ABSTRACT
Arbuscular mycorrhizal (AM) fungi form symbiotic associations with land plants and supply soil minerals including phosphorus to their hosts. AM fungi accumulate polyphosphate (polyP), a linear phosphate polymer, in their mycelia, which functions in phosphorus storage and translocation. In the budding yeast Saccharomyces cerevisiae, it has been demonstrated that the vacuolar transporter chaperone 4 (VTC4) protein, a subunit of the VTC complex, is responsible for polyP synthesis. Here, we conducted a comprehensive survey of VTC proteins in eight AM fungal genomes by Blast analysis and characterized the biochemical properties of the Rhizophagus irregularis VTC4. The genomes of AM fungal species encode VTC1, VTC2, and VTC4. The recombinant protein RiVTC4* (RiVTC4183–474) containing the catalytic tunnel domain was expressed in E. coli cells and purified. RiVTC4* is capable of catalyzing polyP polymerization using ATP as a substrate. Pyrophosphate enhanced polyP-polymerizing activity >10-fold. RiVTC4* exhibited maximum activity at neutral pH and required divalent metal ions, preferentially Mn2+. In the presence of high concentrations of ADP, the reverse reaction (the regeneration of ATP from polyP) by RiVTC4* occurred. In the range of 0.2–5 mM ADP, polyP depolymerization by the reverse reaction was observed at the ATP/ADP ratio of less than 2–5. These results suggest that AM fungal VTC4 not only synthesizes polyP but also regenerates ATP from polyP and ADP, which has potential implications for the modulation of polyP and ATP levels in AM fungi.
1. Introduction
Arbuscular mycorrhizal (AM) fungi belonging to the subphylum Glomeromycotina (Spatafora et al. Citation2016) are symbiotic microorganisms that form mutualistic relationships with most land plants (Brundrett and Tedersoo Citation2018). In exchange for plant carbon, AM fungi provide mineral nutrients, especially phosphorus (P), to the host plants via hyphae that interconnect the roots and the surrounding soil (Smith and Read Citation2008). The improvement of plant P nutrition through AM symbiosis is particularly important under P-deficient conditions.
Inorganic orthophosphate (Pi) in the soil is taken up by extraradical hyphae of AM fungi through Pi transporters localized on the plasma membrane (Harrison and van Buuren Citation1995; Maldonado-Mendoza, Dewbre, and Harrison Citation2001; Benedetto et al. Citation2005), rapidly polymerized into inorganic polyphosphate (polyP), a linear polymer of Pi, and then accumulated in the tubular vacuoles and cell walls (Uetake et al. Citation2002; Ezawa et al. Citation2004; Kuga et al. Citation2008; Hijikata et al. Citation2010; Kikuchi et al. Citation2014). Vacuolar polyP is dynamically turned over through depolymerization, export to the cytosol, incorporation into ATP, and polymerization, after which it is translocated to the roots, likely by water flow mediated by fungal aquaporins (Kikuchi et al. Citation2014, Citation2016). PolyP is depolymerized in arbuscules, highly branched hyphal termini formed in plant cortical cells, and then transferred to the host plants across the periarbuscular space (Solaiman et al. Citation1999; Viereck, Hansen, and Jakobsen Citation2004; Ohtomo and Saito Citation2005; Takanishi et al. Citation2009). Although the molecular mechanisms underlying Pi export from AM fungi are largely unknown, several hypothetical pathways have been proposed. The first hypothesis is that Pi released by hydrolysis of polyP is exported by Pi exporters on the fungal plasma membrane or by Golgi/trans-Golgi network-mediated vesicle trafficking (Saito and Ezawa Citation2016; Ezawa and Saito Citation2018). The second hypothesis is that polyP is directly exported from arbuscules (Saito and Ezawa Citation2016; Nguyen and Saito Citation2021). PolyP metabolism is an important for symbiotic P delivery, but the molecular mechanism underlying polyP synthesis and degradation in AM fungi has not yet been fully elucidated.
PolyP metabolism has been extensively studied in prokaryotes. The polyphosphate kinases PPK1 and PPK2 that are widely distributed in Gram-negative bacteria catalyze polyP biosynthesis using ATP as a substrate (forward reaction) and also ATP generation using polyP as a phosphate donor (reverse reaction) (Rao, Gómez-García, and Kornberg Citation2009). In eukaryotes, polyP metabolic enzymes have been well documented in the budding yeast Saccharomyces cerevisiae (Secco et al. Citation2012; Azevedo and Saiardi Citation2017; Austin and Mayer Citation2020; Denoncourt and Downey Citation2021). Degradation of vacuolar polyP is mediated by the endopolyphosphatases PPN1 (Kumble and Kornberg Citation1996; Shi and Kornberg Citation2005) and PPN2 (Gerasimaitė and Mayer Citation2017) that show both exo– and endopolyphosphatase activities. The NUDIX-family hydrolase DDP1, originally identified as inositol pyrophosphate phosphatase (Safrany et al. Citation1998), also shows endopolyphosphatase activity (Safrany et al. Citation1998; Lonetti et al. Citation2011). S. cerevisiae possesses the cytosolic exopolyphosphatase PPX1, but its function is unclear (Wurst and Kornberg Citation1994; Wurst, Shiba, and Kornberg Citation1995). Despite extensive studies on polyP-degrading enzymes in the past decades, polyP-polymerizing enzymes in eukaryotes have only recently been identified in the yeast. The vacuolar transporter chaperone 4 (VTC4) is responsible for the biosynthesis of polyP by transferring the γ (gamma)-Pi of ATP to the acceptor Pi or the terminal Pi residue of polyP (Hothorn et al. Citation2009). VTC4 that forms a complex with VTC1 and VTC3 (VTC1/3/4 complex) is localized in the tonoplast, whereas the VTC1/2/4 complex is mainly present in the endoplasmic reticulum (Hothorn et al. Citation2009). VTC4 contains an SYG1/Pho81/XPR1 (SPX) domain at the N-terminus, a catalytic tunnel domain, and a transmembrane domain at the C-terminus, with the first two domains facing the cytoplasm (Muller et al. Citation2003; Wild et al. Citation2016). VTC2 and VTC3 show a domain structure similar to that of VTC4, but their tunnel domains lack the functional active sites. VTC1 consists only of a transmembrane domain. VTC5 was identified as an accessory protein that associates with the VTC complex and enhances the polyP-polymerizing activity (Desfougères et al. Citation2016). The VTC complex embedded in the tonoplast synthesizes polyP using ATP in the cytosol as a substrate and simultaneously translocates the polymer into the vacuoles, which requires an electrochemical gradient generated by the vacuolar H+-ATPase (Hothorn et al. Citation2009; Gerasimaitė et al. Citation2014).
VTC proteins are widely present in fungi, protozoan parasites, and green algae and play significant roles in various cellular processes (Rooney et al. Citation2011; Gomes-Vieira et al. Citation2018; Austin and Mayer Citation2020; Denoncourt and Downey Citation2021; Wang et al. Citation2021). Null mutants of yeast VTC proteins not only reduce polyP levels (Ogawa, DeRisi, and Brown Citation2000) but also affect membrane transport and vesicular traffic (Cohen et al. Citation1999; Murray and Johnson Citation2000; Müller et al. Citation2002; Muller et al. Citation2003; Uttenweiler et al. Citation2006). In the phytopathogenic fungus Ustilago maydis, VTC4 is involved in fungal morphogenesis and virulence in maize (Boyce, Kretschmer, and Kronstad Citation2006). VTC4 knockout and knockdown mutants in protozoan parasites decrease polyP accumulation in the acidocalcisomes, which influences the infectivity of the parasites in the host (Lander, Ulrich, and Docampo Citation2013; Ulrich et al. Citation2014; Kohl et al. Citation2018). In the green algae Chlamydomonas reinhardtii, polyP polymerization by VTC proteins affects acclimation to sulfur deprivation and cellular ATP homeostasis (Aksoy, Pootakham, and Grossman Citation2014; Sanz-Luque et al. Citation2020).
AM fungi can accumulate a massive amount of polyP (> 60% of total cellular P) without perturbation of cytosolic Pi levels, which probably provide a large P pool for supplying P to their host plants (Hijikata et al. Citation2010). Tani et al. (Citation2009) demonstrated that AM fungus Rhizophagus clarus possesses polyP-polymerizing activity using ATP as a direct substrate in an organelle fraction. The AM fungi R. clarus and R. irregularis have three genes encoding VTC1, VTC2, and VTC4 in their genomes (Tisserant et al. Citation2013; Ezawa and Saito Citation2018), and the transcript levels of these genes in extraradical mycelia of R. clarus were increased by applying Pi to the mycelia (Kikuchi et al. Citation2014; Ezawa and Saito Citation2018). These findings suggest that AM fungal VTCs are responsible for polyP synthesis, but there has been no direct evidence for their biochemical activity. In this study, we characterized the biochemical properties of a recombinant catalytic domain of R. irregularis VTC4, with emphasis on the kinetics of the forward (polyP polymerization) and reverse (polyP depolymerization) reactions.
2. Materials and methods
2.1. Expression and purification of recombinant proteins
The gene encoding the catalytic domain of R. irregularis VTC4 (from glycine 183 to glutamine 474, RiVTC4*) was amplified from cDNA of R. irregularis colonizing Lotus japonicus roots by PCR with the primers 5’-AACTGCAGGCCAACAACAAAATTTTGTTCG-3’ and 5’-CGGAATTCCTATTGTGGAAGCCAGAAAGGA-3’. The PCR product was first inserted into pGEM-T (Promega, Madison, WI, USA) to create pGEM-RiVTC4*. The gene was then inserted into pTrcHisB (Invitrogen, Carlsbad, CA, USA), from the PstI (5’) to the EcoRI (3’) site, yielding pTrc-HisB-RiVTC4*. E. coli ArcticExpress (DE3) RIL (Agilent Technologies, Santa Clara, CA, USA) harboring pTrc-HisB-RiVTC4* was cultured in 250 ml Terrific Broth with 50 µg ml−1 carbenicillin at 30°C. The culture was induced with 0.1 mM isopropylthio-β-D-galactoside at an OD600 of 0.5 at 18°C. After incubation for 19 h, cells were harvested by centrifugation and resuspended in 14 ml lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 48 mM imidazole, and 0.5 mM phenylmethylsulfonyl fluoride). Bacterial cells were lysed using a sonicator and centrifuged at 15,300 × g for 10 min at 4°C. The supernatant was filtered through a 0.45-µm cellulose acetate membrane filter (Advantec, Tokyo, Japan). The crude extract was loaded onto a HisTrap HP column (Cytiva, Tokyo, Japan), washed with 50 column volumes of buffer A (50 mM Tris-HCl pH 7.4, 150 mM NaCl, and 70 mM imidazole), and the recombinant protein was placed on a step gradient against buffer B (50 mM Tris-HCl pH 7.4, 150 mM NaCl, and 300 mM imidazole). The buffer was changed to 50 mM Tris-HCl pH 7.4 using a PD-10 column (Cytiva). The recombinant protein was concentrated using an Amicon Ultra-4 10 K centrifugal unit (Merck, Kenilworth, NJ, USA). Protein concentrations were measured using the Qubit Protein Assay Kit (Invitrogen). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Oriole (Bio-Rad, Hercules, CA, USA) were performed.
2.2. Assays for polyP-polymerizing reaction
The polyP-polymerizing activity of RiVTC4* was assayed in triplicate at 24°C in a reaction mixture (10 µl) containing 50 mM PIPES-KOH pH 6.8, 150 mM NaCl, 1 mM MnCl2, 0.005–7.5 mM ATP-Na (Oriental Yeast, Tokyo, Japan), and 200 nM RiVTC4*. When indicated, 5 mM sodium pyrophosphate was added to the reaction. The reaction was initiated by the addition of ATP and terminated with 1 µl of 0.15 M EDTA after 60 min. The amount of ADP generated by ATP hydrolysis during polyP polymerization was determined using the Fluorospark Kinase/ADP Multi-Assay Kit (FUJIFILM Wako Chemicals, Osaka, Japan) following the manufacturer’s instructions after the reaction solution was diluted five or ten times with MilliQ H2O. Fluorescence (excitation: 540 nm, emission: 590 nm) was measured using a microplate reader (Corona Electric, Ibaraki, Japan). One unit of polyP-polymerizing activity was defined as the amount of enzyme required to catalyze the generation of 1 μmol of ADP equivalents per min. The kinetic parameters (Vmax and apparent Km) were calculated by nonlinear regression using the function drm in the add-on package drc of R version 4.0.0 (Ritz et al. Citation2016). The enzyme kinetics at varying concentrations of disodium hydrogenphosphate, sodium pyrophosphate, sodium tripolyphosphate (Sigma-Aldrich), and short-chain polyP EXP-S (EXP-S, mean chain length 14-mer; RegeneTiss, Nagano, Japan) were also analyzed using 1 mM ATP under the same reaction conditions, since these polyP stimulate the polyP-polymerizing activity of ScVTC4, possibly by a priming effect (Hothorn et al. Citation2009; Lander, Ulrich, and Docampo Citation2013). To determine the optimum pH for polyP-polymerizing activity, the assay was performed at a pH range of 5.1 to 8.9. The reaction mixture contained 50 mM MES-KOH, PIPES-KOH, HEPES-KOH, or Tris-HCl buffer, 150 mM NaCl, 1 mM MnCl2, 1 mM ATP, 1 mM sodium pyrophosphate, and 50 nM RiVTC4*. Assays to examine the effects of metals (1 mM CoCl2, MgCl2, MnCl2, NiCl2, and ZnCl2) and 1 mM EDTA on the enzyme activity were conducted under the same reaction conditions as for the pH assay, except that PIPES-KOH pH 6.8 was added to a final concentration of 50 mM. To determine the polyP chain length produced by the polyP-polymerizing reaction, RiVTC4* proteins (1 μM final concentration) were assayed at 24°C for 1, 2, 4, and 8 h in an ATP-regenerating mixture (50 mM PIPES-KOH pH 6.8, 150 mM NaCl, 1 mM MnCl2, 1 mM ATP, 40 mM creatine phosphate, and 50 IU ml−1 creatine kinase [Oriental Yeast]) with 1 mM sodium pyrophosphate or 0.1 mM (as Pi) of the fractionated polyP PP5 with a mean chain length of 37-mer (Ohtomo et al. Citation2008). The synthesized polyP was analyzed by polyacrylamide gel electrophoresis (PAGE) as described below. The effects of ADP on polyP kinase activity were determined by PAGE after incubating 1 μM RiVTC4* at 24°C for 4 h in a reaction mixture containing 50 mM PIPES-KOH pH 6.8, 150 mM NaCl, 1 mM MnCl2, 1 mM sodium pyrophosphate, 1 mM ATP, and the indicated concentrations of ADP (0–2 mM).
2.3. Assays for polyP-depolymerizing reaction
The polyP-depolymerizing reaction to produce ATP from polyP and ADP by RiVTC4* was determined. The reaction mixture contained 50 mM PIPES-KOH pH 6.8, 150 mM NaCl, 1 mM MnCl2, 0.125–25.6 mM ADP-Na (Oriental Yeast), 0.4–500 µM (as Pi) EXP-S, and 50 or 100 nM RiVTC4* in a final volume of 10 µl. Six independent reactions were performed for each enzyme assay. The reaction was initiated by the addition of ADP at 24°C and terminated by heating at 70°C for 5 min. The reaction time was 60 min. After the reaction solution was diluted with four volumes of 250 mM Tricine-KOH pH 7.8, ATP concentration was determined using an ATP Bioluminescence Assay Kit CLS II (Roche, Mannheim, Germany) following the manufacturer’s instructions. Since luciferase activity is known to be affected by pyrophosphate and tripolyphosphate (Fontes et al. Citation2008), an ATP standard curve was prepared for each concentration of polyP. One unit of polyP-depolymerizing activity was defined as the amount of enzyme that catalyzes the generation of 1 μmol of ATP equivalents per min. The apparent optimal pH for the polyP-depolymerizing activity was determined by incubating the enzyme in 50 mM MES-KOH, PIPES-KOH, HEPES-KOH, and Tris-HCl buffer. As substrates, 5 mM ADP and 0.5 mM (as Pi) EXP-S were included in the reactions. An assay to examine the effects of divalent metal cations (1 mM CoCl2, MgCl2, MnCl2, NiCl2, and ZnCl2) and 1 mM EDTA on polyP-depolymerizing activity was performed. To monitor polyP chain length during the reaction, 1 mM (as Pi) EXP-S and 1 mM long-chain polyP EXP-L (EXP-L, mean chain length 130-mer; RegeneTiss) were incubated with 100 nM RiVTC4* at 24°C in an ADP-regenerating mixture (50 mM PIPES-KOH pH 6.8, 150 mM NaCl, 1 mM MnCl2, 5 mM ADP, 50 mM glucose, and 50 IU ml−1 hexose kinase [Oriental Yeast]) and separated by PAGE as described below. To detect Pi, pyrophosphate, and tripolyphosphate generated during the polyP-depolymerizing reaction, these P compounds contained in solutions incubated with 10 mM (as Pi) EXP-S and 500 nM RiVTC4* in the ADP-regenerating mixture were separated by PAGE and thin-layer chromatography (TLC) as described below.
2.4. Effects of ATP:ADP ratios on polyP polymerization and depolymerization
EXP-S (1 mM as Pi) were incubated at 24°C for 4 h in a reaction solution containing 50 mM PIPES-KOH pH 6.8, 150 mM NaCl, 1 mM MnCl2, 1 μM RiVTC4*, and the indicated concentrations of ATP (0–10 mM) and ADP (0–5 mM). Control experiments without enzyme addition were also performed in parallel. PolyP was separated by PAGE as described below.
2.5. Electrophoresis of polyP
Solutions containing polyP were neutralized with a NaOH solution after adding phenolphthalein indicator, and then mixed with 5× loading dye solution (5× Tris-borate-EDTA [TBE], 15% Ficoll 400, and 0.1% bromophenol blue [BPB] or orange G). Samples and the polyP size markers PP4, PP5, and PP6 (Ohtomo et al. Citation2008) were loaded onto polyacrylamide gels (33 or 15% acrylamide:bis-acrylamide (19:1), 1× TBE, 0.07% ammonium persulfate, 0.08% TEMED). The running buffer used was a 1× TBE. After a pre-run at 300 V for 1 h, electrophoresis was performed at 300 V until BPB migrated 4.5 cm from the top of the gel. The gel was stained with 2 µg ml−1 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) in a fixative solution (25% methanol, 5% glycerol, and 50 mM Tris) for 30 min and destained in the fixative solution for 15 min (Smith and Morrissey Citation2007). The gels were then exposed to 360 nm light on a UV transilluminator for several minutes to induce photobleaching, after which digital images of DAPI negative staining for polyP were captured (Smith and Morrissey Citation2007).
2.6. TLC of polyP
Pi, pyrophosphate, tripolyphosphate, and longer polyPs were separated by TLC according to Scott and Haight (Citation1975). After the polyP-depolymerizing assay with 10 mM EXP-S, the solutions were neutralized with NaOH solution after adding the phenolphthalein indicator. Samples (1 µl) were spotted 2 cm from the bottom of a 7-cm PEI cellulose F 25 plastic sheet (Merck, Darmstadt, Germany). We also spotted 3.33 nmol disodium hydrogenphosphate, 1.66 nmol sodium pyrophosphate, and 1 nmol sodium tripolyphosphate as markers. Ascending chromatography was performed using a developing solvent containing 1.5 M LiCl and 1 M formic acid. After drying, the TLC sheets were sequentially sprayed with 1% ammonium heptamolybdate, 10% trichloroacetic acid, and 2% ascorbic acid. The sheets were then placed in an oven at 100°C for 3 min.
2.7. Gene expression analysis
Gene expression analysis was performed as previously described (Kobae et al. Citation2015). Briefly, Lotus japonicus B-129 ‘Gifu’ was grown in a mesh bag culture system (Kikuchi et al. Citation2014), in which mycorrhizal and hyphal compartments were separated by a cone-shaped nylon mesh bag. Plants were inoculated with 1,000 spores of R. irregularis DAOM197198 and cultivated in a growth chamber at 25°C with a 16 h day/8 h night cycle for four weeks. Mycorrhizal roots and extraradical mycelia were harvested from the mycorrhizal and hyphal compartments, respectively. Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Genomic DNA was digested using RNase-free DNase I (Qiagen) and Turbo DNA-free (Thermo Fisher Scientific, Waltham, MA, USA). First-strand cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Quantitative PCR was performed using a StepOne Real-Time PCR System (Thermo Fisher Scientific) with the Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) and the primers RiVTC1 (5’-AGTACGGGGAACATTTTT-3’ and 5’-GAAAATAGCAAATTGATCGAAA-3’), RiVTC2 (5’-GGTTCGCGAGGATAATTTTGAC-3’ and 5’-TTGCGACGCCACTCATCTT-3’), and RiVTC4 (5’-CATGGTGTCGCAACACTTATGG-3’ and 5’-GTGGAAGCCAGAAAGGAAAAAG-3’). Expression levels were normalized to the quantity of R. irregularis EF1β (Kobae et al. Citation2015). All reactions were performed using three biological replicates. Expression of the VTC genes between intraradical and extraradical mycelia was analyzed using Welch’s t-test.
2.8. Bioinformatics analysis
Amino acid sequences of AM fungal VTC proteins were obtained by BlastP searches against AM fungal genome databases () using S. cerevisiae VTC1–VTC5 as queries (https://www.yeastgenome.org). In these searches, those that showed similarity to VTC3 were not considered because ScVTC2 and ScVTC3 were derived by a recent duplication in the lineage, thus sharing high sequence similarity (Gomes-Vieira et al. Citation2018). InterPro (https://www.ebi.ac.uk/interpro/) was used to predict the domain of VTC proteins in R. irregularis. The transmembrane region was predicted using TMHMM (http://www.cbs.dtu.dk/services/TMHMM/). Amino acid sequences of AM fungal and S. cerevisiae VTC proteins were aligned using ClustalW with manual adjustments. Maximum-likelihood tree inference and bootstrapping with 100 replicates were performed with RAxML-NG version 1.0.1 (Kozlov et al. Citation2019) using the LG+I + G amino acid substitution model. The best-fitted evolutionary model was identified using ModelTest-NG version 0.1.3 (Darriba et al. Citation2020). Trees were visualized and annotated using the iTOL platform version 5.7 (Letunic and Bork Citation2019). The alignment and tree files presented were deposited in the Dryad Digital Repository (10.5061/dryad.ghx3ffbp7).
Table 1. List of VTC protein sequences in arbuscular mycorrhizal fungi and Saccharomyces cerevisiae.
3. Results
3.1. AM fungal VTCs
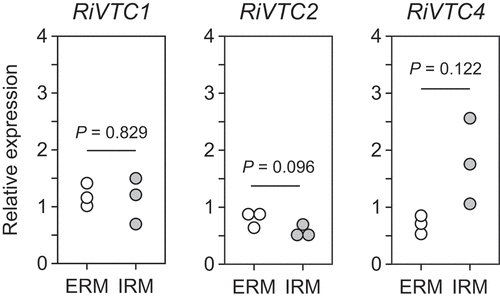
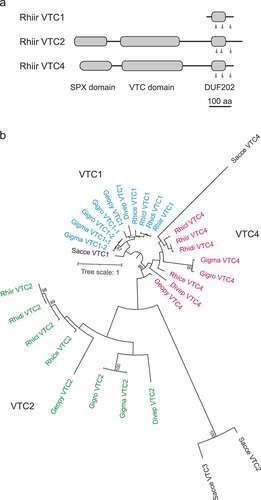
AM fungi have homologs of ScVTC1, ScVTC2, and ScVTC4, but not ScVTC5, in their genome (). Gigaspora margarita and G. rosea possess two ScVTC1 homologs, whereas other fungi have only one, and single homologs of VTC2 and VTC4 were found in all fungi. The AM fungal VTCs share structural similarities with the ScVTCs. VTC1 consists of a DUF202 domain (IPR003807) with three transmembrane regions, while VTC2 and VTC4 possess an SPX domain (IPR004331) at the N-terminal, a VTC domain (IPR018966) with the catalytic tunnel domain in the middle, and a DUF202 domain at the C-terminal (). Key residues involved in polyP polymerizing activity of ScVTC4 are conserved among AM fungal VTC4 proteins (Figure S1). Maximum likelihood phylogenetic trees were drawn based on the shared C-terminal sequences, indicating that there are three clades corresponding to VTC1, VTC2, and VTC4 in the fungi (). The tree also suggests that the two homologs of VTC1 in the two Gigaspora spp. likely arose from a gene duplication event after speciation. Transcripts of VTC1, VTC2, and VTC4 were detected in both extraradical and intraradical mycelia of R. irregularis, and their expression levels were not significantly different between the mycelia, confirming that all three genes were expressed in this fungus ().
Figure 1. Phylogenetic analysis of VTC proteins in arbuscular mycorrhizal fungi. (a) Domain structures of VTC1, VTC2, and VCT4 in Rhizophagus irregularis. Arrowheads show transmembrane regions predicted by TMHMM search. Scale: 100 amino acids. (b) Maximum likelihood tree of C-terminal sequences containing DUF202 domain of VTC1, VTC2, and VTC4 in eight arbuscular mycorrhizal fungi and Saccharomyces cerevisiae. Bootstrap values (from 100 replicates) greater than 75% are shown next to the branches. Accession no. of VTC protein sequences used in these analyses were listed in . Abbreviations of the species names are: Rhice, R. cerebriforme; Rhicl, R. clarus; Rhidi, R. diaphanus; Rhiir, R. irregularis; Divep, Diversispora epigaea; Gigma, Gigaspora margarita; Gigro, G. rosea; Geopy, Geosiphon pyriformis; Sacce, S. cerevisiae.
3.2. Isolation of recombinant RiVTC4* protein
To examine the biochemical properties of R. irregularis VTC4, we produced recombinant RiVTC4* (RiVTC4183–474) containing the catalytic tunnel domain. Initially, we used the bacterial strain ArcticExpress which expresses chaperones at low temperatures to produce soluble RiVCT4* proteins. However, RiVTC4* was strongly bound to the chaperones, which interfered with the analysis of its biochemical properties. A strain lacking the chaperone plasmid was obtained from the ArcticExpress colonies harboring pTrc-HisB-RiVTC4*, resulting in the production of soluble protein with polyP-polymerizing activity. The RiVTC4* protein purified using an Ni2+-charged affinity column showed a single band with SDS-PAGE that had a molecular mass of approximately 38.5 kDa, which is close to the predicted size (Figure S2).
3.3. PolyP-polymerizing activity of RiVTC4*
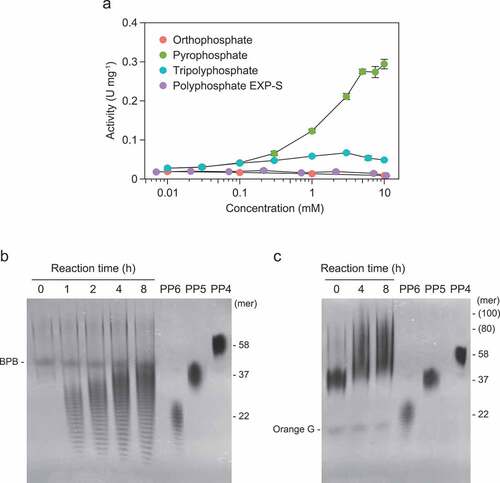
Pyrophosphate increased the activity of RiVTC4* by up to 5 mM (). Tripolyphosphate slightly stimulated the reaction, but Pi and EXP-S had no effect. The addition of pyrophosphate resulted in the synthesis of short-chain polyP of various sizes below 35-mer within 1 h of incubation, with chain lengths of approximately 30-mer as the most abundant (). The chain length increased with incubation duration (up to 8 h) to 40–50-mer with a major size range of approximately 35-mer. Longer polymers (80–100-mer) were synthesized in the presence of PP5, indicating the priming effect of short-chain polyP on the polyP-polymerizing activity of RiVTC4* ().
Figure 3. Polyphosphate (polyP)-polymerizing reaction of RiVTC4*. (a) Effect of phosphate compounds on the polyP-polymerizing activity. RiVTC4* protein (200 nM) was incubated with 1 mM ATP and various concentrations of orthophosphate, pyrophosphate, tripolyphosphate, and polyP EXP-S for 60 min at pH 6.8. The molar concentration of polyP EXP-S was calculated based on its mean chain length of 14-mer. After stopping the reaction with EDTA, the concentration of ADP was determined. Other experimental conditions were as described in the ‘Materials and methods’ section. One unit (U) is defined as 1 μmol of ADP generated per min; units mg−1 of protein gives the specific activity. Mean ± SD, n = 3. (b, c) Time course of polyP polymerization by RiVTC4*. PolyP was synthesized by incubating 1 μM RiVTC4* with an ATP-regenerating system for the indicated periods. The reaction mixtures contained 1 mM pyrophosphate (b) or 0.1 mM (as phosphate) polyP PP5 with a mean chain length of 37-mer (c). The reaction mixtures were resolved on 33% polyacrylamide gels. PolyP was visualized with DAPI-negative staining. Numbers on the right side indicate chain lengths of the polyP size markers PP4, PP5, and PP6. Chain lengths in parentheses were estimated from the relative mobility of PP4–PP6 in the gel. BPB and Orange G are loading dyes. A representative gel of three independent experiments is shown.
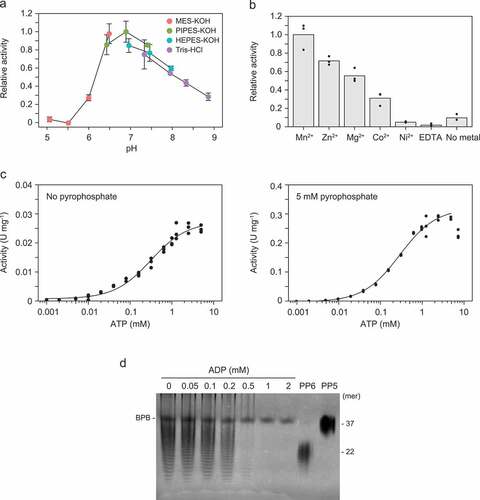
RiVTC4* exhibited higher activity in the neutral pH range of 6.5 to 7.5, with optimal activity at pH 6.9 (). The activity was highest in the presence of Mn2+ and was completely inhibited by the chelating agent EDTA (). We further investigated ATP (Pi donor)-dependence of the RiVTC4* polyP-polymerizing activity in the absence and presence of pyrophosphate. Km values for ATP were approximately 300 μM regardless of the presence of pyrophosphate, but the catalytic potency (Vmax and kcat) of the enzyme was >10 times greater in the presence of pyrophosphate ( and ). However, at 7.5 mM ATP, the activity of RiVTC4* was slightly decreased in the presence of pyrophosphate, suggesting product inhibition by the ADP generated from ATP hydrolysis. Indeed, this activity was inhibited at higher concentrations of ADP ().
Table 2. Km,Vmax, and kcat values of the forward (polyP polymerization) and reverse (ATP generation) reactions of Rhizophagus irregularis VTC4*
Figure 4. Kinetics of polyphosphate (polyP)-polymerizing reaction of RiVTC4*. (a) Optimum pH of the polyP-polymerizing activity. 1 mM pyrophosphate was included in all cases. Mean ± SD, n = 3. (b) Activation of the polyP-polymerizing activity by divalent metals. The final concentration of each metal ion and EDTA was 1 mM. In all cases, 1 mM pyrophosphate was included. Bars and dots show means and data points, respectively. (c) ATP dependence of the polyP-polymerizing activity in the presence or absence of pyrophosphate (5 mM). The activity was determined, and data were fitted between 0.001–7.5 mM ATP as indicated in the ‘Materials and methods’ section. (d) Inhibition by ADP to the polyP-polymerizing activity of RiVTC4*. RiVTC4* protein (1 μM) was incubated with various concentrations of ADP in the presence of 1 mM ATP and 1 mM pyrophosphate for 4 h. PolyP generated in the reaction mixture was analyzed by 33% polyacrylamide gels. Numbers on the right indicate the mean chain lengths of the polyP markers PP5 and PP6. BPB is a loading dye. A representative gel of three independent experiments is shown.
3.4. PolyP-depolymerizing activity of RiVTC4*
Both EXP-S and EXP-L were gradually depolymerized in the presence of 5 mM ADP and became undetectable by DAPI that visualizes 14-mer and longer polyPs (Smith, Wang, and Morrissey Citation2018) after 8 h of incubation with RiVTC4* (, b). In the TLC analysis, the staining intensity of polyP decreased with increasing incubation time, consistent with the PAGE analysis, in which tripolyphosphate appeared after 8 h and then disappeared after 24 h (). This led us to examine occurrence of the reverse reaction (ATP regeneration from polyP) by RiVTC4*, and thus the amount of ATP produced from ADP and EXP-S was measured. High activity was observed in the neutral pH range of 7–8 (). The pH profile in the alkaline range, however, could not be properly evaluated because of the very low activity in the HEPES-KOH and Tris-HCl buffer solutions. The enzyme showed the highest activity in the presence of the divalent metal cation Mn2+, which is consistent with the forward (polyP-polymerizing) reaction (). The Km of EXP-S (in terms of 14-mer polyP) was less than 0.1 µM with a Vmax of 0.048 U mg−1 protein in the presence of 5 mM ADP, whereas that of ADP was 2 mM with a Vmax of 0.069 U mg−1 protein in the presence of 0.5 mM EXP-S (, d and ). The apparent turnover rates (kcat) of the reverse reaction were 0.031 and 0.044 which were less than that of the forward reaction with 5 mM pyrophosphate, but two times higher than that without pyrophosphate (). Taken together, although the affinity of RiVTC4* for ADP in the reverse reaction is an order of magnitude lower than that for ATP in the forward reaction, the apparent turnover rates of the reverse reaction are within the range of those in the forward reaction, suggesting that the reverse reaction could proceed at certain ATP:ADP ratios in the cell. This issue is further addressed subsequently.
Figure 5. Polyphosphate (polyP)-depolymerizing activity of RiVTC4*. (a–c) Time course of polyP depolymerization by RiVTC4*. Long-chain polyP EXP-L (a) and short-chain polyP EXP-S (b, c) were incubated with RiVTC4* in an ADP-regenerating mixture for the indicated periods. The concentration of polyP was 1 mM (a, b) and 10 mM (c) as phosphate. Other experimental conditions were as described in the ‘Materials and methods’ section. After the reaction, polyP was resolved on 15% (a) or 33% (b, c) polyacrylamide gels (PAGE), and visualized with DAPI-negative staining. DNA markers (DNA) were also resolved to estimate the chain length of polyP according to Smith, Wang, and Morrissey (Citation2018). Mean chain lengths of the polyP size markers PP4 and PP5 were shown on the right side of the gels. BPB is a loading dye. (c) Orthophosphate (P1), pyrophosphate (P2), and tripolyphosphate (P3) were separated by thin-layer chromatography (TLC). M: P1–P3 markers. A representative gel and TLC sheet of three independent experiments are shown.
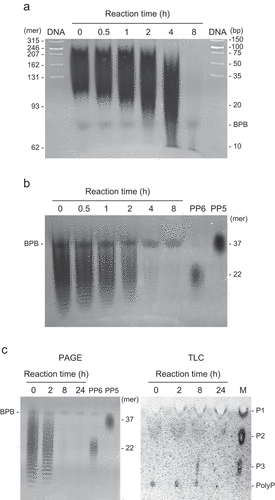
Figure 6. Kinetics of the reverse reaction (the regeneration of ATP from polyphosphate [polyP]) by RiVTC4*. (a) Optimum pH of the reverse reaction. RiVTC4* protein (100 nM) was incubated with 5 mM ADP and 1 mM polyP EXP-S at various pH levels. After stopping the reaction by heating, the concentration of ATP generated was determined. Other experimental conditions were as described in the ‘Materials and methods’ section. Mean ± SD, n = 6. (b) Activation of the reverse reaction by divalent metals. The final concentration of each metal ion and EDTA was 1 mM. In all cases, 5 mM ADP and 1 mM polyP EXP-S were included. Bars and dots show means and data points, respectively. (c) PolyP (EXP-S) dependence of the reverse reaction in the presence of 5 mM ADP. One unit (U) is defined as 1 μmol of ATP generated per min; units mg−1 of protein denotes the specific activity. The activity was determined, and data were fitted as indicated in the ‘Materials and methods’ section. (d) ADP dependence of the reverse reaction in the presence of 1 mM polyP EXP-S. Data were fitted between 0.25–7.59 mM ADP.
![Figure 6. Kinetics of the reverse reaction (the regeneration of ATP from polyphosphate [polyP]) by RiVTC4*. (a) Optimum pH of the reverse reaction. RiVTC4* protein (100 nM) was incubated with 5 mM ADP and 1 mM polyP EXP-S at various pH levels. After stopping the reaction by heating, the concentration of ATP generated was determined. Other experimental conditions were as described in the ‘Materials and methods’ section. Mean ± SD, n = 6. (b) Activation of the reverse reaction by divalent metals. The final concentration of each metal ion and EDTA was 1 mM. In all cases, 5 mM ADP and 1 mM polyP EXP-S were included. Bars and dots show means and data points, respectively. (c) PolyP (EXP-S) dependence of the reverse reaction in the presence of 5 mM ADP. One unit (U) is defined as 1 μmol of ATP generated per min; units mg−1 of protein denotes the specific activity. The activity was determined, and data were fitted as indicated in the ‘Materials and methods’ section. (d) ADP dependence of the reverse reaction in the presence of 1 mM polyP EXP-S. Data were fitted between 0.25–7.59 mM ADP.](/cms/asset/c256b447-6078-474b-b3be-710f78a425bc/tssp_a_2029220_f0006_oc.jpg)
3.5. ATP:ADP ratio drives reaction direction
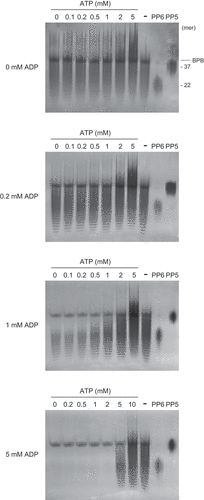
At 0 and 0.2 mM ADP, no polyP depolymerization was observed, and apparent polyP polymerization, that is, shifts in the chain length distribution of the products from those of the substrate EXP-S to higher molecular weights, were observed in the presence of 1 mM and higher ATP concentrations (). At 1 and 5 mM ADP, polyP polymerization occurred in the presence of 2 mM and higher and 10 mM ATP, respectively, but below these ATP concentrations, polyP depolymerization was observed. These results implied that the direction of the reaction was switched at ATP:ADP ratios of 2:1–5:1.
Figure 7. Effects of ATP and ADP ratios on polyphosphate (polyP) polymerizing and depolymerizing activity of RiVTC4*. RiVTC4* proteins (1 μM) were incubated with various combinations of ATP and ADP in the presence of 1 mM polyP EXP-S for 4 h. Control experiments without enzyme addition (-) were also performed in parallel. PolyP generated in the reaction mixture and the polyP markers PP5 and PP6 were analyzed by 33% polyacrylamide gels. Numbers on the right indicate the mean chain lengths of PP5 and PP6. BPB is a loading dye. Representative gels of three independent experiments are shown.
4. Discussion
Our experiments demonstrate that the catalytic domain of R. irregularis VTC4 is capable of synthesizing polyP using ATP as the phosphoryl donor and also of generating ATP using polyP as the phosphoryl donor; the VTC4 catalyzes both the forward and reverse reactions as the bacterial PPKs (Rao, Gómez-García, and Kornberg Citation2009). PolyP is the major form of P storage and translocation in AM fungi (Saito and Ezawa Citation2016). PolyP polymerization is prominent in extraradical mycelia (Ezawa et al. Citation2004; Tani et al. Citation2009), while depolymerization of polyP is observed in intraradical mycelia (Solaiman et al. Citation1999; Viereck, Hansen, and Jakobsen Citation2004; Ohtomo and Saito Citation2005; Takanishi et al. Citation2009). Our study suggests the involvement of VTC4 in both polymerization and depolymerization.
As expected, the enzymatic properties of RiVTC4* were similar to those of S. cerevisiae VTC4. RiVTC4* is likely to synthesize polyP in a non-processive manner because various chain lengths of polyP were generated during the reaction. This is consistent with ScVTC4 (Hothorn et al. Citation2009; Lander, Ulrich, and Docampo Citation2013), but in contrast to the bacterial PPKs that act in a processive mode (Robinson and Wood Citation1986; Zhang, Ishige, and Kornberg Citation2002; Ishige, Zhang, and Kornberg Citation2002). The other properties, including the acceleration of the forward reaction by pyrophosphate (i.e., the priming effect), the optimal pH in the neutral range, and the requirement of Mn2+ were also in agreement with those of ScVTC4 (Hothorn et al. Citation2009; Lander, Ulrich, and Docampo Citation2013). In ScVTC4, Mn2+ interacts with the α-, β-, and γ-Pi of ATP and Glu426 located in the tunnel center (Hothorn et al. Citation2009). The Glu residue corresponding to Glu426 of ScVTC4 is conserved among AM fungal VTC4 proteins (Figure S1), indicating the coordination of Mn2+ with ATP in their tunnel center in a similar manner to ScVTC4.
We demonstrated for the first time that VTC4 catalyzes the reverse reaction by using AM fungal VTC4. Given that the nucleotide substrate ATP is supplied in the cytosol in the forward reaction in S. cerevisiae (Muller et al. Citation2003; Hothorn et al. Citation2009), it is highly likely that the catalytic domain of RiVTC4 faces the cytosol. This idea is indirectly supported by our result that the optimum pH of the reaction was between 7 and 8, consistent with the cytosolic pH range of the fungi (Jolicoeur et al. Citation1998; Funamoto et al. Citation2015). Therefore, we consider that the direction of the reactions is dependent on the ATP:ADP concentration ratios in the cytosol. In S. cerevisiae, intracellular ATP ranges from submillimolar to millimolar concentrations and is only several-fold higher than that of ADP, and the ATP:ADP ratios vary, mainly depending on the availability of the carbon source (Walther et al. Citation2010; Ozalp et al. Citation2010; Nguyen et al. Citation2019; Takaine et al. Citation2019). Although there is no information about cytosolic ADP levels in AM fungi, ATP levels in the extraradical mycelia of R. clarus were estimated to be 10–20 nmol mg−1 protein, which was maintained even during polyP accumulation, suggesting that ATP homeostasis is tightly regulated in the mycelia (Kikuchi et al. Citation2016). Given that the polyP levels in extraradical mycelia are likely to be dynamically regulated by the balance between synthesis and degradation during translocation (Ezawa, Smith, and Smith Citation2001), the reverse reaction (i.e., ATP regeneration from polyP) by VTC4 could provide an energetically more favorable mechanism for polyP turnover than simple degradation by hydrolases such as PPN/PPX. The in vivo occurrence of the reverse reaction in AM fungi should be investigated in the future.
The absence of the SPX domain in the truncated RiVTC4 (i.e., RiVTC4*) that consists only of the catalytic domain may alter the kinetic parameters. SPX domains occur not only in VTCs but also in Pi transporters and Pi-signaling proteins and act as sensors for the regulation of cellular Pi homeostasis via interaction with transcription factors and inositol polyphosphates (InsPs), through which the function of the SPX-containing proteins is modulated (Wild et al. Citation2016). In S. cerevisiae VTCs, the binding of InsP to the SPX domains enhanced polyP-polymerizing activity and thus polyP accumulation in the vacuoles (Gerasimaite et al. Citation2017; Wild et al. Citation2016). Therefore, it is possible that the kinetic parameters estimated using RiVTC4* are likely underestimated compared with those of the native protein in the presence of InsP in vivo. Modulation of the kinetic/enzymatic properties by SPX-InsP interactions should be assessed by different approaches, for example, heterologous expression of intact RiVTC4 in yeast.
5. Conclusions
Our study provides a new insight into the mechanism of symbiotic P delivery in the plant-AM fungal association. PolyP plays a central role in the long-distance P translocation toward the host (Saito and Ezawa Citation2016; Ezawa and Saito Citation2018), but the biochemical processes of the polymerization and depolymerization have largely unknown. Our detailed characterization of the VTC4 revealed that the protein catalyzes both forward and reverse reactions, suggesting that energy required for polyP biosynthesis could be preserved during the translocation through the hyphae. Given that the biosynthetic reaction consumes a large amount of ATP, the feasibility of ATP regeneration from polyP may have a significant impact on the carbon budget of the fungi that are obligate biotrophs dependent on host-derived carbon source. The feasibility of ATP regeneration by VTC4 also has an impact on Pi homeostasis in eukaryotic cells. Cellular Pi homeostasis could be maintained by the metabolic and signaling interplay between polyP, ATP, and InsPs via SPX domain (Azevedo and Saiardi Citation2017), in which VTC4 could be one of key enzymes that have a regulatory role in the maintenance of Pi homeostasis, e.g., during polyP translocation in the fungi (Hijikata et al. Citation2010). However, it remains unclear whether the reverse reaction by the AM fungal VTC complex occurs in vivo. Therefore, the biological significance of the reverse reaction in AM symbiosis should be addressed in the future.
Supplemental Material
Download PDF (995.3 KB)Acknowledgments
We thank Ryo Ohtomo in the National Agriculture and Food Research Organization (NARO) for providing the polyP size markers and comments on the work. We also thank Reika Oguchi, Mabi Koide, and Minori Kamiya in Shinshu University for technical assistance and Yusuke Kikuchi in Hokkaido University for providing RNA for the expression analysis of R. irregularis ERM. We appreciate Research Center for Supports to Advanced Science, Shinshu University for technical support and Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions of this study are included in this article. Further inquiries can be directed to the corresponding author. Data of the alignments and phylogenetic trees of AM fungal VTC proteins are openly available in the Dryad digital repository at 10.5061/dryad.ghx3ffbp7.
Supplementary material
Supplemental data for this article can be accessed here
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Aksoy, M., W. Pootakham, and A. R. Grossman. 2014. “Critical Function of a Chlamydomonas reinhardtii Putative Polyphosphate Polymerase Subunit during Nutrient Deprivation.” The Plant Cell 26 (10): 4214–4229. doi:10.1105/tpc.114.129270.
- Austin, S., and A. Mayer. 2020. “Phosphate Homeostasis − a Vital Metabolic Equilibrium Maintained through the INPHORS Signaling Pathway.” Frontiers in Microbiology 11: 1367. doi:10.3389/fmicb.2020.01367.
- Azevedo, C., and A. Saiardi. 2017. “Eukaryotic Phosphate Homeostasis: The Inositol Pyrophosphate Perspective.” Trends in Biochemical Sciences 42 (3): 219–231. doi:10.1016/j.tibs.2016.10.008.
- Benedetto, A., F. Magurno, P. Bonfante, and L. Lanfranco. 2005. “Expression Profiles of a Phosphate Transporter Gene (GmosPT) from the Endomycorrhizal Fungus Glomus mosseae.” Mycorrhiza 15 (8): 620–627. doi:10.1007/s00572-005-0006-9.
- Boyce, K. J., M. Kretschmer, and J. W. Kronstad. 2006. “The vtc4 Gene Influences Polyphosphate Storage, Morphogenesis, and Virulence in the Maize Pathogen Ustilago maydis.” Eukaryotic Cell 5 (8): 1399–1409. doi:10.1128/EC.00131-06.
- Brundrett, M. C., and L. Tedersoo. 2018. “Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity.” New Phytologist 220 (4): 1108–1115. doi:10.1111/nph.14976.
- Cohen, A., N. Perzov, H. Nelson, and N. Nelson. 1999. “A Novel Family of Yeast Chaperons Involved in the Distribution of V-ATPase and Other Membrane Proteins.” Journal of Biological Chemistry 274 (38): 26885–26893. doi:10.1074/jbc.274.38.26885.
- Darriba, D., A. M. David Posada, A. S. Kozlov, B. Morel, and T. Flouri. 2020. “ModelTest-NG: A New and Scalable Tool for the Selection of DNA and Protein Evolutionary Models.” Molecular Biology and Evolution 37 (1): 291–294. doi:10.1093/molbev/msz189.
- Denoncourt, A., and M. Downey. 2021. “Model Systems for Studying Polyphosphate Biology: A Focus on Microorganisms.” Current Genetics 67 (3): 331–346. doi:10.1007/s00294-020-01148-x.
- Desfougères, Y., R. Gerasimaitė, H. J. Jessen, and A. Mayer. 2016. “Vtc5, a Novel Subunit of the Vacuolar Transporter Chaperone Complex, Regulates Polyphosphate Synthesis and Phosphate Homeostasis in Yeast.” Journal of Biological Chemistry 291 (42): 22262–22275. doi:10.1074/jbc.M116.746784.
- Ezawa, T., and K. Saito. 2018. “How Do Arbuscular Mycorrhizal Fungi Handle Phosphate? New Insights into Fine-tuning of Phosphate Metabolism.” New Phytologist 220 (4): 1116–1121. doi:10.1111/nph.15187.
- Ezawa, T., S. E. Smith, and F. A. Smith. 2001. “Differentiation of Polyphosphate Metabolism between the Extra- and Intraradical Hyphae of Arbuscular Mycorrhizal Fungi.” New Phytologist 149 (3): 555–563. doi:10.1046/j.1469-8137.2001.00040.x.
- Ezawa, T., T. R. Cavagnaro, S. E. Smith, F. A. Smith, and R. Ohtomo. 2004. “Rapid Accumulation of Polyphosphate in Extraradical Hyphae of an Arbuscular Mycorrhizal Fungus as Revealed by Histochemistry and a Polyphosphate Kinase/luciferase System.” New Phytologist 161 (2): 387–392. doi:10.1046/j.1469-8137.2003.00966.x.
- Fontes, R., D. Fernandes, F. Peralta, H. Fraga, I. Maio, and J. C. Esteves Da Silva. 2008. “Pyrophosphate and Tripolyphosphate Affect Firefly Luciferase Luminescence because They Act as Substrates and Not as Allosteric Effectors.” FEBS Journal 275 (7): 1500–1509. doi:10.1111/j.1742-4658.2008.06309.x.
- Funamoto, R., K. Saito, H. Oyaizu, T. Aono, and M. Saito. 2015. “pH Measurement of Tubular Vacuoles of an Arbuscular Mycorrhizal Fungus, Gigaspora margarita.” Mycorrhiza 25 (1): 55–60. doi:10.1007/s00572-014-0588-1.
- Gerasimaitė, R., and A. Mayer. 2017. “Ppn2, a Novel Zn2+-dependent Polyphosphatase in the Acidocalcisome-like Yeast Vacuole.” Journal of Cell Science 130 (9): 1625–1636. doi:10.1242/jcs.201061.
- Gerasimaite, R., I. Pavlovic, S. Capolicchio, A. Hofer, A. Schmidt, H. J. Jessen, and A. Mayer. 2017. “Inositol Pyrophosphate Specificity of the SPX-dependent Polyphosphate Polymerase VTC.” ACS Chemical Biology 12 (3): 648–653. doi:10.1021/acschembio.7b00026.
- Gerasimaitė, R., S. Sharma, Y. Desfougères, A. Schmidt, and A. Mayer. 2014. “Coupled Synthesis and Translocation Restrains Polyphosphate to Acidocalcisome-like Vacuoles and Prevents Its Toxicity.” Journal of Cell Science 127 (23): 5093–5104. doi:10.1242/jcs.159772.
- Gomes-Vieira, A. L., J. G. Wideman, L. Paes-Vieira, S. L. Gomes, T. A. Richards, and J. R. Meyer-Fernandes. 2018. “Evolutionary Conservation of a Core Fungal Phosphate Homeostasis Pathway Coupled to Development in Blastocladiella emersonii.” Fungal Genetics and Biology 115: 20–32. doi:10.1016/j.fgb.2018.04.004.
- Harrison, M. J., and M. L. van Buuren. 1995. “A Phosphate Transporter from the Mycorrhizal Fungus Glomus versiforme.” Nature 378 (6557): 626–629. doi:10.1038/378626a0.
- Hijikata, N., M. Murase, C. Tani, R. Ohtomo, M. Osaki, and T. Ezawa. 2010. “Polyphosphate Has a Central Role in the Rapid and Massive Accumulation of Phosphorus in Extraradical Mycelium of an Arbuscular Mycorrhizal Fungus.” New Phytologist 186 (2): 285–289. doi:10.1111/j.1469-8137.2009.03168.x.
- Hothorn, M., H. Neumann, E. D. Lenherr, M. Wehner, V. Rybin, Hassa, P. O., Uttenweiler, A., et al. 2009. “Catalytic Core of a Membrane-associated Eukaryotic Polyphosphate Polymerase.” Science 324 (5926): 513–516. doi:10.1126/science.1168120.
- Ishige, K., H. Zhang, and A. Kornberg. 2002. “Polyphosphate Kinase (PPK2), a Potent, Polyphosphate-driven Generator of GTP.” Proceedings of the National Academy of Sciences of the United States of America 99 (26): 16684–16688. doi:10.1073/pnas.262655299.
- Jolicoeur, M., S. Germette, M. Gaudette, M. Perrier, and G. Bécard. 1998. “Intracellular pH in Arbuscular Mycorrhizal Fungi. A Symbiotic Physiological Marker.” Plant Physiology 116 (4): 1279–1288. doi:10.1104/pp.116.4.1279.
- Kikuchi, Y., N. Hijikata, K. Yokoyama, R. Ohtomo, Y. Handa, M. Kawaguchi, K. Saito, and T. Ezawa. 2014. “Polyphosphate Accumulation Is Driven by Transcriptome Alterations that Lead to Near-synchronous and Near-equivalent Uptake of Inorganic Cations in an Arbuscular Mycorrhizal Fungus.” New Phytologist 204 (3): 638–649. doi:10.1111/nph.12937.
- Kikuchi, Y., N. Hijikata, R. Ohtomo, Y. Handa, M. Kawaguchi, K. Saito, C. Masuta, and T. Ezawa. 2016. “Aquaporin-mediated Long-distance Polyphosphate Translocation Directed Towards the Host in Arbuscular Mycorrhizal Symbiosis: Application of Virus-induced Gene Silencing.” New Phytologist 211 (4): 1202–1208. doi:10.1111/nph.14016.
- Kobae, Y., M. Kawachi, K. Saito, Y. Kikuchi, T. Ezawa, M. Maeshima, S. Hata, and T. Fujiwara. 2015. “Up-regulation of Genes Involved in N-acetylglucosamine Uptake and Metabolism Suggests a Recycling Mode of Chitin in Intraradical Mycelium of Arbuscular Mycorrhizal Fungi.” Mycorrhiza 25 (5): 411–417. doi:10.1007/s00572-014-0623-2.
- Kohl, K., H. Zangger, M. Rossi, N. Isorce, L. F. Lye, K. L. Owens, S. M. Beverley, A. Mayer, and N. Fasel. 2018. “Importance of Polyphosphate in the Leishmania Life Cycle.” Microbial Cell 5 (8): 371–384. doi:10.15698/mic2018.08.642.
- Kozlov, A. M., D. Darriba, T. Flouri, B. Morel, and A. Stamatakis. 2019. “RAxML-NG: A Fast, Scalable and User-friendly Tool for Maximum Likelihood Phylogenetic Inference.” Bioinformatics 35 (21): 4453–4455. doi:10.1093/bioinformatics/btz305.
- Kuga, Y., K. Saito, K. Nayuki, R. L. Peterson, and M. Saito. 2008. “Ultrastructure of Rapidly-frozen and Freeze-substituted Germ Tubes of an Arbuscular Mycorrhizal Fungus and Localization of Polyphosphate.” New Phytologist 178 (1): 189–200. doi:10.1111/j.1469-8137.2007.02345.x.
- Kumble, K. D., and A. Kornberg. 1996. “Endopolyphosphatases for Long Chain Inorganic Polyphosphate in Yeast and Mammals.” Journal of Biological Chemistry 271 (43): 27146–27151. doi:10.1074/jbc.271.43.27146.
- Lander, N., P. N. Ulrich, and R. Docampo. 2013. “Trypanosoma brucei Vacuolar Transporter Chaperone 4 (TbVtc4) Is an Acidocalcisome Polyphosphate Kinase Required for in Vivo Infection.” Journal of Biological Chemistry 288 (47): 34205–34216. doi:10.1074/jbc.M113.518993.
- Letunic, I., and P. Bork. 2019. “Interactive Tree of Life (Itol) V4: Recent Updates and New Developments.” Nucleic Acids Research 47 (W1): W256–W9. doi:10.1093/nar/gkz239.
- Lonetti, A., Z. Szijgyarto, D. Bosch, O. Loss, C. Azevedo, and A. Saiardi. 2011. “Identification of an Evolutionarily Conserved Family of Inorganic Polyphosphate Endopolyphosphatases.” Journal of Biological Chemistry 286 (37): 31966–31974. doi:10.1074/jbc.M111.266320.
- Maldonado-Mendoza, I. E., G. R. Dewbre, and M. J. Harrison. 2001. “A Phosphate Transporter Gene from the Extra-radical Mycelium of an Arbuscular Mycorrhizal Fungus Glomus intraradices Is Regulated in Response to Phosphate in the Environment.” Molecular Plant-Microbe Interactions 14 (10): 1140–1148. doi:10.1094/MPMI.2001.14.10.1140.
- Müller, O., H. Neumann, M. J. Bayer, and A. Mayer. 2003. “Role of the Vtc Proteins in V-ATPase Stability and Membrane Trafficking.” Journal of Cell Science 116 (6): 1107–1115. doi:10.1242/jcs.00328.
- Müller, O., M. J. Bayer, J. S. Christopher Peters, M. M. Andersen, and A. Mayer. 2002. “The Vtc Proteins in Vacuole Fusion: Coupling NSF Activity to V0 Trans-complex Formation.” The EMBO Journal 21 (3): 259–269. doi:10.1093/emboj/21.3.259.
- Murray, J. M., and D. I. Johnson. 2000. “Isolation and Characterization of Nrf1p, a Novel Negative Regulator of the Cdc42p GTPase in Schizosaccharomyces pombe.” Genetics 154 (1): 155–165. doi:10.1093/genetics/154.1.155.
- Nguyen, C. T., and K. Saito. 2021. “Role of Cell Wall Polyphosphates in Phosphorus Transfer at the Arbuscular Interface in Mycorrhizas.” Frontiers in Plant Science 12: 725939. doi:10.3389/fpls.2021.725939.
- Nguyen, P., T. Mai, Y. Ishiwata-Kimata, and Y. Kimata. 2019. “Monitoring ADP/ATP Ratio in Yeast Cells Using the Fluorescent-protein Reporter PercevalHR.” Bioscience, Biotechnology, and Biochemistry 83 (5): 824–828. doi:10.1080/09168451.2019.1574204.
- Ogawa, N., J. DeRisi, and P. O. Brown. 2000. “New Components of a System for Phosphate Accumulation and Polyphosphate Metabolism in Saccharomyces cerevisiae Revealed by Genomic Expression Analysis.” Molecular Biology of the Cell 11 (12): 4309–4321. doi:10.1091/mbc.11.12.4309.
- Ohtomo, R., and M. Saito. 2005. “Polyphosphate Dynamics in Mycorrhizal Roots during Colonization of an Arbuscular Mycorrhizal Fungus.” New Phytologist 167 (2): 571–578. doi:10.1111/j.1469-8137.2005.01425.x.
- Ohtomo, R., Y. Sekiguchi, T. Kojima, and M. Saito. 2008. “Different Chain Length Specificity among Three Polyphosphate Quantification Methods.” Analytical Biochemistry 383 (2): 210–216. doi:10.1016/j.ab.2008.08.002.
- Ozalp, V. C., T. R. Pedersen, L. J. Nielsen, and L. F. Olsen. 2010. “Time-resolved Measurements of Intracellular ATP in the Yeast Saccharomyces cerevisiae Using a New Type of Nanobiosensor.” The Journal of Biological Chemistry 285 (48): 37579–37588. doi:10.1074/jbc.M110.155119.
- Rao, N. N., M. R. Gómez-García, and A. Kornberg. 2009. “Inorganic Polyphosphate: Essential for Growth and Survival.” Annual Review of Biochemistry 78 (1): 605–647. doi:10.1146/annurev.biochem.77.083007.093039.
- Ritz, C., F. Baty, J. C. Streibig, and D. Gerhard. 2016. “Dose-response Analysis Using R.” PLoS One 10 (12): e0146021. doi:10.1371/journal.pone.0146021.
- Robinson, N. A., and H. G. Wood. 1986. “Polyphosphate Kinase from Propionibacterium shermanii. Demonstration that the Synthesis and Utilization of Polyphosphate Is by a Processive Mechanism.” Journal of Biological Chemistry 261 (10): 4481–4485. doi:10.1016/S0021-9258(18)61176-7.
- Rooney, P. J., L. Ayong, C. M. Tobin, S. N. J. Moreno, and L. J. Knoll. 2011. “TgVTC2 Is Involved in Polyphosphate Accumulation in Toxoplasma gondii.” Molecular and Biochemical Parasitology 176 (2): 121–126. doi:10.1016/j.molbiopara.2010.12.012.
- Safrany, S. T., J. J. Caffrey, X. Yang, M. E. Bembenek, M. B. Moyer, W. A. Burkhart, and S. B. Shears. 1998. “A Novel Context for the ‘Mutt’ Module, A Guardian of Cell Integrity, in A Diphosphoinositol Polyphosphate Phosphohydrolase.” The EMBO Journal 17 (22): 6599–6607. doi:10.1093/emboj/17.22.6599.
- Saito, K., and T. Ezawa. 2016. “Phosphorus Metabolism and Transport in Arbuscular Mycorrhizal Symbiosis.” In Molecular Mycorrhizal Symbiosis, edited by F. Martin, 197–216. New Jersey: John Wiley & Sons.
- Sanz-Luque, E., S. Saroussi, W. Huang, N. Akkawi, and A. R. Grossman. 2020. “Metabolic Control of Acclimation to Nutrient Deprivation Dependent on Polyphosphate Synthesis.” Science Advances 6 (40): eabb5351. doi:10.1126/sciadv.abb5351.
- Scott, R. A., and G. P. Haight Jr. 1975. “Separation and Detection of Ortho-, Pyro-, and Tripolyphosphate by Anion Exchange Thin Layer Chromatography.” Analytical Chemistry 47 (14): 2439–2440. doi:10.1021/ac60364a021.
- Secco, D., C. Wang, H. Shou, and J. Whelan. 2012. “Phosphate Homeostasis in the Yeast Saccharomyces cerevisiae, the Key Role of the SPX Domain-containing Proteins.” FEBS Letters 586 (4): 289–295. doi:10.1016/j.febslet.2012.01.036.
- Shi, X., and A. Kornberg. 2005. “Endopolyphosphatase in Saccharomyces cerevisiae Undergoes Post-translational Activations to Produce Short-chain Polyphosphates.” FEBS Letters 579 (9): 2014–2018. doi:10.1016/j.febslet.2005.02.032.
- Smith, S. A., and J. H. Morrissey. 2007. “Sensitive Fluorescence Detection of Polyphosphate in Polyacrylamide Gels Using 4’,6-diamidino-2-phenylindol.” Electrophoresis 28 (19): 3461–3465. doi:10.1002/elps.200700041.
- Smith, S. A., Y. Wang, and J. H. Morrissey. 2018. “DNA Ladders Can Be Used to Size Polyphosphate Resolved by Polyacrylamide Gel Electrophoresis.” Electrophoresis 39 (19): 2454–2459. doi:10.1002/elps.201800227.
- Smith, S. E., and D. J. Read. 2008. Mycorrhizal Symbiosis. 3rd ed. San Diego: Academic Press.
- Solaiman, M. Z., T. Ezawa, T. Kojima, and M. Saito. 1999. “Polyphosphates in Intraradical and Extraradical Hyphae of an Arbuscular Mycorrhizal Fungus, Gigaspora margarita.” Applied and Environmental Microbiology 65 (12): 5604–5606. doi:10.1128/AEM.65.12.5604-5606.1999.
- Spatafora, J. W., Y. Chang, G. L. Benny, K. Lazarus, M. E. Smith, M. L. Berbee, Bonito, G., et al. 2016. “A Phylum-level Phylogenetic Classification of Zygomycete Fungi Based on Genome-scale Data.” Mycologia 108 (5): 1028–1046. doi:10.3852/16-042.
- Takaine, M., M. Ueno, K. Kitamura, H. Imamura, and S. Yoshida. 2019. “Reliable Imaging of ATP in Living Budding and Fission Yeast.” Journal of Cell Science 132 (8): jcs230649. doi:10.1242/jcs.230649.
- Takanishi, I., R. Ohtomo, M. Hayatsu, and M. Saito. 2009. “Short-chain Polyphosphate in Arbuscular Mycorrhizal Roots Colonized by Glomus spp.: A Possible Phosphate Pool for Host Plants.” Soil Biology & Biochemistry 41 (7): 1571–1573. doi:10.1016/j.soilbio.2009.04.002.
- Tani, C., R. Ohtomo, M. Osaki, Y. Kuga, and T. Ezawa. 2009. “ATP-dependent but Proton Gradient-independent Polyphosphate-synthesizing Activity in Extraradical Hyphae of an Arbuscular Mycorrhizal Fungus.” Applied and Environmental Microbiology 75 (22): 7044–7050. doi:10.1128/aem.01519-09.
- Tisserant, E., M. Malbreil, A. Kuo, A. Kohler, A. Symeonidi, R. Balestrini, P. Charron, et al. 2013. “Genome of an Arbuscular Mycorrhizal Fungus Provides Insight into the Oldest Plant Symbiosis.” Proceedings of the National Academy of Sciences of the United States of America 110 (50): 20117–20122. doi:10.1073/pnas.1313452110.
- Uetake, Y., T. Kojima, T. Ezawa, and M. Saito. 2002. “Extensive Tubular Vacuole System in an Arbuscular Mycorrhizal Fungus, Gigaspora margarita.” New Phytologist 154 (3): 761–768. doi:10.1046/j.1469-8137.2002.00425.x.
- Ulrich, P. N., N. Lander, S. P. Kurup, L. Reiss, J. Brewer, L. C. Soares Medeiros, K. Miranda, and R. Docampo. 2014. “The Acidocalcisome Vacuolar Transporter Chaperone 4 Catalyzes the Synthesis of Polyphosphate in Insect-stages of Trypanosoma brucei and T. Cruzi.” Journal of Eukaryotic Microbiology 61 (2): 155–165. doi:10.1111/jeu.12093.
- Uttenweiler, A., H. Schwarz, H. Neumann, and A. Mayer. 2006. “The Vacuolar Transporter Chaperone (VTC) Complex Is Required for Microautophagy.” Molecular Biology of the Cell 18 (1): 166–175. doi:10.1091/mbc.e06-08-0664.
- Viereck, N., P. E. Hansen, and I. Jakobsen. 2004. “Phosphate Pool Dynamics in the Arbuscular Mycorrhizal Fungus Glomus intraradices Studied by in Vivo 31P NMR Spectroscopy.” New Phytologist 162 (3): 783–794. doi:10.1111/j.1469-8137.2004.01048.x.
- Walther, T., M. Novo, K. Rössger, F. Létisse, M.-O. Loret, J.-C. Portais, and F. Jean-Marie. 2010. “Control of ATP Homeostasis during the Respiro-fermentative Transition in Yeast.” Molecular Systems Biology 6 (1): 344. doi:10.1038/msb.2009.100.
- Wang, L., X. Q. Jia, Y. X. Zhang, L. Xu, B. Menand, H. Y. Zhao, H. Q. Zeng, L. Dolan, Y. Y. Zhu, and K. K. Yi. 2021. “Loss of Two Families of SPX Domain-containing Proteins Required for Vacuolar Polyphosphate Accumulation Coincides with the Transition to Phosphate Storage in Green Plants.” Molecular Plant 14 (5): 838–846. doi:10.1016/j.molp.2021.01.015.
- Wild, R., R. Gerasimaite, J.-Y. Jung, V. Truffault, I. Pavlovic, A. Schmidt, A. Saiardi, et al. 2016. “Control of Eukaryotic Phosphate Homeostasis by Inositol Polyphosphate Sensor Domains.” Science 352 (6288): 986–990. doi:10.1126/science.aad9858.
- Wurst, H., and A. Kornberg. 1994. “A Soluble Exopolyphosphatase of Saccharomyces cerevisiae. Purification and Charcterization.” Journal of Biological Chemistry 269 (15): 10996–11001. doi:10.1016/S0021-9258(19)78082-X.
- Wurst, H., T. Shiba, and A. Kornberg. 1995. “The Gene for a Major Exopolyphosphatase of Saccharomyces cerevisiae.” Journal of Bacteriology 177 (4): 898–906. doi:10.1128/jb.177.4.898-906.1995.
- Zhang, H., K. Ishige, and A. Kornberg. 2002. “A Polyphosphate Kinase (PPK2) Widely Conserved in Bacteria.” Proceedings of the National Academy of Sciences of the United States of America 99 (26): 16678–16683. doi:10.1073/pnas.262655199.