase, since there was a significant relationship between SCS and available P (). As reported by Dossa et al. (Citation2018), the bulk of P applied remains in the soil as a result of immobilization by microbial biomass and sorption onto soil organic and inorganic colloids. The soil exchangeable K and Ca values for each of the cocoa plantations were below their critical values of 0.25 cmolc kg−1 and 7.5 cmolc kg−1, respectively, while Mg values except in the 14 and 23 years old plantations were below the established critical value of 1.33 cmolc kg−1 for cocoa soils in Ghana (Ahenkorah Citation1981). The soil pH values recorded in all the cocoa plantations were below the critical value of 5.6–7.2 considered adequate for cocoa growth. The texture of the soils was generally sandy loam indicating that the water retention characteristics of the soils might be poor. Provision of irrigation facilities especially in the dry season (November to February) would be needed to ensure sufficient moisture in the soil throughout the cropping season. As noted by Abo-Hamed, Collin, and Hardwick (Citation1983), abscisic acids accumulation is high under internal moisture stress conditions and this prevents the development of new leaf flushes. The soils could be better managed by ensuring that there is adequate desirable shade tree species on the farms, while the floor was covered with leaf litter falls to serve as preventive measures against loss of soil water through evaporation (Ofori-Frimpong, Afrifa, and Acquaye Citation2010; Asare Citation2016).
5. Conclusion
The study shows the importance of cocoa plantations in storing carbon. The 23 years old cocoa plantation gave the highest SCS implied the importance of long-term cocoa plantations for C storage. There were differential variations in some of the measured soil nutrient parameters such as N, P, K, Ca, and Mg. Among five different cocoa plantation ages, only TN, available P, and exchangeable K increased significantly after 23 years of cocoa plantations compared to 5 and 10 years. However, differential variations in soil C storage and some of the soil nutrient parameters are needed to study in the future for understanding how much chemical fertilizer application rates would be more appropriate for different cocoa age stands and whether long-term effect would be increased under more than 23 years.
Acknowledgments
The authors are grateful to the technical staff of the Soil Science Division of CRIG for assisting with the soil sampling and analyses. This paper is published with the kind permission of the Executive Director of CRIG.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Abo-Hamed, S., H. A. Collin, and K. Hardwick. 1983. “Biochemical and Physiological Aspects of Leaf Development in Cocoa (Theobroma Cacao L.) VII. Growth, Orientation, Surface Structure and Water Loss from Developing Flush Leaves.” New Phytologist 95 (1): 9–17. doi:https://doi.org/10.1111/j.1469-8137.1983.tb03463.x.
- Adu, S. V., and R. D. Asiamah. 1992. Soils of the Ayensu-Densu Basin, Memoir No. 9. Kumasi, Ghana: Soil Research Institute/Council for Scientific and Industrial Research.
- Ahenkorah, Y., B. J. Halm, M. R. Appiah, G. S. Akrofi, and J. E. K. Yirenkyi. 1987. “Twenty Years’ Results from a Shade and Fertilizer Trial on Amazon Cocoa (Theobroma Cacao) in Ghana.” Experimental Agriculture 23: 31–39. doi:https://doi.org/10.1017/S0014479700003380.
- Ahenkorah, Y. 1981. “The Influence of Environment on Growth and Production of the Cacao Tree: Soils and Nutrition.” In Proceedings of the 7th International Cocoa Research Conference, 1979, 167–176. Douala, Cameroon.
- Appiah, M. R., S. T. Sackey, K. Ofori-Frimpong, and A. A. Afrifa. 1997. “The Consequences of Cocoa Production on Soil Fertility in Ghana – A Review.” Ghana Journal of Agricultural Science 30: 183–190. doi:https://doi.org/10.4314/gjas.v30i2.1970.
- Asare, R. 2016. The Relationship between On-farm Shade Trees and Cocoa Yields in Ghana, 1–46. Department of Geosciences and Natural Resource Management, University of Copenhagen, IGN Rapport.
- Asumadu-Sarkodie, S., and P. A. Owusu. 2016. “The Relationship between Carbon Dioxide and Agriculture in Ghana: A Comparison of VECM and ARDL Model.” Environmental Science and Pollution Research 23 (11): 10968–10982. doi:https://doi.org/10.1007/s11356-016-6252-x.
- Chagas, T., R. O’Sullivan, C. Bracer, and C. Streck. 2010. “Consolidating National REDD + Accounting and Subnational Activities in Ghana. UNDP.” Global Environment Facility, NORAD, and Gordon and Betty Moore Foundation 2010: 36.
- Cheng, W. 2020. “Soil Carbon and Nitrogen Dynamics by Land Use and Management Changes in East and Southeast Asian Countries (Soil C and N by LUMC).” Soil Science and Plant Nutrition 66 (1): 34–36. doi:https://doi.org/10.1080/00380768.2020.1718923.
- Dawoe, E. 2009. “Conversion of Natural Forest to Cocoa Agroforest in Lowland Humid Ghana: Impact on Plant Biomass Production, Organic Carbon and Nutrient Dynamics.”279. Ph.D. Thesis, Kwame Nkrumah University of Science and Technology.
- Dixon, R. 1995. “Agroforestry Systems: Sources of Sinks of Greenhouse Gases?” Agroforestry System 31 (2): 99–116. doi:https://doi.org/10.1007/BF00711719.
- Dossa, J. E., A. Arthur, W. Dogbe, A. Mando, A. A. Afrifa, and S. Acquaye. 2018. “An Assessment of Inherent Chemical Properties of Soils for Balanced Fertilizer Recommendations for Cocoa in Ghana .” In Improving the Profitability, Sustainability and Efficiency of Nutrients through Site Specific Fertilizer Recommendations in West Africa, Agro-Ecosystems, edited by Bationo A., Ngaradoum D., Youl S., Lompo F., Fening J., 325–336. Cham: Springer. https://doi.org/https://doi.org/10.1007/978-3-319-58789-9_18I
- Egbe, N. E., E. A. Ayodele, and C. R. Obatolu. 1989. “Soils and Nutrition of Cacao, Coffee, Kola, Cashew and Tea.“ In: Progress in Tree Crop Research in Nigeria, 2nd Edition, 27–38. CRIN Ibadan.
- Gockowski, J., and D. J. Sonwa. 2010. “Cocoa Intensification Scenarios and Their Predicted Impact on CO2 Emissions, Biodiversity Conservation, and Rural Livelihoods in the Guinea Rain Forest of West Africa.” Environmental Management 8 (2): 307–321.
- Hartemink, A. E. 2005. “Nutrient Stocks, Nutrient Cycling and Soil Changes in Cocoa Ecosystems: A Review.” Advances in Agronomy 8: 227–253.
- Inubushi, K., M. Yashima, S. Hanazawa, A. Goto, K. Miyamoto, T. Tsuboi, and G. Asea. 2020. “Long-term Fertilizer Management in NERICA Cultivated Upland Affects on Soil Bio-chemical Properties.” Soil Science and Plant Nutrition 66 (1): 247–253. doi:https://doi.org/10.1080/00380768.2019.1705738.
- Isaac, M. E., A. Gordon, N. Thevathasan, S. Oppong, and J. Quashie-Sam. 2005. “Temporal Changes in Soil Carbon and Nitrogen in West African Multistrata Agroforestry Systems: A Chronosequence of Pools and Fluxes.” Agroforestry System 65 (1): 23–31. doi:https://doi.org/10.1007/s10457-004-4187-6.
- Isaac, M. E., V. R. Timmer, and S. J. Quashie-Sam. 2007. “Shade Tree Effects in an 8-year-old Cocoa Agroforestry System: Biomass and Nutrient Diagnosis of Theobroma Cacao by Vector Analysis.” Nutrient Cycling in Agroecosystem 78 (2): 155–165. doi:https://doi.org/10.1007/s10705-006-9081-3.
- IUSS Working Group WRB. 2014. “International Soil Classification System for Naming Soils and Creating Legends for Soil Maps.” World Soil Resources Reports No. 106. Rome: FAO.
- Jobbagy, E. G., and R. B. Jackson. 2000. “The Vertical Distribution of Soil Organic Carbon and Its Relation to Climate and Vegetation.” Ecological Applications 10 (2): 423–436. doi:https://doi.org/10.1890/1051-0761(2000)010[0423:TVDOSO]2.0.CO;2.
- Kautsar, V., W. Cheng, K. Tawaraya, S. Yamada, K. Toriyama, and K. Kobayashi. 2020. “Carbon and Nitrogen Stocks and Their Mineralization Potentials are Higher under Organic than Conventional Farming Practices in Japanese Andosols.” Soil Science and Plant Nutrition 66: 144–151. doi:https://doi.org/10.1080/00380768.2019.1705739.
- Lal, R. 2020. “Managing Soils for Negative Feedback to Climate Change and Positive Impact on Food and Nutritional Security.” Soil Science and Plant Nutrition 66: 1–6. doi:https://doi.org/10.1080/00380768.2020.1718548.
- Ling, A. H. 1984. “Litter Production and Nutrient Cycling in a Mature Cocoa Plantation on Inland Soils of Peninsular Malaysia.” In Proceedings of International Conference on Cocoa and Coconuts: Progress and Outlook, edited by E. Pushparajah, and C. P. Soon, (451–465. Kuala Lumpar, Malaysia: The Incorporated Society of Planters.
- Mohammed, A. M., J. S. Robinson, D. Midmore, and A. Verhoef. 2016. “Carbon Storage in Ghanaian Cocoa Ecosystems.” Carbon Balance and Management 11 (6): 1–8. doi:https://doi.org/10.1186/s13021-016-0045-x.
- Monroe, P. H. M., E. F. Gama-Rodrigues, A. C. Gama-Rodrigues, and J. R. B. Marques. 2016. “Soil Carbon Stocks and Origin under Different Cacao Agroforestry Systems in Southern Bahia, Brazil.” Agriculture, Ecosystems and Environment 221: 99–108. doi:https://doi.org/10.1016/j.agee.2016.01.022.
- Norgrove, L., and S. Hauser. 2013. “Carbon Stocks in Shaded Theobroma Cacao Farms and Adjacent Secondary Forests of Similar Age in Cameroon.” Tropical Ecology 54 (1): 15–22.
- Ofori-Frimpong, K., A. A. Afrifa, and S. Acquaye. 2010. “Impact of Shade and Cocoa Plant Densities on Soil Organic Carbon Sequestration Rates in a Cocoa Growing Soil of Ghana.” African Journal of Environmental Science and Technology 4 (9): 621–624.
- Sparks, D. L., A. L. Page, P. A. Helmke, R. H. Loeppert, P. N. Soltanpour, M. A. Tabatabai, and C. T. Johnston. 1996. “Methods of Soil Analysis, Part 3.” SSSA Book series No. 5. Madison, USA: Soil Science Society of America and America Society of Agronomy.
- Stoorvogel, J. J., and E. M. A. Smaling. 1990. “Assessment of Soil Nutrient Depletion in Sub-Saharan Africa. 1983 – 2000.” Report No. 28 Vol. 114. Wageningen, The Netherlands: Winand Staring Center.
- Umrani, R., and C. K. Jain. 2010. Agroforestry: Systems and Practices. Jaipur, Rajasthan, India: Oxford Book Company.
- Vermeulen, S. J., B. M. Campbell, and J. S. I. Ingram. 2012. “Climate Change and Food Systems.” Annual Review of Environmental Resources 37 (1): 195–222. doi:https://doi.org/10.1146/annurev-environ-020411-130608.