ABSTRACT
Soil microbial biomass (microbial biomass) contains substantial amounts of potassium (K) as a reservoir of K in soils. However, information about K dynamics and K flux through the microbial biomass are lacking. In the present study, the turnover time of microbial biomass K was estimated for the first time using paddy field soils. The paddy field soils, either amended with substrates of carbon, nitrogen, and phosphorus, or not, were aerobically incubated for 60 days, during which the amounts of microbial biomass K were periodically determined by the chloroform fumigation-extraction procedure. Microbial biomass K increased to twice (51.4–72.3 mg K kg−1 soil) of that in the unamended control 5 days after addition of the substrates and gradually decreased to the values in the control (25.2–44.0 mg K kg−1 soil) until 60 days of the incubation. The turnover time of microbial biomass K was estimated from the declines in the substrate amended soils. The turnover time of microbial biomass K ranged from 80.4 to 98.5 days, which roughly corresponded to the cultivation period of rice (about 100 days). These results indicated that microbial biomass K plays important roles in the supply of K source as well as the reservoir of K in paddy field soil.
1. Introduction
Nutrient cycling and energy flow in agroecosystems are closely linked with soil microbial biomass (microbial biomass) via microbial turnover. Soil microorganisms decompose and metabolize organic substances and are a driving force behind nutrient transformations in soils, which contribute to the soil fertility as a storage and supply of important nutrients for plants such as nitrogen (N), phosphorus (P), and sulfur (S) (Kanazawa, Asakawa, and Takai Citation1988; Smith and Paul Citation1990; Banerjee and Chapman Citation1996; Brookes Citation2001). In silt loam soils in Pacific Northwest, contents of microbial biomass N, P, and S were 180, 17, and 9 kg ha−1, respectively, corresponding to 60%, 47%, and 28% of the nutrient requirements of the winter wheat (Smith and Paul Citation1990). In an unfertilized plot of the Broadbalk Continuous Wheat Experiment, the flux of N through microbial biomass was 38 kg ha−1 y−1 (Jenkinson and Ladd Citation1981), which was almost equivalent to the removal of N for the grain and straw of 24 kg ha−1 y−1 (Jenkinson Citation1977). Thus, microbial biomass, although comprising only 1–3% and 1–5% of total soil organic C (Jenkinson and Ladd Citation1981) and total soil N (Smith and Paul Citation1990), respectively, plays a critical role in supplying source and sink of plant nutrients (Brookes Citation2001).
The turnover time of microbial biomass C, N, or P has been estimated by tracing isotopes in the microbial biomass pools. In the estimation, dynamics of total and labeled microbial biomass were monitored with time after soils were amended with labeled substrates, 13C, 14C, 15N, or 32P (e.g., van Veen, Ladd, and Amato Citation1985; Okano, Nishio, and Sawada Citation1987; Sakamoto and Hodono Citation2000; Chen and He Citation2002; Kouno, Wu, and Brookes Citation2002; Wessels Perelo and Munch Citation2005; Wessels Perelo, Jimenez, and Munch Citation2006). The turnover time was estimated from the decline in the labeled microbial biomass by first-order kinetics. Okano, Nishio, and Sawada (Citation1987) estimated the turnover time of microbial biomass N for 1.9 y from the decline of the microbial biomass 15N in the root mat layer of pasture. The N flux of 21 kg N ha−1 y−1 calculated from the turnover time and the size of the microbial biomass N (40 kg N ha−1) corresponded to about 20% of the N uptake of the pasture. Kouno, Wu, and Brookes (Citation2002) showed that about 60 kg P ha−1 of microbial biomass P was released through the microbial biomass 32P turnover of 40 days. The size and the turnover of the microbial biomass are important for the evaluation of the source of the plant nutrients.
Potassium (K) is one of the major essential nutrients in plants, along with N and P, and its requirement by crops is equivalent to or larger than that of N (Owa Citation2006). In addition to plants, potassium ion (K+) is the most abundant intracellular cation in eukaryotic and prokaryotic microbial cells (Tanudjaja et al. Citation2017). Bacteria and fungi actively accumulate K+ inside the cells via uniporter system for the important functions such as maintenance of intracellular osmolarity, formation of membrane potential, and regulation of enzyme activities (Rodríguez-Navarro Citation2000; Epstein Citation2003). Concentrations of K+ greater than 0.18–0.2 M are maintained inside the fungal and bacterial cells and higher than in the external environment (Brown Citation1964; Slayman and Tatum Citation1964). Therefore, K in the cells of soil microorganisms could importantly influence the cycling and phytoavailability of K in the agroecosystems.
Perrott, Sarathchandra, and Waller (Citation1990) investigated seasonal changes in microbial biomass K in a grassland soil and showed the implication of K sink-source function from the finding that the decrease in microbial biomass K from 150 to 200 mg K kg−1 soil in winter to 50–100 mg K kg−1 soil in spring and summer was associated with nutrient requirement of grasses. Khan, Heinze, and Joergensen (Citation2009) suggested that microbial biomass C was an important factor for K uptake by microbial cells as microbial biomass K was positively correlated with microbial biomass C. In these studies, however, the rate of recovery of K from microbial biomass in the fumigation treatment was not evaluated, and a conversion factor (kK) was not used for the determination of microbial biomass K. Lorenz et al. (Citation2010) developed the assay for the determination of microbial biomass K by the chloroform fumigation-extraction method for the first time and determined the kK factor in a range of agricultural soils. In the soils of upland corn (Zea mays L.) field in Ohio with different soil textures – fine sand, silt loam, and clay, the amounts of microbial biomass K corresponded to 15–37% of the exchangeable K (Lorenz et al. Citation2010). In the soil of a paddy field without fertilizer application for 38 years, the amount of microbial biomass K corresponded to 115% of the exchangeable K (Yamashita et al. Citation2014). Although these studies showed the importance of microbial biomass K as the reservoir of K in soils potentially available for plants, estimation of turnover time of microbial biomass K is necessary for estimating the rate of K supply to plants from this source. However, no information is available on the turnover time of microbial biomass K so far.
Paddy field soils are managed under flooded conditions during the rice-growing period (about 100 days). In the rest of the period (about 250 days) after rice harvest, the soils are kept drained under unflooded conditions. The dynamics of microbial biomass K in soil is important under both of the conditions with regard to the K supply to rice plants in the rice-growing period and also changes in the pool size of microbial biomass K associated with organic matter application such as rice straw after harvesting.
In the present study, we estimated the turnover time of microbial biomass K in paddy field soils under drained (aerobic) conditions. The measurement of stable isotope of K (41K) is not feasible because the techniques for the isotopic analysis in 41K/39K are not common, and the 41K isotope substances are highly costly. We therefore estimated the turnover time from the decline of total amounts of microbial biomass K, which was increased by adding nutrients (C, N, and P), as an apparent turnover time for microbial biomass K for the first time.
2. Materials and methods
2.1. Soils and incubation
Paddy soils from the Crop Institute, Aichi Agricultural Research Center, Anjo, Aichi, Japan (Anjo; 34°58´N, 137°4´E) and from two fields (Omagari-1 and −2) in the NARO Tohoku Agricultural Research Center, Daisen, Akita, Japan (Omagari; 39°29´N, 140°29´E) were sampled at a depth of 0–10 cm, sieved (<2 mm), and stored at 4°C. A sampling plot in Anjo was 0.5 ha with mono-culture of paddy rice (Oryza sativa L.). Chemical fertilizers (51 kg N ha−1 y−1, 20 kg P2O5 ha−1 y−1, and 30 kg K2O ha−1 y−1 in total) were applied as a basal and top-dressing fertilizers. Sampling plots in Omagari-1 and −2 were used for monoculture of paddy rice and applied with chemical fertilizers as a basal and top-dressing fertilizers: 80 kg N ha−1 y−1, 80 kg P2O5 ha−1 y−1, and 80 kg K2O ha−1 y−1 in total in Omagari-1, with the same amounts of P and K, but 60 kg N ha−1 y−1 in total in Omagari-2. Soil type and physicochemical properties of soils used are shown in .
Table 1. Soil type and physicochemical properties of the soils
A batch incubation was conducted in 50 ml glass vials (SV-50A, Nichiden-rika glass, Hyogo, Japan) for a period of 60 days at 25°C, where water content of the soil samples was kept at 60% of water-holding capacity (WHC) during the incubation in the present study, assuming the drained period of paddy fields. Treatments were conducted as follows: 10 grams of wet soil in each vial (n = 3) were treated with no addition (control) or supplemented with a solution mixed with C as glucose (1,000 mg C kg−1 soil), N and P as NH4Cl and NH4H2PO4 (72 mg N kg−1 soil and 79 mg P kg−1 soil) to increase microbial biomass (CNP treatment). The treatments were conducted without K addition because addition of K as KCl to the soil did not increase microbial biomass K in the preliminary study (data not shown). Each of these vials of the soil preparations was taken out at 0, 5, 10, 20, 30, 40, 50, and 60 days of the incubation followed by the measurements of microbial biomass K, C, and N contents. The incubation experiment in triplicate was repeated three times.
2.2. Measurements of microbial biomass K, C, and N
Microbial biomass K was measured by the chloroform fumigation-extraction procedure, where K in the fumigated or unfumigated 10 g of wet soil in each vial was extracted with 100 ml of 1 M ammonium acetate (NH4OAc) solution for the determination of microbial biomass K (Lorenz et al. Citation2010; Yamashita et al. Citation2014). The concentration of K in the NH4OAc extracts was quantified using a flame photometer (ANA-135, Tokyokoden, Tokyo, Japan). Microbial biomass K was calculated as the following equation: [(K extracted from the fumigated soil) − (K extracted from the unfumigated soil)]/kK, where the value of 0.28 was used for the conversion factor (Yamashita et al. Citation2018). Fifty milliliters of 0.5 M K2SO4 solution were used for the extraction of microbial biomass C and N (Brookes et al. Citation1985; Vance, Brookes, and Jenkinson Citation1987), and total organic C and total N in the K2SO4 extracts were determined using a TOC analyzer (TOC-VCPH, Shimadzu, Kyoto, Japan). The values of 0.45 (Joergensen Citation1996) and 0.45 (Urashima Citation2013) were used for the conversion factors for microbial biomass C (kC) and N (kN), respectively. All the measurements in both the control and CNP treated soils were performed in triplicate.
2.3. Calculation of turnover time of microbial biomass K, C, and N
Turnover time was calculated from the decline in the values of microbial biomass K, C, and N with time (d). The decline in microbial biomass K, C, and N were expressed using the first-order equation: Bt = B0 e−kt, where Bt and B0 are the amounts of microbial biomass K, C, and N at time t = t and t = 0, and k is decay rate constant (Kouno, Wu, and Brookes Citation2002; Wessels Perelo and Munch Citation2005). In the present study, time 0 represents the day just before the amount of microbial biomass K, C, and N were decreased, i.e., the day when the maximum microbial biomass K, C, and N contents were observed. The turnover time T (d) was expressed as 1/k.
2.4. Statistical analysis
Mean value of triplicate data was calculated for each incubation experiment and then the values were expressed as the averages ± SE of the three sets of the mean data from the repeated measurements three times. Differences in the exchangeable K or microbial biomass K, C, and N between treatments were analyzed by using the Student’s t-test at 5% level (P < 0.05). Differences in the values of microbial biomass K, C, and N, and turnover time among the soils were tested by using the one-way analysis of variance (one-way ANOVA) followed by the Tukey’s test at 5% level (P < 0.05).
3. Results
3.1. Change in exchangeable K
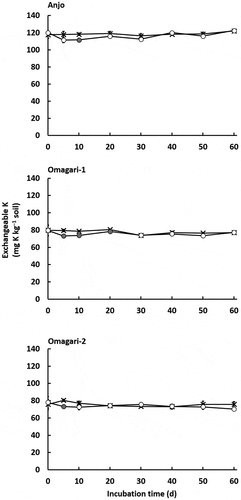
The amount of exchangeable K in the control remained constant until 60 days of the incubation () with the average values in entire period of 119 ± 1, 75.6 ± 0.8, and 73.7 ± 0.8 mg K kg−1 soil for the soils in Anjo, Omagari-1, and −2, respectively. The amounts of the exchangeable K in the CNP treatment were relatively stable except on day 5 or 10 when slight but significant decreases ranging from 6 to 7 mg K kg−1 soil were observed (P < 0.05). The average values in entire period in the CNP treatment were similar to those in respective controls: 116 ± 1, 77.8 ± 0.7, and 75.6 ± 1.3 mg K kg−1 soil for the soils in Anjo, Omagari-1, and −2, respectively.
3.2. Change in microbial biomass K, C, and N
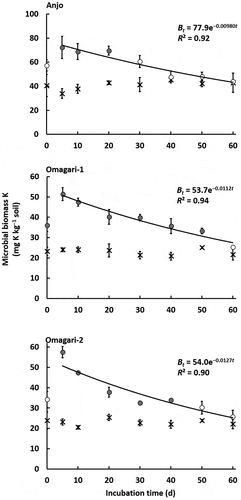
Stable microbial biomass K content over the incubation period was observed in the control with the average values of 40.9 ± 1.2, 23.0 ± 0.5, and 22.9 ± 0.5 mg K kg−1 soil for the soils in Anjo, Omagari-1, and −2, respectively (). The microbial biomass K value in Anjo soil was significantly higher than that in Omagari-1 and −2 soils (P < 0.01), but no significant difference was detected between the soils in Omagari. In the CNP treatment, microbial biomass K increased until day 5 and decreased gradually until day 60 when the contents reached the values in the control (44.0 ± 1.5, 25.2 ± 0.6, and 25.6 ± 3.3 mg K kg−1 soil for the soils in Anjo, Omagari-1, and −2, respectively). Five days after the CNP addition, microbial biomass K increased about two-fold greater than that in the control and reached maximum: 72.3 ± 9.2, 51.4 ± 3.2, and 57.5 ± 2.7 mg K kg−1 soil in Anjo, Omagari-1, and −2 soils, respectively. The differences in the microbial biomass K on day 5 between the control and the CNP treatment ranged from 23.2 to 33.9 mg K kg−1 soil, which were greater than the differences in the exchangeable K (6–7 mg K kg−1 soil) observed between the treatments.
Figure 2. Changes in microbial biomass K (cross symbols, control; circle symbols, CNP treatment). Decay rate constants k in Anjo, Omagari-1, and −2 soils are 9.80 × 10−3, 1.12 × 10−2, and 1.27 × 10−2, respectively. Closed symbols in CNP treatment indicate significant differences between control and CNP treatments (P < 0.05). Lines express a first order exponential function. Bars indicate S.E. (n = 3).
During the entire period, microbial biomass C and N for the soils in Anjo, Omagari-1 and −2 were stable in the control, and averaged 481 ± 7, 240 ± 9, and 234 ± 4 mg C kg−1 soil, respectively, for microbial biomass C, and 41.3 ± 0.8, 14.4 ± 1.4, and 16.2 ± 1.0 mg N kg−1 soil, respectively, for microbial biomass N (). The value of microbial biomass C and N in Anjo soil was significantly higher than that in Omagari-1 and −2 soils (P < 0.01), but no significant difference was detected between the soils in Omagari-1 and −2. The changes in microbial biomass C and N in the CNP treatment showed a similar trend to those in the microbial biomass K. The amounts of microbial biomass C and N reached maximum values at 5 days after CNP addition and gradually decreased until day 60 (). The amounts of microbial biomass C in Anjo, Omagari-1, and −2 soils were increased by 49%, 43%, and 57%, respectively, compared with those in the control (). Microbial biomass N increased up to about two-fold greater than that in the control (90%, 105%, and 102% for the soils in Anjo, Omagari-1, and −2, respectively) ().
Figure 3. Changes in microbial biomass C (cross symbols, control; circle symbols, CNP treatment). Decay rate constants k in Anjo, Omagari-1, and −2 soils are 5.96 × 10−3, 1.11 × 10−2, and 9.94 × 10−3, respectively. Closed symbols in CNP treatment indicate significant differences between control and CNP treatments (P < 0.05). Lines express a first order exponential function. Bars indicate S.E. (n = 3).
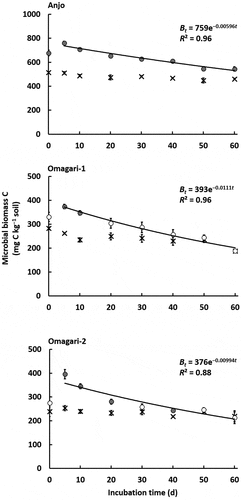
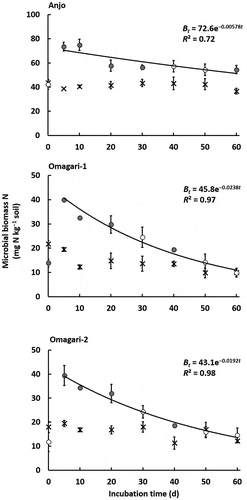
Figure 4. Changes in microbial biomass N (cross symbols, control; circle symbols, CNP treatment). Decay rate constants k in Anjo, Omagari-1, and −2 soils are 5.78 × 10−3, 2.38 × 10−2, and 1.92 × 10−2, respectively. Closed symbols in CNP treatment indicate significant differences between control and CNP treatments (P < 0.05). Lines express a first order exponential function. Bars indicate S.E. (n = 3).
3.3. Turnover time of microbial biomass K, C, and N
The turnover time of microbial biomass K, C, and N was estimated by fitting the first-order equation to the declines of microbial biomass beginning with the maximum values on day 5 as shown in . The decay rate constants (k [d−1]) for microbial biomass K were calculated to be 9.80 × 10−3 in Anjo soil, 1.12 × 10−2 in Omagari-1 soil, and 1.27 × 10−2 in Omagari-2 soil (). Averaged values of turnover time from the repeated measurements three times ranged from 80.4 to 98.5 d (). There was no significant difference in the turnover time of microbial biomass K among the soils.
Table 2. Turnover time of microbial biomass K, C, and N. *
The decay rate constant calculated for microbial biomass C was 5.96 × 10−3 in Anjo soil, 1.11 × 10−2 in Omagari-1 soil, and 9.94 × 10−3 in Omagari-2 soil (), and the averaged values of turnover time ranged from 92.1 to 173 d (). The decay rate constant for microbial biomass N was calculated to be 5.78 × 10−3 in Anjo soil, 2.38 × 10−2 in Omagari-1 soil, and 1.92 × 10−2 in Omagari-2 soil (), and the averaged values of turnover time ranged from 40.3 to 175 d (). The turnover time of microbial biomass C and N in Anjo soil was significantly slower than those in Omagari-1 and −2 soils (P < 0.05), and no significant differences were detected between Omagari-1 and −2 soils. In Omagari-1 and −2 soils, the turnover time of microbial biomass K was not significantly different from that of microbial biomass C, but significantly slower than that of microbial biomass N (P < 0.05). In Anjo soil, no significant differences were detected among the turnover time of microbial biomass K, C, and N.
4. Discussion
4.1. Turnover time of microbial biomass K in paddy field soil
We estimated the apparent turnover time of microbial biomass K in paddy field soil for the first time in the present study by the addition of CNP to the soils. The values from 80.4 to 98.5 d in the soils were not significantly different among the soils and averaged 90.4 d (). The amounts of microbial biomass K in the CNP treatment were increased up to 72.3, 51.4, and 57.5 mg K kg−1 soil for the soils in Anjo, Omagari-1, and −2, respectively, which were about two-fold higher than those in the untreated control. These values corresponded to 51.4–72.3 kg microbial biomass K ha−1 assuming that the bulk density of the soils was 1.0, and the depth of the plow layer was 10 cm. The turnover time of microbial biomass K obtained in this study (90.4 d) is almost equivalent to the cultivation period of rice (about 100 d). The K supplied from microbial biomass for 100 d is estimated to be 40–60% of the amount of K uptake by rice plants (130 kg K ha−1; Owa Citation2006).
In paddy field soils in Omagari with long-term application of cattle manure and rice straw composts, amounts of microbial biomass K ranged between 60 and 80 kg K ha−1 (Yamashita et al. Citation2014). Combining these values with the turnover time presented in , K released from microbial biomass was estimated to be 46–62% of K uptake by rice plants. In paddy field soil with chemical fertilizer application in Anjo (Yamashita et al. Citation2014) approximately 40 kg K ha−1 was released from microbial biomass within 100 d based on the turnover time in the present study. In addition, Yamashita et al. (submitted for publication) indicated that the role of microbial biomass K as a K reservoir is more important in paddy field soil than in upland and orchard soils. These estimations suggest that microbial biomass K could play an important role as a source of available K for rice plants.
We estimated the turnover time of microbial biomass K, C, and N in the paddy field soils with the water content of 60% WHC throughout the incubation. The turnover time of microbial biomass K under flooded conditions could be faster than the value (90.4 d) in the present study under drained conditions. Previous reports indicate that flooded soils have faster turnover time of microbial biomass C and N than upland soils. In the flooded paddy field soils without rice transplantation in the International Rice Research Institute (IRRI), Philippines, the turnover time of microbial biomass C was about 40–60 d based on the estimation by Witt et al. (Citation2000), which was faster than the values (92.1–173 d) in the present study (). Turnover time of microbial biomass N in flooded paddy field soils ranged between 10 and 20 d (Inubushi and Watanabe Citation1986; Shibahara Citation2002), which was also faster than those in the upland soil (1.5 y; Jenkinson and Parry Citation1989) and the grassland soil (1.9 y; Okano, Nishio, and Sawada Citation1987). The turnover time of microbial biomass C (92.1–173 d) and N (40.3–175 d) in the present study () was obtained from the soils under the conditions similar to upland soils with the water content of 60% WHC, which may have caused the relatively slow turnover time.
The turnover time of microbial biomass K in the present study was obtained from the incubation with a microbial metabolism being activated by the CNP application, in which the calculation may have been overestimated. Even so, these results give an indicator for the assessment of the K fertility in paddy fields with organic matter application, in particular application of plant residues such as rice straw to paddy fields under drained conditions after rice harvest.
4.2. Dynamics of microbial biomass K following CNP addition
Carbon is the energy source and the most limiting element to the soil microbial community in most ecosystems. Therefore, nutrient cycles in soils are closely linked with C metabolism as the driving force (van Veen, Ladd, and Amato Citation1985). In the control, amounts of microbial biomass K in Anjo soil were significantly higher than those in Omagari-1 and −2 soils. In addition, amounts of microbial biomass C in Anjo soil was significantly higher than those in Omagari-1 and −2 soils (). These findings agree with the reports by Khan, Heinze, and Joergensen (Citation2009) and Lorenz et al. (Citation2010), in which positive correlations between microbial biomass K and C were detected. In the CNP treatment, the changes in the microbial biomass K were similar to that of the microbial biomass C and N (). Christie and Wasson (Citation2001) reported the addition of glucose and inorganic-N (ammonium and nitrate) enhanced the immobilization of N compared with the addition of inorganic-N alone. Preliminary experiments showed that addition of KCl alone did not increase microbial biomass K (data not shown). Thus, the enhancement of microbial biomass K as well as microbial biomass N could be caused by the addition of glucose rather than K and NP. The reduction of microbial biomass K followed by the subsequent incubation was mostly attributed to the simultaneous reduction of the size of microbial biomass C and N, indicating that microbial K uptake and release were regulated by cellular functions.
4.3. Potassium balance between microbial biomass K and exchangeable K
In the present study, because of no addition of K, the K used for the increase in microbial biomass K was derived from K originally present in the soils. In a closed system in a batch experiment where K loss by leaching did not occur, water-soluble and exchangeable K contents remained constant over the incubation period as shown in the control (). On the early days of incubation (day 5 and 10) in the CNP treatment, slight but significant decrease in the amounts of exchangeable K ranging from 6 to 7 mg K kg−1 soil was observed in the soil samples (), indicating the microbial uptake of exchangeable K. Interestingly, however, the range of increase in the amounts of microbial biomass K (27.4–38.2 mg K kg−1 soil) did not match the decrease in exchangeable K (6.3–7.2 mg K kg−1 soil). Furthermore, the amount of exchangeable K did not increase accompanied with the decrease in the amount of microbial biomass K in the subsequent incubation (). For microbial biomass N, the increase in total N in unfumigated soils accompanied with the decrease in the microbial biomass N was observed in the CNP treatment (data not shown), indicating the sink-source function of microbial biomass N, although denitrification was not taken into account.
We cannot explain why exchangeable K was steadily constant and unaffected by the fluctuations of microbial biomass K. This might implicate that microbial biomass K was originated from K unextractable with NH4OAc, i.e., non-exchangeable K and that exchangeable K was rapidly replenished from non-exchangeable K after K uptake by microorganisms. However, the release rate of non-exchangeable K is much slower than that of exchangeable K. Release rate coefficients of the non-exchangeable K (1.6 × 10−3–2.9 × 10−3 h−1) in loamy sand in Kenansville, USA, were much lower than those of Ca2+-K+ exchange release rate (0.96–1.50 h−1), indicating the low rates of non-exchangeable K release in the soil (Martin and Sparks Citation1983; Sparks and Jardine Citation1984).
On the other hand, incongruities between changes in exchangeable K reserves and K uptake by crops were prevalently acknowledged from the long-term trials in agricultural soils (Khan, Mulvaney, and Ellsworth Citation2014). Khan, Mulvaney, and Ellsworth (Citation2014) also suggested the importance of non-exchangeable K as a reservoir for exchangeable and water-soluble K from the decrease in the amount of non-exchangeable K (from 930–1120 to 240–280 kg K ha−1) over time in the surface soil samples of arable land cropped for 17–20 years without K application. In addition, several reports showed the importance of non-exchangeable K for the plant available K, in which K-bearing minerals such as mica and illite are subject to the biological weathering in rhizosphere (Hinsinger and Jaillard Citation1993; Moritsuka et al. Citation2003; Moritsuka, Yanai, and Kosaki Citation2004). A variety of soil microorganisms can excrete organic acids like citric, oxalic, and tartaric acids, which either directly dissolves mineral or chelating Fe and/or Al ions to bring the K into solution (Liu et al. Citation2006; Basak and Biswas Citation2009; Meena, Maurya, and Verma Citation2014; Khani, Enayatizamir, and Masir Citation2019).
These observations implicit that the biological activities enhance the release rate of non-exchangeable K from minerals, which might lead to replenishing of K into the exchangeable pool. The results observed in the present study further suggested that non-exchangeable K might be released associated with increase in the pool size of the microbial biomass K even under the conditions without K uptake by rice plants during the drained period after harvest. These points need further investigation.
4.4. Difference in microbial turnover time among soils
In Anjo soil, values of the turnover time of 173 and 175 d in microbial biomass C and N were significantly slower than those of microbial biomass C (92.1–105 d) and N (40.3–51.5 d) in Omagari soils. This may be related to the differences in soil texture. The turnover time of microbial biomass C, N, and P in clayey soils was generally slower than that in sandy soils (van Veen, Ladd, and Amato Citation1985; Gregorich, Voroney, and Kachanoski Citation1991; Sakamoto and Hodono Citation2000; Chen and He Citation2002). Sakamoto and Hodono (Citation2000) reported a slower turnover time of microbial biomass C of 215 d in red dark soil with a soil texture of LiC than that of 97 d in brown forest soil with a soil texture of CL. The difference in the turnover time between Anjo soil (LiC) and the Omagari soils (CL) might be related to the physicochemical properties of the soils.
The turnover time of microbial biomass K in Anjo soil (98.5 d) tended to be faster than that of microbial biomass C and N (), although the differences were not statistically significant (P = 0.09). Kouno, Wu, and Brookes (Citation2002) estimated the turnover time of microbial biomass P and C to be 37 and 82 d, respectively, where glucose and KH2PO4 were added to soil. For the faster turnover time of microbial biomass P, they suggested that the P was mostly located in the labile intracellular fraction, while the C was in resistant forms such as the cell wall. The tendency of faster turnover time of microbial biomass K than that of microbial biomass C in the present study may be explained in a similar way; K+ in microbial cells acts more labile than C since K represents as monovalent cation and does not form organic compounds. Chen and He (Citation2002) estimated the turnover time of microbial biomass P in red soils of China to be 63.3 d in a sandy soil, and 96.2 and 119 d in two clayey soils, indicating the effect of the difference in the soil texture as mentioned above. However, there was no significant difference in the turnover time of microbial biomass K among the soils in the present study (). The effect of soil texture on the turnover time of microbial biomass K still needs further investigation.
4.5. Conclusion
The turnover time of microbial biomass K was quantitatively evaluated for the first time using paddy field soils and estimated to be 80.4 to 98.5 d in the present study. The turnover time of microbial biomass K, together with the pool size of microbial biomass K in paddy field soil in the previous studies (40–80 kg microbial biomass K ha−1; Yamashita et al. Citation2014), indicates the importance of microbial biomass K as a source as well as a reservoir of K for rice. The present experiment was conducted under the laboratory conditions with constant temperature of 25°C and water content of 60% WHC, i.e., under aerobic conditions. Further studies will be necessary for the soils under flooded conditions and under field conditions with fertilizer application.
Acknowledgments
We thank Professors A. Watanabe, J. Murase, T. Watanabe, and Dr. F. Shibahara for the valuable comments on this study. We also thank staff members in the Crop Institute, Aichi Agricultural Research Center and the NARO Tohoku Agricultural Research Center for providing soil samples. This work was supported in part by the JSPS KAKENHI (Grant No. 15K07337).
Disclosure statement
No potential competing interest was reported by the authors.
Additional information
Funding
References
- Banerjee, M. R., and S. J. Chapman. 1996. “The Significance of Microbial Biomass Sulphur in Soil.” Biology and Fertility of Soils 22 (1–2): 116–125. doi:10.1007/BF00384442.
- Basak, B. B., and D. R. Biswas. 2009. “Influence of Potassium Solubilizing Microorganism (Bacillus mucilaginosus) and Waste Mica on Potassium Uptake Dynamics by Sudan Grass (Sorghum vulgare Pers.) Grown Under Two Alfisols.” Plant and Soil 317 (1): 235–255. doi:10.1007/s11104-008-9805-z.
- Brookes, P. 2001. “The Soil Microbial Biomass: Concept, Measurement and Applications in Soil Ecosystem Research.” Microbes and Environments 16 (3): 131–140. doi:10.1264/jsme2.2001.131.
- Brookes, P. C., A. Landman, G. Pruden, and D. S. Jenkinson. 1985. “Chloroform Fumigation and the Release of Soil Nitrogen: a Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil.” Soil Biology & Biochemistry 17 (6): 837–842. doi:10.1016/0038-0717(85)90144-0.
- Brown, A. D. 1964. “Aspects of Bacterial Response to the Ionic Environment.” Bacteriological Reviews 28 (3): 296–329. doi:10.1128/br.28.3.296-329.1964.
- Chen, G. C., and Z. L. He. 2002. “Microbial Biomass Phosphorus Turnover in Variable-Charge Soils in China.” Communications in Soil Science and Plant Analysis 33 (13–14): 2101–2117. doi:10.1081/CSS-120005751.
- Christie, P., and E. A. Wasson. 2001. “Short-Term Immobilization of Ammonium and Nitrate Added to a Grassland Soil.” Soil Biology & Biochemistry 33 (9): 1277–1278. doi:10.1016/S0038-0717(00)00237-6.
- Epstein, W. 2003. “The Roles and Regulation of Potassium in Bacteria.” Progress in Nucleic Acid Research and Molecular Biology 75: 293–320. doi:10.1016/s0079-6603(03)75008-9.
- Gregorich, E. G., R. P. Voroney, and R. G. Kachanoski. 1991. “Turnover of Carbon Through the Microbial Biomass in Soils with Different Textures.” Soil Biology & Biochemistry 23 (8): 799–805. doi:10.1016/0038-0717(91)90152-A.
- Hinsinger, P., and B. Jaillard. 1993. “Root-Induced Release of Interlayer Potassium and Vermiculitization of Phlogopite as Related to Potassium Depletion in the Rhizosphere of Ryegrass.” Journal of Soil Science 44 (3): 525–534. doi:10.1111/j.1365-2389.1993.tb00474.x.
- Inubushi, K., and I. Watanabe. 1986. “Dynamics of Available Nitrogen in Paddy Soils. II. Mineralized N of Chloroform-Fumigated Soil as a Nutrient Source for Rice.” Soil Science and Plant Nutrition 32 (4): 561–577. doi:10.1080/00380768.1986.10557538.
- Jenkinson, D. S. 1977. “The Nitrogen Economy of the Broadbalk Experiments. I. Nitrogen Balance in the Experiments.” Rothamsted Experimental Station Report for 1976. Part 2: 103–109. doi: 10.23637/ERADOC-1-34449.
- Jenkinson, D. S., and J. N. Ladd. 1981. “Microbial Biomass in Soil: Measurement and Turnover.” In Soil Biochemistry. Vol. 5, edited by E. A. Paul, and J. N. Ladd, 415–471. New York: Marcel Dekker.
- Jenkinson, D. S., and L. C. Parry. 1989. “The Nitrogen Cycle in the Broadbalk Wheat Experiment: a Model for the Turnover of Nitrogen through the Soil Microbial Biomass.” Soil Biology & Biochemistry 21 (4): 535–541. doi:10.1016/0038-0717(89)90127-2.
- Joergensen, R. G. 1996. ”The Fumigation-Extraction Method to Estimate Soil Microbial Biomass: Calibration of the kEC Value.” Soil Biology & Biochemistry 28 (1): 25–31. doi:10.1016/0038-0717(95)00102-6.
- Kanazawa, S., S. Asakawa, and Y. Takai. 1988. “Effect of Fertilizer and Manure Application on Microbial Numbers, Biomass, and Enzyme Activities in Volcanic Ash Soils. I. Microbial Numbers and Biomass Carbon.” Soil Science and Plant Nutrition 34 (3): 429–439. doi:10.1080/00380768.1988.10415698.
- Khan, K. S., S. Heinze, and R. G. Joergensen. 2009. “Simultaneous Measurement of S, Macronutrients, and Heavy Metals in the Soil Microbial Biomass with CHCl3 Fumigation and NH4NO3 Extraction.” Soil Biology & Biochemistry 41 (2): 309–314. doi:10.1016/j.soilbio.2008.11.001.
- Khan, S. A., R. L. Mulvaney, and T. R. Ellsworth. 2014. “The Potassium Paradox: Implications for Soil Fertility, Crop Production and Human Health.” Renewable Agriculture and Food Systems 29 (1): 3–27. doi:10.1017/S1742170513000318.
- Khani, A. G., N. Enayatizamir, and M. N. Masir. 2019. “Impact of Plant Growth Promoting Rhizobacteria on Different Forms of Soil Potassium Under Wheat Cultivation.” Letters in Applied Microbiology 68 (6): 514–521. doi:10.1111/lam.13132.
- Kouno, K., J. Wu, and P. C. Brookes. 2002. “Turnover of Biomass C and P in Soil Following Incorporation of Glucose or Ryegrass.” Soil Biology & Biochemistry 34 (5): 617–622. doi:10.1016/S0038-0717(01)00218-8.
- Liu, W., X. Xu, X. Wu, Q. Yang, Y. Luo, and P. Christie. 2006. “Decomposition of Silicate Minerals by Bacillus mucilaginosus in Liquid Culture.” Environmental Geochemistry and Health 28 (1–2): 133–140. doi:10.1007/s10653-005-9022-0.
- Lorenz, N., K. Verdell, C. Ramsier, and R. P. Dick. 2010. “A Rapid Assay to Estimate Soil Microbial Biomass Potassium in Agricultural Soils.” Soil Science Society of America Journal 74 (2): 512–516. doi:10.2136/sssaj2008.0391.
- Martin, H. W., and D. L. Sparks. 1983. “Kinetics of Nonexchangeable Potassium Release from Two Coastal Plain Soils.” Soil Science Society of America Journal 47 (5): 883–887. doi:10.2136/sssaj1983.03615995004700050008x.
- Meena, V. S., B. R. Maurya, and J. P. Verma. 2014. “Does a Rhizospheric Microorganism Enhance K+ Availability in Agricultural Soils?” Microbiological Research 169 (5–6): 337–347. doi:10.1016/j.micres.2013.09.003.
- Moritsuka, N., J. Yanai, A. Fujii, S. Sano, and T. Kosaki. 2003. “Evaluation of Readily Available Nonexchangeable Potassium in Soil by Sequential Extractions with 0.01 Molar Hydrochloric Acid.” Soil Science and Plant Nutrition 49 (4): 631–639. doi:10.1080/00380768.2003.10410053.
- Moritsuka, N., J. Yanai, and T. Kosaki. 2004. “Possible Processes Releasing Nonexchangeable Potassium from the Rhizosphere of Maize.” Plant and Soil 258 (1): 261–268. doi:10.1023/B:PLSO.0000016556.79278.7f.
- Okano, S., M. Nishio, and Y. Sawada. 1987. “Turnover Rate of Soil Biomass Nitrogen in the Root Mat Layer of Pasture.” Soil Science and Plant Nutrition 33 (3): 373–386. doi:10.1080/00380768.1987.10557583.
- Owa, N. 2006. “The Fundamentals of Fertilization.” In Encyclopedia of Fertilizers. edited by N. Owa, M. Kimura, M. Koshino, M. Saigusa, T. Tadano, I. Hasegawa, and M. Yoshiba, 207–212. Tokyo: Asakura Publishing. (In Japanese).
- Perrott, K. W., S. U. Sarathchandra, and J. E. Waller. 1990. “Seasonal Storage and Release of Phosphorus and Potassium by Organic Matter and the Microbial Biomass in a High-Producing Pastoral Soil.” Australian Journal of Soil Research 28 (4): 593–608. doi:10.1071/SR9900593.
- Rodríguez-Navarro, A. 2000. “Potassium Transport in Fungi and Plants.” Biochimica et Biophysica Acta 1469 (1): 1–30. doi:10.1016/S0304-4157(99)00013-1.
- Sakamoto, K., and N. Hodono. 2000. “Turnover Time of Microbial Biomass Carbon in Japanese Upland Soils with Different Textures.” Soil Science and Plant Nutrition 46 (2): 483–490. doi:10.1080/00380768.2000.10408801.
- Shibahara, F. 2002. “Studies on Nitrogen Dynamics of Soil Microbial Biomass and Its Significance in Paddy Field Ecosystems.” PhD diss., Nagoya University. (In Japanese).
- Slayman, C. W., and E. L. Tatum. 1964. “Potassium Transport in Neurospora. I. Intracellular Sodium and Potassium Concentrations, and Cation Requirements for Growth.” Biochimca et Biophysica Acta 88 (3): 578–592. doi:10.1016/0926-6577(64)90101-9.
- Smith, J. L., and E. A. Paul. 1990. “The Significance of Soil Microbial Biomass Estimations.” In Soil Biochemistry. Vol. 6, edited by J. M. Bollag, and G. Stotzky, 357–396. New York: Marcel Dekker.
- Soil Survey Staff. 2014. Keys to Soil Taxonomy. 12th ed. Washington, DC: United States Department of Agriculture.
- Sparks, D. L., and P. M. Jardine. 1984. “Comparison of Kinetic Equations to Describe Potassium-Calcium Exchange in Pure and in Mixed Systems.” Soil Science 138 (2): 115–122. doi:10.1097/00010694-198408000-00004.
- Tanudjaja, E., N. Hoshi, Y. H. Su, S. Hamamoto, and N. Uozumi. 2017. “Kup-Mediated Cs+ Uptake and Kdp-Driven K+ Uptake Coordinate to Promote Cell Growth During Excess Cs+ Conditions in Escherichia Coli.” Scientific Reports 7 (2122): 1–9. doi:10.1038/s41598-017-02164-7.
- Urashima, Y. 2013. “Chloroform-Fumigation Method.” In Experimental Methods for Soil Microorganisms. 3rd Ed. edited by Japanese Society of Soil Microbiology, 87–90. Tokyo: Yokendo. (In Japanese).
- van Veen, J. A., J. N. Ladd, and M. Amato. 1985. “Turnover of Carbon and Nitrogen Through the Microbial Biomass in a Sandy Loam and a Clay Soil Incubated with [14C(U)]glucose and [15N](NH4)2SO4 Under Different Moisture Regimes.” Soil Biology & Biochemistry 17 (6): 747–756. doi:10.1016/0038-0717(85)90128-2.
- Vance, E. D., P. C. Brookes, and D. S. Jenkinson. 1987. “An Extraction Method for Measuring Soil Microbial Biomass C.” Soil Biology & Biochemistry 19 (6): 703–707. doi:10.1016/0038-0717(87)90052-6.
- Wessels Perelo, L., M. Jimenez, and J. C. Munch. 2006. “Microbial Immobilisation and Turnover of 15N Labelled Substrates in Two Arable Soils Under Field and Laboratory Conditions.” Soil Biology & Biochemistry 38 (5): 912–922. doi:10.1016/j.soilbio.2005.07.013.
- Wessels Perelo, L., and J. C. Munch. 2005. “Microbial Immobilisation and Turnover of 13C Labelled Substrates in Two Arable Soils Under Field and Laboratory Conditions.” Soil Biology & Biochemistry 37 (12): 2263–2272. doi:10.1016/j.soilbio.2005.02.039.
- Witt, C., U. Biker, C. C. Galicia, and J. C. G. Ottow. 2000. “Dynamics of Soil Microbial Biomass and Nitrogen Availability in a Flooded Rice Soil Amended with Different C and N Sources.” Biology and Fertility of Soils 30 (5): 520–527. doi:10.1007/s003740050031.
- Yamashita, K., H. Honjo, M. Nishida, M. Kimura, and S. Asakawa. 2014. “Estimation of Microbial Biomass Potassium in Paddy Field Soil.” Soil Science and Plant Nutrition 60 (4): 512–519. Corrigendum [2016] 62 (5–6): 570–573. doi:10.1080/00380768.2016.1234137.
- Yamashita, K., H. Honjo, M. Shinohara, and S. Asakawa. 2018. “Conversion Factor (k Factor) for Estimation of Soil Microbial Biomass Potassium by the Chloroform-Fumigation Extraction Method.” Soil Science and Plant Nutrition 64 (4): 465–468. doi:10.1080/00380768.2018.1469953.