?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Wastewater containing high concentrations of ammonium-nitrogen (NH4+-N) is considered a major concern because its untreated discharge has a variety of adverse effects on the environment and human health. Adsorption using biochars is an easy and cost-effective wastewater treatment method. However, aquatic plants such as water hyacinth for biochar feedstock are considered unsuitable for adsorbent use due to limited NH4+-N adsorption capacity. In this study, biochar made from water hyacinth was modified with potassium hydroxide (KOH) and hydrogen peroxide (H2O2) to obtain highly efficient adsorbent. This study aimed to enhance NH4+-N adsorption capacity by KOH- and H2O2-treatments and identify NH4+-N adsorption mechanism of the modified biochars derived from water hyacinth. The NH4+-N adsorption of all biochars was dependent on the initial solution pH increasing from pH 2 to 4, then relatively constant from pH 4 to 8. Pseudo-second-order model and Langmuir model were found to be the best fit for NH4+-N adsorption data. The maximum NH4+-N adsorption capacity of biochars increased about 8 times (17.1 mg g−1) and 10 times (21.5 mg g−1) after KOH- and H2O2-modification, respectively, compared to pristine biochar (2.14 mg g−1). The main NH4+-N adsorption mechanisms were suggested as cation exchange for both biochars particularly KOH-modified biochar, and hydrogen bonding by oxygen-containing surface functional groups for H2O2-modified biochar. This study suggested that aquatic plant-based biochar, which has been considered difficult to use, had potential as a promising alternative adsorbent for removing NH4+-N from wastewater through modification.
1. Introduction
Recent fast economic growth and rapid population expansion have inevitably led to a large amount of wastewaters from human activities (Fulazzaky et al. Citation2015; Van Drecht et al. Citation2009). Ammonium-nitrogen (NH4+-N) is one of the most common pollutants in various wastewaters such as municipal wastewater (Fulazzaky et al. Citation2015) and industrial wastewater (Cho et al. Citation2016). When NH4+-N is not adequately treated from wastewaters before being discharged into aquatic environment, it can cause severe environmental problems such as eutrophication and oxygen depletion in water bodies as well as odor and human health risks (Cao et al. Citation2009; Zhu et al. Citation2012). Therefore, NH4+-N in wastewater must be treated on-site to meet water quality regulations.
There have been developed various methods of treating NH4+-N from wastewater such as air stripping, struvite precipitation, and biological nitrification and denitrification (Cao et al. Citation2021; Liao, Chen, and Lo Citation1995; Rajaniemi et al. Citation2021; Zhao et al. Citation2021). However, these technologies have some disadvantages. For instance, air stripping requires chemicals for pH adjustment, and decreases removal rate in low temperature (<15°C) environments (Taşdemir et al. Citation2020). Struvite precipitation has limited application conditions such as necessity of adding phosphate (PO43–) and magnesium (Mg2+) ions and keeping solution in alkaline pH (>9.5) and low temperature for crystal precipitation (Hakimi, Jegatheesan, and Navaratna Citation2020). A commonly used process in wastewater NH4+-N removal technology is biological nitrification and denitrification, by which NH4+-N is oxidized to nitrate-nitrogen (NO3−-N) and NO3−-N is converted to nitrogen gas (N2), respectively. Although highly efficient, this process is also expensive because it requires aeration for nitrification (Keith, Stephenson, and Semmens Citation1998) and methanol as an external carbon source for denitrification depending on C/N ratio of wastewater. This makes it difficult to implement this technology in regions such as developing countries. Nevertheless, these wastewater treatment technologies should be carefully selected according to the characteristics (e.g., NH4+-N concentrations and pH) of the targeted wastewater.
On the other hand, one of cost-effective and low-tech wastewater treatment technologies that can be used in developing countries is using adsorbents for NH4+-N (Fan et al. Ruemei et al. Citation2019), although NH4+-N removal efficiency may be lower than the above-mentioned physical, chemical, and biological removal technologies. Besides NH4+-N removal from wastewater, adsorbents to which NH4+-N is adsorbed can be used for soil application purposes such as fertilizer and soil amendments, which have also been well studied (Kizito et al. Citation2019; Zhixiong et al. Citation2019). Artificial adsorbents such as zeolite (Mingyu et al. Citation2011), bentonite (Angar, Eddine Djelali, and Kebbouche-Gana Citation2017), and ion exchange resin (Sica et al. Citation2014) are highly effective for NH4+-N removal. However, issues remain in terms of the complexity of the production process and low yield as final products (Han et al. Citation2021). Therefore, it is necessary to select appropriate feedstock for adsorbents that are renewable and easy to collect. For instance, one of typical examples of unutilized plant biomass is water hyacinth (Eichhornia crassipes), which has been overgrowing in watershed areas around the world in recent years (e.g., Lake Tana in Ethiopia). This invasive plant can cause problems such as reducing oxygen production in water bodies (Muche, Mucheye, and Tadesse Citation2020) and reduced biodiversity of native aquatic species (Jianbo et al. Citation2007). For these reasons, there is an urgent need to establish treatment and effective utilization methods of water hyacinth biomass.
Treatment methods of water hyacinth biomass such as composting and agricultural materials (i.e., mulching) have been developed (Jiwan and Kalamdhad Citation2013). However, problems of such methods include necessity of space and time for process, odor produced during process, and inability of deactivation of seeds. On the other hand, one of effective treatments of water hyacinth biomass is thermal treatment (e.g., pyrolysis) to convert to biochar. Biochar is obtained by the pyrolysis of biomass under oxygen-limited conditions, and can be a potentially efficient adsorbent material (Han et al. Citation2021). This is because biochar generally has porous structure (i.e., macro, meso, and micro pores), abundant amount of oxygen-containing functional groups on its surface (i.e., carboxylic and phenolic hydroxyl groups), and high cation exchange capacity (CEC) (Haipeng et al. Citation2017). In particular, the surface charge of biochar is generally negative (Yin, Wang, and Zhao Citation2018). Therefore, these properties make biochar suitable as adsorbent for NH4+-N.
However, pristine biochars have limited NH4+-N adsorption capacity. Previous reports have compared NH4+-N adsorption capacity between clinoptilolite (commercially available adsorbent) and biochars made from various biomass including agricultural residues, wood, animal wastes, and aquatic wastes (Song et al. Citation2019). It was found that clinoptilolite had significantly higher NH4+-N adsorption capacity than any other biochars. Among the biochars, aquatic plant-based biochar (i.e., water hyacinth) showed the lowest NH4+-N adsorption capacity. This is considered to be due to the lack of carboxylic and phenolic groups in aquatic plant-based biochar.
To improve adsorption capacity of biochars for NH4+-N, various modification methods (i.e., alkali-treatment, oxidation, magnetic coating, and metal oxides-treatment) on biochars have been studied (Wu et al. Citation2020; Yin, Wang, and Zhao Citation2018). Different modification methods are employed to improve NH4+-N adsorption capacity through changing physicochemical properties of biochars for different purposes such as removal of nutrients, heavy metals, and chemical dyes, inclusively called modified/engineered/designer biochars (Rajapaksha et al. Citation2016). The type of activator (e.g., H2O2, HNO3, HCl, H2SO4, KOH, NaOH, Fe, Mg, Al, Ca, and La), modification method (e.g., before or after pyrolysis, impregnation, and stirring), modification duration, and modification temperature all affect the properties of the modified biochars (Yang et al. Citation2019).
Among those modifications, for example, metal oxides treatment is very costly depending on the kind of metal used, and there was not much improvement in the adsorption capacity of NH4+-N (Yin, Wang, and Zhao Citation2018). Magnetic coating is easy to recover from the water where it is applied (Kołodyńska et al. Citation2017), but a very dangerous process due to the use of highly toxic iron chloride and secondary pollution. Therefore, alkali- and oxidation-treatment, which are relatively easy and inexpensive to treat, are more practical. It has been reported that the NH4+-N adsorption capacity could be greatly improved by the modification of various feedstocks with potassium hydroxide (KOH) and hydrogen peroxide (H2O2). Previous studies have suggested that the main mechanisms for improved NH4+-N adsorption by KOH-treatment included (1) increased exchangeable potassium cation (K+) from sylvite (KCl) crystal for exchanging with NH4+-N (Hsu et al. Citation2019), and (2) increased pore volume and specific surface area caused by KOH-activation during pyrolysis for increased NH4+-N filling and surface adsorption. In case of H2O2-treatment, surface oxidation caused an increase in deprotonated (negatively charged) oxygen-containing surface functional groups such as carboxyl, carbonyl, phenolic hydroxyl, and lactone groups, resulting in improved NH4+-N adsorption by electrostatic attraction (Wang et al. Citation2015). Yet, such mechanisms have not been fully understood. In addition, there have been no reports for improved NH4+-N adsorption capacity by KOH- and H2O2-modifications for biochars derived from waste biomass particularly water hyacinth, of which pristine biochar are considered difficult to utilize as adsorbent for NH4+-N. Therefore, the objectives of this study were to: (1) enhance the NH4+-N adsorption capacity by KOH- and H2O2-treatments; and (2) identify the NH4+-N adsorption mechanism of the modified biochars derived from water hyacinth.
2. Materials and methods
2.1. Collection and preparation of water hyacinth
Water hyacinth (WH) sample used in this study was collected from rice paddies adjacent to a roadside station in Kazo City, Saitama Prefecture, Japan. The collected WH was immediately transported to laboratory at Soka University and crushed and squeezed to separate liquid and solid fractions. Then, the solid fraction of WH was dried in a dryer at 80°C for 48 h. The dried WH was washed several times with reverse osmosis water and then dried in the dryer at 45°C for 48 h. The re-dried WH was crushed and sieved to a size of 0.5–2.0 mm. The sieved WH was used to make three different biochar samples: pristine biochar without any modifications (BC), KOH-treated biochar (KBC), and H2O2-treated biochar (HBC).
2.2. Production of pristine and KOH- and H2O2-treated biochars
For BC production, the WH was pyrolyzed at 350°C in a steel container placed in an electric muffle furnace (FUW242PA, ADVANTEC) with a heating rate of 5°C min−1 under limited oxygen supply, and 2 h of retention time at the highest heating temperature. Based on a preliminary experiment, KOH-modified biochar pyrolyzed at 350°C showed higher NH4+-N adsorption amount (5.03 mg g−1) than that at 550°C (2.60 mg g−1). Therefore, the pyrolysis temperature of 350°C was selected for this study. After pyrolysis, biochar and deionized water were mixed in a plastic bottle at a solid–liquid ratio of 1:100, shaken for 24 h using a reciprocating shaker (SR-1, TAITEC), filtered, and dried in the oven at 105°C for 24 h.
For KBC production, the sieved WH material (0.5–2.0 mm) was mixed with a solution of the optimum KOH concentration (0.5 mol L−1; determined in Section 3.2) at a solid–liquid ratio of 1:50 and treated with stirring for 6 h at room temperature and dried in the oven at 105°C for 24 h. The KOH-pretreated WH was then pyrolyzed at the same conditions as BC. After pyrolysis, KBC was washed at the same method as BC, filtered, and dried in the oven at 105°C for 24 h.
The HBC production was referred to Wang et al. (Citation2015a). The sieved BC (<500 µm) and 30% H2O2 solution were pre-treated by stirring for 12 h at room temperature with a solid–liquid ratio of 1:50. Then, H2O2-treated BC was filtered by filter paper (Whatman No. 1), washed with deionized water, dried in the oven at 105°C for 24 h, and dried HBC was mixed with deionized water at a solid–liquid ratio of 1:100. The pH of the mixture was adjusted to the optimum pH of 12.0 (determined in Section 3.2) to show the maximum NH4+-N adsorption amount using 0.1 mol L−1 NaOH by constant stirring with pH adjustment every hour until an equilibrium reached. After adjusting the pH of the HBC, the mixture was dried in the oven at 105°C without filtration until only solids remained.
After above mentioned treatments, all biochars were sieved by a mesh with a pore diameter of <500 µm and stored in the dryer at 45°C until further uses.
2.3. Selection of the optimal modification methods for the modified biochars
Preliminary adsorption experiments were carried out to select the optimal modification methods for biochars; the optimum KOH concentration and pH level for KBC and HBC, respectively. Different KOH concentrations (0.1, 0.25, and 0.5 mol L−1) to treat WH were tested in the process of KOH preparation, and different pH levels of the HBC mixture (pH 7.0, 8.0, 9.0, 10.0, 11.0, and 12.0) were tested in the process of HBC preparation.
Ammonium chloride (NH4Cl) powder was dissolved in deionized water to prepare ammonium solution with a concentration of 100 mg-N L−1. The pH of the solution was adjusted to 7.00 ± 0.05 using 0.1 mol L−1 HCl or NaOH solutions. Then, 0.05 g of each biochars (BC, KBC, and HBC) were added into the 50 mL centrifuge tube with 25 mL of ammonium solution. The same solid–liquid ratio (1:500) was used in all subsequent adsorption experiments. The mixture was placed in a reciprocating shaker to shake at 160 strokes min−1 for 24 h at room temperature. After shaking, the mixture was filtered by 0.45 µm pore size nylon membrane filter. Then, the concentration of NH4+-N in the filtrate was measured using an auto-analyzer (FIAlyzer-1000, FIAlab Instruments). All adsorption experiments were performed in triplicate. The concentration of NH4+-N adsorbed onto the biochars were calculated by EquationEquation (1)(1)
(1) .
where qe (mg g−1) is the amount of NH4+-N adsorbed by biochar; C0 (mg L−1) and Ce (mg L−1) are the initial and equilibrium concentration of NH4+-N, respectively; V (L) is the volume of solution; and M (g) is the mass of biochar. Volatilized NH3 in tubes during adsorption experiment was insignificant for the amount of NH4+-N adsorbed by biochar.
2.4. Batch ammonium adsorption experiments
2.4.1. Adsorption kinetics experiment
To assess the effects of contact time, adsorption kinetic studies were carried out where the samples were shaken at different interval times (15, 30, 60, 120, 240, 480, 720, and 1440 min). The initial NH4+-N solution concentration and pH were 20 mg-N L−1 and 7.00 ± 0.05, respectively. The concentration of NH4+-N adsorbed onto the biochars were calculated by the EquationEquation (1)(1)
(1) . The experimental results were fitted with the following three adsorption kinetic models (2, 3, and 4):
where qe and qt (mg g−1) refer to the adsorption amount at equilibrium and the adsorption amount homologous to the reaction time t (min) of NH4+-N; k1 (min−1), k2 (g mg−1 min−1), and k3 (mg g−1 min−0.5) are the respective rate constants of the three models; C (mg g−1) is a desorption constant of intra-particle diffusion model.
2.4.2. Effect of different ammonium solution pH
The effect of different pH of the initial ammonium solution on NH4+-N adsorption was investigated with different pH levels (2.0, 4.0, 6.0, and 8.0 ± 0.05). To prevent solution pH change due to CO2 absorption (particularly for pH 8.0 solution), all pH-adjusted solutions were immediately inserted to the centrifuge tube and mixed with the biochar. The initial NH4+-N concentration and contact time were 20 mg N L−1 and 240 min (determined in Section 2.4.2), respectively. The pH of the filtrate (equilibrium pH) was measured after every adsorption experiment and the concentration of NH4+-N was calculated by EquationEquation (1)(1)
(1) .
2.4.3. Adsorption isotherm experiment
To assess the effect of the initial NH4+-N concentration on adsorption, different NH4+-N concentrations were used (10, 20, 40, 80, 100, 150, 200, 300, 500, and 1000 mg-N L−1). The initial NH4+-N solution pH and contact time were 4.0 ± 0.05 (determined in Section 2.4.2) and 240 min (determined in Section 2.4.1), respectively. The concentration of NH4+-N adsorbed onto the biochars was calculated by the EquationEquation (1)(1)
(1) . The experimental results were fitted with the following two adsorption isotherm models (5 and 6):
where qe (mg g−1) and Ce (mg L−1) refer to the adsorption amount of NH4+-N in equilibrium and the concentration of NH4+-N in solution, respectively; qm (mg g−1) represents the maximum adsorption capacity of NH4+-N by different adsorbents; KL is the adsorption affinity constant; KF stands for Freundlich constant; 1/n is non-linear constant.
2.5. Biochar characterization
The pH of the biochar was measured using an automatic electric pH meter (F-71AC, HORIBA) after shaking for 1 h in deionized water at a solid–liquid ratio of 1:10. Total potassium (T-K) of biochars after nitric acid/sulfuric acid digestion was analyzed by measuring the absorbance at a wavelength of 766 nm with a flame atomic absorption spectrometer (AAnalyst 200, Perkin Elmer). N2 adsorption/desorption was performed using Micromeritics ASAP 2020 at 77 K and relative pressure of 0.0 to 1.0 to measure pore structure characteristics. Each sample was degassed at 573 K for 10 h. The specific surface area and pore volume were calculated by Brunauer-Emmett-Teller (BET) method and Barrett-Joyner-Halenda (BJH) method, respectively. Determination of CEC was carried out according to the standard protocol (Graber et al. Citation2017). The point of zero charge (pHPZC) of biochars was determined by solid addition method (Hafshejani et al. Citation2016).
2.6. FTIR analysis
Spectral analysis was carried out for each biochar before and after NH4+-N adsorption. The biochar samples after NH4+-N adsorption were prepared separately for Fourier transform infrared spectroscopy (FTIR) analysis using the same initial NH4+-N concentration of 1000 mg-N L−1 which was the highest initial concentration under the same experimental conditions as the adsorption isotherm experiment (Section 2.4.3). The biochar samples were filtered by filter paper (Whatman No. 1) and dried in an oven together with the paper at 105°C for 24 h. After drying, the NH4+-N adsorbed biochars were separated from the paper and sieved to <500 µm. The adsorbed NH4+-N amounts by these biochar samples were confirmed by analyzing the remaining NH4+-N in the filtrates, and were equivalent to those by biochar samples from the adsorption isotherm experiment. Biochar samples were analyzed by FTIR after mixing 1.0 mg of sample with spectral-grade 100 mg KBr and ground in an agate mortar. The mixture was then compressed at about 7845 kPa for 2 min into a 13 mm sample pellet. A background spectrum was obtained each time before the samples were processed. All spectra between 4000 and 400 cm−1 were collected at a spectral resolution of 2 cm−1 and 64 scans. Spectral analysis was performed using Omnic 9 software (Thermo Scientific).
2.7. Statistical analyses
Statistical analyses were carried out using the statistical software Statistica 6.1 (StatSoft. Inc., Tulsa, OK, U.S.A.). Treatment effects were analyzed by one-way analysis of variance (ANOVA). A Tukey honestly significant difference (HSD) analysis was performed for multiple comparisons of the treatment effects. Statistical significances were determined at p < 0.05.
3. Results and discussion
3.1. Selection of the optimal modification methods for the modified biochars
Results of adsorbed NH4+-N of KBC treated with different KOH concentrations showed that no significant difference was observed between 0.1 mol L−1 KBC (3.71 mg g−1) and 0.25 mol L−1 KBC (3.74 mg g−1; ). The highest NH4+-N adsorption was observed at 0.5 mol L−1 KBC (6.39 mg g−1) and significantly higher than all other KBC. The reason for the increased NH4+-N adsorption of KBC treated with KOH from 0.1 to 0.5 mol L−1 compared to BC (1.48 mg g−1) was probably due to increased biochar CEC (Takaya et al. Citation2019). Therefore, KOH of 0.5 mol L−1 was selected as optimum modification method for production of KBC for further experiments in this study (detailed explanation of biochar physicochemical properties and adsorption mechanism are in Section 3.2 and Section 3.5, respectively).
Figure 1. Adsorbed NH4+-N from (a) KOH-treated biochars (KBC) and (b) H2O2-treated biochars (HBC).
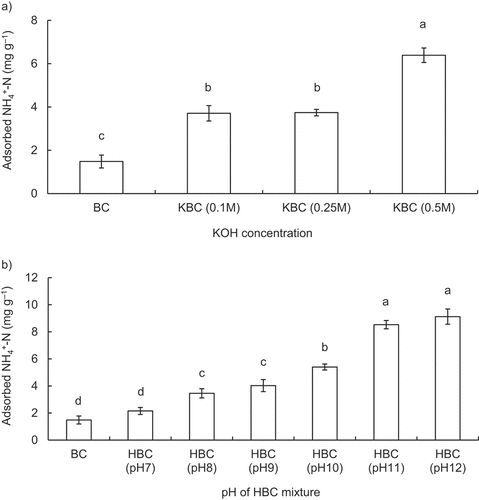
Results of adsorbed NH4+-N of HBC-treated with different pH of HBC mixture showed an increasing trend from pH 7.0 up to 12.0 (). The highest NH4+-N adsorption was observed with HBC treated with pH 12 mixture (9.12 mg g−1), and significantly higher than all other pH levels except for 11.0. This might be attributed to the fact that the biochar surface functional groups became more negatively charged due to pH adjustment, resulting in increased NH4+-N adsorption by electrostatic attraction. According to Wang et al. (Citation2015a), the maximum NH4+-N adsorption (5.44 mg g−1) was found when pH of H2O2-treated biochar was adjusted from 3.69 to 7.0, while the amount of NH4+-N adsorption decreased for biochars when pH was adjusted from 4.37 (2.77 mg g−1) and 5.97 (1.22 mg g−1) to 7.0, respectively. It was suggested that deprotonation of functional groups by pH adjustment of biochar was a factor in improving NH4+-N adsorption. Therefore, in this study, when the original pH of HBC (3.40; ) was adjusted to pH 7.0 up to 12.0, the deprotonation may have proceeded more, resulted in more negatively charged surface on HBC. In fact, the equilibrium pH and the amount of adsorbed NH4+-N of HBC adjusted pH to 7.0 to 12.0 showed a significant positive correlation (Figure S1b; R2 = 0.943; p < 0.01), suggesting that in this pH adjustment range negatively charged oxygen-containing surface functional groups caused by deprotonation may have adsorbed more NH4+-N by more electrostatic attraction. Therefore, pH adjustment to 12.0 for HBC was selected as the optimum modification method for production of HBC for further experiments in this study.
Table 1. Selected physicochemical properties of water hyacinth biochars.
3.2. Basic properties of selected biochars
The physicochemical properties of biochars used in this study are shown in . In general, the biochar pH increased as the pyrolysis temperature increased due to the degradation of acidic surface functional groups (Xiaojian et al. Citation2020). Since BC was pyrolyzed at a low temperature (350°C) in this study, its pH value was weakly acidic (6.39). On the other hand, KBC showed a high pH value (10.1) due to the strong alkali treatment, and HBC showed strong acidic pH values (3.40) before pH adjustment due to the oxidation-treatment using acidic hydrogen peroxide (pH 2–4). The pHPZC value of BC showed almost the same value as pH itself of BC (6.52). If the biochar pH does not differ from the biochar pHPZC, biochar has no charges (Hellen, Mlsna, and Wipf Citation2021). The pHPZC values for KBC (9.42) and HBC (9.22) increased compared to that of BC, as shown in other study (Zhang et al. Citation2021). Although the changes of pHPZC by alkali-treatments of biochars have not been well documented, one of possible reasons may include increases of oxygen-containing surface functional groups such as carboxylic (–COOH) and phenolic (–OH) groups on biochar surface by the treatment, as seen later in FTIR results for the biochars used in this study (i.e., ; detailed explanation of the surface functional groups of biochars are in Section 3.5). Biochar CEC increased by both treatments resulted in 107 and 104 cmol+ kg−1 for KBC and HBC, respectively, compared to that for BC (76.6 cmol+ kg−1). One of reasons for the higher CEC in KBC and HBC may be due to new exchangeable sites created by deprotonation (negatively charged) through alkali- and oxidation (following high pH adjustment)-treatments on biochar surfaces. The BET surface area and pore volume were detected only in KBC (6.77 m2 g−1 and 0.0255 cm3 g−1), but not in BC and HBC. Previous studies showed that small or non-detectable specific surface area of biochars pyrolyzed at low temperatures was due to the formation of tar during pyrolysis process preventing the formation of pores. However, when pyrolysis was carried out at high temperatures, pores were easily generated due to the volatilization of tar compounds (Ahmad et al. Citation2013). Although pyrolysis temperature was same as BC and HBC (350°C), BET surface area and pore structure were observed only in KBC because KOH attached to the biochar surface acted as an activator during pyrolysis, which activated the biochar surface and formed the pore structure.
3.3. Adsorption kinetics of biochars
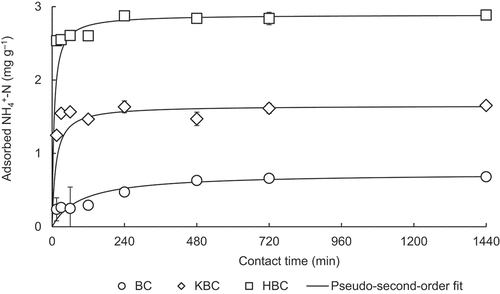
The pseudo-first-order model is used to describe reversible physical adsorption (Yin, Wang, and Zhao Citation2018) and chemical adsorption processes (Largitte and Pasquier Citation2016), while the pseudo-second-order model can describe chemical adsorption between adsorbent and adsorbate (Yin, Wang, and Zhao Citation2018).
The pseudo-second-order model (R2 = 0.991–0.999; ; Figure S2b) better fitted to kinetic data from all biochars than the pseudo-first-order model did (R2 = 0.172–0.986; ; Figure S2a). In particular, since KBC and HBC kinetics data were nearly perfectly fitted by the second-order model, NH4+-N adsorption of both biochars was considered to be mainly due to chemical adsorption (Yin, Liu, and Ren Citation2019). Chemical adsorption mainly involves exchange or sharing of ions, precipitation, complexation, and chemical reactions with functional groups (Defu et al. Citation2019; Takaya et al. Citation2016).
Table 2. Parameters of adsorption kinetic models for NH4+-N on biochars.
On the other hand, kinetic data from only BC were fitted to both pseudo-first-order and pseudo-second-order models with relatively similar goodness of fit (R2 = 0.986–0.991; ; Figure S2a; Figure S2b). It is suggesting that NH4+-N adsorption on BC might have been regulated by a combined physical and chemical adsorption mechanisms (Yin, Wang, and Zhao Citation2018). To clarify possible physical adsorption mechanism in more details, intra-particle diffusion model was applied to evaluate rate-controlled steps during physical adsorption on biochars (Yin, Wang, and Zhao Citation2018). Diffusion rate of solvent is approximately in proportion to the half of squared time (t0.5) rather than t, and both film diffusion and solvent diffusion controls the intra-particle diffusion process (Önal, Akmil-Başar, and Sarici-Özdemir Citation2007). In general, this adsorption process includes three steps: (1) external mass transfer (e.g., boundary layer diffusion); (2) intra-particle diffusion; and (3) saturation (Yang, Zifu, and Mahmood Citation2014). However, in this study only two major steps appeared to be found ( and Figure S2c). The intra-particle diffusion model showed a high goodness of fit (R2 = 0.970) up to the first 480 min (first stage) of contact with a steep gradient on BC (k3a = 0.106 mg g−1 min−0.5; ; Figure S2c). It indicates a rapid external diffusion step that represents mass transfer taking place in the diffusion boundary layer during the first stage (Zhong et al. Citation2019). The intercept of the straight line at the first stage does not pass through the origin (C1 = 0.219), indicating that intra-particle diffusion was not the only factor affecting the adsorption rate, rather involving other adsorption processes such as liquid membrane diffusion and surface adsorption (Jiang et al. Citation2019). Next, the diffusion rate in the second stage (>480 min) clearly decreased with a slope of 0.00571 mg g−1 min−0.5 ( and Figure S2c), suggesting that the slow intra-particle diffusion and saturation reach to gradual equilibrium during the second stage (Yang, Zifu, and Mahmood Citation2014). On the other hand, KBC and HBC did not completely fit the intra-particle diffusion model (R2 = 0.283–0.558; ; Figure S2c). Thus, NH4+-N adsorption of these treated biochar was clearly related to chemical factors rather than diffusion reactions.
Based on the parameter, k2 (the rate constant), of the pseudo-second-order models (), KBC and HBC resulted in faster adsorption process compared to BC. The NH4+-N adsorption by KBC and HBC appeared to have reached equilibrium at around 240 min, and that by BC at around 480 min (). Contact time required to reach 90% of the adsorbed amounts at 1440 min was 57, 114, and 503 min for HBC, KBC, and BC, respectively. Therefore, it can be inferred that faster chemical adsorption could have occurred with KBC and HBC than with BC. A similar NH4+-N adsorption trend was observed by Wang et al. (Citation2020), where H2O2-treated biochar exhibited rapid adsorption behavior compared to untreated, possibly due to relatively fast chemical interactions. Based on the results of this experiment, the contact time used for further adsorption experiments was set to 240 min.
3.4. Optimization of initial solution pH for adsorption
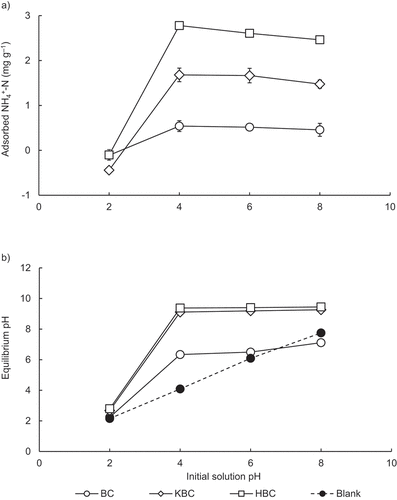
In this study, NH4+-N adsorption did not occur at the initial solution pH of 2.0, however increased at pH 4.0, and remained relatively constant at higher pH up to 8.0 for all biochars (). When the initial solution pH was 2.0, the equilibrium pH after NH4+-N adsorption was also around 2.0 for all biochars (), suggesting that a large amount of H+ in the solution occupied negatively charged surface of adsorption sites (oxygen-containing surface functional groups) negating electrostatic attraction for NH4+. Therefore, around neutral conditions may generally be preferred for NH4+-N adsorption on biochars (Zhang, Tang, and Guan Citation2020). While, in this study, the initial solution pH was increased to 4.0 to 8.0, the equilibrium pH ranged 6.3–7.1, 9.1–9.3, and around 9.4 for BC, KBC, and HBC, respectively, probably favoring NH4+-N adsorption on biochars (). Zheng and Wang (Citation2009) also found similar results with hydrogel composites resulting in maximum NH4+ adsorption at a pH value of 5.0, followed by a gradual decrease in NH4+-N adsorption with increasing pH thereafter. Based on the results of this experiment, the initial pH of the NH4+-N solution used for further adsorption experiments was set to 4.0.
3.5. Adsorption isotherm and mechanism of biochars
As the equilibrium concentration of the solution increased, NH4+-N adsorption was clearly enhanced by KBC and HBC compared to BC (). Adsorption isotherms of all biochars fit well to both Langmuir and Freundlich isotherm models, overall better to Langmuir (R2 = 0.972–0.996) than Freundlich (R2 = 0.704–0.978) model (; Figure S3). The Langmuir maximum NH4+-N adsorption capacity (qm) was about 8 times higher for KBC (17.1 mg g−1) and about 10 times higher for HBC (21.5 mg g−1) compared to BC (2.14 mg g−1), and those of KBC and HBC were significantly higher than that of BC (). A relationship between adsorbed NH4+-N and equilibrium pH showed a weak negative correlation for BC (R2 = 0.418; p < 0.05), while strong negative correlations were obtained for KBC (R2 = 0.881; p < 0.001) and HBC (R2 = 0.903; p < 0.001), respectively (). This result indicates a tendency for NH4+-N adsorption decreases as the equilibrium pH increases. Equilibrium pH for BC (6.03–6.40) remained almost same as pH of BC itself (6.39) being slightly acidic, which could have caused low NH4+-N adsorption due to strong competition with H+ on adsorption sites. Strong alkalinity of the treated biochars (pH 10.1 and 12.0 for KBC and HBC, respectively) may have only slightly neutralized by small amounts of NH4+ (low range in adsorption isotherm) electrostatically attracted to OH– on the biochar surface, which resulted in still high equilibrium pH after NH4+-N adsorption. On the other hand, when large amounts of NH4+ was added (high range in adsorption isotherm), alkalinity of the treated biochars could have largely neutralized by NH4+ electrostatic attraction to OH– on the surface, which resulted in lowered equilibrium pH after NH4+-N adsorption ().
Figure 4. Adsorption isotherms of NH4+-N on biochars for (a) Langmuir model fit of experimental data plot and (b) correlation between amount of adsorbed NH4+-N and equilibrium pH.
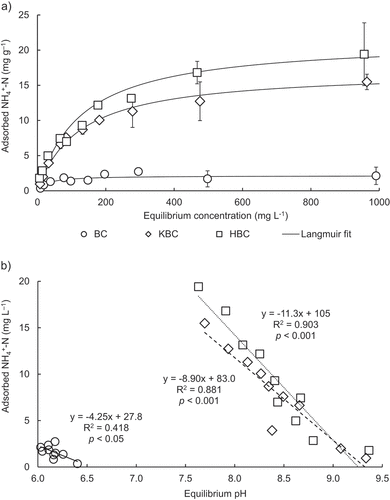
Table 3. Parameters of adsorption isotherm models for NH4+-N on biochars.
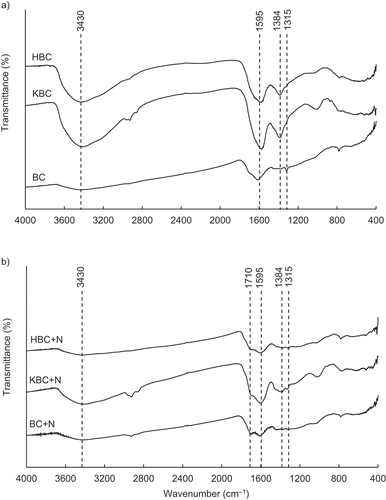
Other possible mechanism for the increased NH4+-N adsorption capacity of KBC compared to that of BC may include cation exchange reaction associated with increased K+ attached to the biochar deprotonated oxygen-containing sites (), as mentioned above (Section 3.2). To elucidate adsorption mechanism of NH4+-N on HBC, FTIR of biochars before and after NH4+-N adsorption process was measured (). The FTIR results before NH4+-N adsorption showed that the peaks of the functional groups were clearly (strongly) seen in HBC than in BC (). This is possibly due to the effect of oxidation by H2O2-treatment. In general, the functional groups used in adsorption can be identified by examining the peak changes before and after adsorption (i.e., appearance of new peaks or decrease/disappearance in peak strength). The reduction of peaks was observed at 1595 cm−1 after NH4+-N adsorption possibly due to C=C stretching that is attributed to the aromatic C – C bonds and asymmetric – COO– stretching (Farinella, Matos, and Arruda Citation2007) of the deprotonated carboxylic acid functional groups of cellulose resulting in negatively charged surface on which electrostatic attraction may have occurred with positively charged NH4+. In detail, the H2O2-treatment increased oxygen-containing functional groups (e.g., carboxyl groups) on the biochar surface, and deprotonation during the pH adjustment process (3.40 to 12.0) is considered to have resulted in an increase in negatively charged biochar surface as shown in EquationEquation (7)(7)
(7) :
The deprotonated biochar surface could have an effective capacity to adsorb positively charged NH4+ by electrostatic attraction as shown in EquationEquation (8)(8)
(8) :
The reduction of peaks of 1300–1384 cm−1 between before and after NH4+-N adsorption was observed, which corresponded to stretching vibrations of symmetric or asymmetric ionic carboxylic groups (–COO–) of pectin for another possible adsorption site. KBC and HBC showed a strong peak around 3430 cm−1, which could be considered as a stretching vibration of the hydroxyl group (–OH) formed by the dehydration of cellulose or hemicellulose derived from water hyacinth (Abdulrazzaq et al. Citation2014). These peaks were clearly weakened after NH4+-N adsorption, suggesting that the oxygen-containing surface functional groups were involved in NH4+-N adsorption on all biochars. Moreover, the extent of decrease in the peaks of oxygen-containing surface functional groups of HBC was clearly larger than those of KBC and BC. Generally, surface functional groups that weakened or disappeared their peaks after NH4+-N adsorption could appear as new peaks and be seen at different wavelengths. A new peak appeared after NH4+-N adsorption at 1710 cm−1 indicating carbonyl (C=O) bond stretching vibrations due to nonionic carboxylic groups (e.g., –COOH, –COOCH3), which probably attributed to hydrogen bonding between carboxylic acids or their esters and NH4+ (Wahab, Jellali, and Jedidi Citation2010). Therefore, it is suggested that adsorption by the oxygen-containing surface functional groups can account for a large proportion of NH4+-N adsorption mechanism for HBC.
3.6. NH4+-N adsorption capacity of biochars of different feedstocks and modifications
Among different feedstocks for biochars, the NH4+-N adsorption capacity of biochars (pristine) derived from water hyacinth showed relatively low compared to those derived from other feedstock such as wheat straw, peanut straw, and mixed wood (). Difference in the NH4+-N adsorption capacity may be attributed to their different specific surface physicochemical structures (Gai et al. Citation2014). However, compared to the report by Song et al. (Citation2019) using the unmodified biochar derived from water hyacinth, the pristine biochar (BC) in this study showed somewhat better NH4+-N adsorption capacity.
Table 4. Comparison of the maximum NH4+-N adsorption capacity onto various biochars.
Furthermore, the NH4+-N adsorption capacity of biochars with alkali- and oxidation-treatment (KBC and HBC in this study, respectively) was comparable or slightly higher than those with the similar modification treatments but with different feedstocks (). These results indicate that biochar derived from aquatic plant-based feedstock, which has been considered difficult to use as adsorbent, has a potential to enhance NH4+-N adsorption capacity equal to or higher than other feedstock biochar after modification. Therefore, it is suggested that modified water hyacinth biochar can be a promising alternative adsorbent for the removal of NH4+-N from wastewater.
The improved NH4+-N adsorption of biochar from water hyacinth by different treatments can expand practical applications such as wastewater treatment and agriculture. For example, to treat a domestic wastewater containing 50 mg-N L−1 (Cruz et al. Citation2018), 100 g of the H2O2-treated biochar can treat 10 (ten) liters of the wastewater (based on the adsorption amount of HBC of about 5.0 mg g−1 at an initial concentration of 40 mg L−1; ). However, this may not be a sustainable approach due to substantial amounts of biochar required because if only biochar is used to treat, for example, 1 (one) ton of daily wastewater, 10 kg of HBC is required daily. Therefore, it is necessary to consider combining this method with other widely used and highly efficient treatment methods, such as the ammonia stripping method. Another practical use of HBC adsorbed with NH4+-N may be as fertilizer. If a common application rate of biochar at 20 t ha−1 is used to agricultural field for crop production, approximately 400 kg-N ha−1 can be applied with HBC maximally-adsorbed with NH4+-N. This amount of nitrogen can sufficiently substitute for nitrogen amount applied from common application rates of urea as nitrogen source at 100–200 kg ha−1 (47–94 kg-N ha−1) although the total amount of nitrogen from HBC applied to soil may not be available for plant uptake.
4. Conclusions
In this study, the NH4+-N adsorption capacity of the modified water hyacinth biochars and the respective adsorption mechanism by different modifications of biochar were investigated. The results showed that the contact time, initial solution pH, and initial solution concentration had significant effects on the NH4+-N adsorption capacity of biochars. The KBC and HBC showed significantly higher NH4+-N adsorption capacity than BC, indicating the positive effect of biochar modification on NH4+-N adsorption capacity. For KBC and HBC, the cation exchange reaction was a major contributor to the NH4+-N adsorption mechanism, while for HBC, the oxygen-containing surface functional groups may be the additional main active sites for NH4+-N adsorption. These results indicate that biochars derived from aquatic plant-based feedstocks such as water hyacinth, which show limited NH4+-N adsorption capacity, have the potential to be used as an excellent adsorbent through alkali- and oxidation-modifications for NH4+-N.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abdulrazzaq, H., H. Jol, A. Husni, and R. Abu-Bakr. 2014. “Characterization and Stabilisation of Biochars Obtained from Empty Fruit Bunch, Wood, and Rice Husk.” BioResources 9 (2): 2888–2898. https://doi.org/10.15376/biores.9.2.2888-2898.
- Ahmad, M., S. Soo Lee, A. Upamali Rajapaksha, M. Vithanage, M. Zhang, J. Sik Cho, S. Eun Lee, and O. Yong Sik. 2013. “Trichloroethylene Adsorption by Pine Needle Biochars Produced at Various Pyrolysis Temperatures.” Bioresource Technology 143:615–622. https://doi.org/10.1016/j.biortech.2013.06.033.
- Angar, Y., N. Eddine Djelali, and S. Kebbouche-Gana. 2017. “Investigation of Ammonium Adsorption on Algerian Natural Bentonite.” Environmental Science and Pollution Research 24 (12): 11078–11089. https://doi.org/10.1007/s11356-016-6500-0.
- Cao, Q., L. Xiaochuan, H. Jiang, W. Han, Z. Xie, X. Zhang, N. Li, et al. 2021. “Ammonia Removal Through Combined Methane Oxidation and Nitrification-Denitrification and the Interactions Among Functional Microorganisms.” Water Research 188 (9): 116555. https://doi.org/10.1016/j.watres.2020.116555.
- Cao, T., P. Xie, N. Leyi, M. Zhang, and X. Jun. 2009. “Carbon and Nitrogen Metabolism of an Eutrophication Tolerative Macrophyte, Potamogeton Crispus, Under NH4+ Stress and Low Light Availability.” Environmental and Experimental Botany 66 (1): 74–78. https://doi.org/10.1016/j.envexpbot.2008.10.004.
- Chen, M., F. Wang, D. L. Zhang, Y. Wei Ming, and Y. Liu. 2021. “Effects of Acid Modification on the Structure and Adsorption NH4+-N Properties of Biochar.” Renewable Energy 169:1343–1350. https://doi.org/10.1016/j.renene.2021.01.098.
- Cho, K., S. Gu Shin, J. Lee, T. Koo, W. Kim, and S. Hwang. 2016. “Nitrification Resilience and Community Dynamics of Ammonia-Oxidizing Bacteria with Respect to Ammonia Loading Shock in a Nitrification Reactor Treating Steel Wastewater.” Journal of Bioscience and Bioengineering 122 (2): 196–202. https://doi.org/10.1016/j.jbiosc.2016.01.009.
- Cruz, H., P. Luckman, T. Seviour, W. Verstraete, B. Laycock, and I. Pikaar. 2018. “Rapid Removal of Ammonium from Domestic Wastewater Using Polymer Hydrogels.” Scientific Reports 8 (1): 1–6. https://doi.org/10.1038/s41598-018-21204-4.
- Defu, X., J. Cao, L. Yingxue, A. Howard, and Y. Kewei. 2019. “Effect of Pyrolysis Temperature on Characteristics of Biochars Derived from Different Feedstocks: A Case Study on Ammonium Adsorption Capacity.” Waste Management 87:652–660. https://doi.org/10.1016/j.wasman.2019.02.049.
- Farinella, N. V., G. D. Matos, and M. A. Z. Arruda. 2007. “Grape Bagasse as a Potential Biosorbent of Metals in Effluent Treatments.” Bioresource Technology 98 (10): 1940–1946. https://doi.org/10.1016/j.biortech.2006.07.043.
- Fulazzaky, M. A., N. Hudai Abdullah, A. Rahim Mohd Yusoff, and E. Paul. 2015. “Conditioning the Alternating Aerobic-Anoxic Process to Enhance the Removal of Inorganic Nitrogen Pollution from a Municipal Wastewater in France.” Journal of Cleaner Production 100 (3): 195–201. https://doi.org/10.1016/j.jclepro.2015.03.043.
- Gai, X., H. Wang, J. Liu, L. Zhai, S. Liu, T. Ren, H. Liu, and J. A. Coles. 2014. “Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of Ammonium and Nitrate.” PLoS ONE 9 (12): 1–19. https://doi.org/10.1371/journal.pone.0113888.
- Graber, E., B. Singh, K. Hanley, and J. Lehmann. 2017. “Determintion of cation exchange capacity in biochar.” Biochar: A Guide to Analytical Methods, 74–84 Vol. 1. 1st ed. Clayton: CSIRO Publishing.
- Hafshejani, D., A. H. Laleh, A. Ali Naseri, A. Soltani Mohammadi, F. Abbasi, and A. Bhatnagar. 2016. “Removal of Nitrate from Aqueous Solution by Modified Sugarcane Bagasse Biochar.” Ecological Engineering 95:101–111. https://doi.org/10.1016/j.ecoleng.2016.06.035.
- Haipeng, W., C. Lai, G. Zeng, J. Liang, J. Chen, X. Jijun, J. Dai, et al. 2017. “The Interactions of Composting and Biochar and Their Implications for Soil Amendment and Pollution Remediation: A Review.” Critical Reviews in Biotechnology 37 (6): 754–764. https://doi.org/10.1080/07388551.2016.1232696.
- Hakimi, M. H., V. Jegatheesan, and D. Navaratna. 2020. “The Potential of Adopting Struvite Precipitation as a Strategy for the Removal of Nutrients from Pre-AnMbr Treated Abattoir Wastewater.” Journal of Environmental Management. (October 2019)259:109783. https://doi.org/10.1016/j.jenvman.2019.109783.
- Han, B., C. Butterly, W. Zhang, J. Zheng He, and D. Chen. 2021. “Adsorbent Materials for Ammonium and Ammonia Removal: A Review.” Journal of Cleaner Production 283:124611. https://doi.org/10.1016/j.jclepro.2020.124611.
- Hellen, S., T. E. Mlsna, and D. O. Wipf. 2021. “Functionalized Biochar Electrodes for Asymmetrical Capacitive Deionization.” Desalination 516 (April): 115240. https://doi.org/10.1016/j.desal.2021.115240.
- Hsu, D., L. Changyi, T. Pang, Y. Wang, and G. Wang. 2019. “Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain.” Applied Sciences (Switzerland) 9 (23): 5249. https://doi.org/10.3390/app9235249.
- Jianbo, L., W. Jianguo, F. Zhihui, and L. Zhu. 2007. “Water Hyacinth in China: A Sustainability Science-Based Management Framework.” Environmental Management 40 (6): 823–830. https://doi.org/10.1007/s00267-007-9003-4.
- Jiang, Y. H., L. An Yu, H. Deng, Y. Cheng Hui, W. Yu Qing, Y. Dan Linmu, and H. Lin Hang. 2019. “Characteristics of Nitrogen and Phosphorus Adsorption by Mg-Loaded Biochar from Different Feedstocks.” Bioresource Technology 276:183–189. (November 2018). https://doi.org/10.1016/j.biortech.2018.12.079.
- Jiwan, S., and A. S. Kalamdhad. 2013. “Assessment of Bioavailability and Leachability of Heavy Metals During Rotary Drum Composting of Green Waste (Water Hyacinth).” Ecological Engineering 52:59–69. https://doi.org/10.1016/j.ecoleng.2012.12.090.
- Keith, B., T. Stephenson, and M. J. Semmens. 1998. “Nitrification and Oxygen Utilisation in a Membrane Aeration Bioreactor.” Journal of Membrane Science 144 (1–2): 197–209. https://doi.org/10.1016/S0376-7388(98)00047-7.
- Kizito, S., H. Luo, L. Jiaxin, H. Bah, R. Dong, and W. Shubiao. 2019. “Role of Nutrient-Enriched Biochar as a Soil Amendment During Maize Growth: Exploring Practical Alternatives to Recycle Agricultural Residuals and to Reduce Chemical Fertilizer Demand.” Sustainability 11 (11): 3211. https://doi.org/10.3390/su11113211.
- Kołodyńska, D., J. Bąk, M. Kozioł, and L. V. Pylypchuk. 2017. “Investigations of Heavy Metal Ion Sorption Using Nanocomposites of Iron-Modified Biochar.” Nanoscale Research Letters 12 (1): 12. https://doi.org/10.1186/s11671-017-2201-y.
- Largitte, L., and R. Pasquier. 2016. “A Review of the Kinetics Adsorption Models and Their Application to the Adsorption of Lead by an Activated Carbon.” Chemical Engineering Research and Design 109:495–504. https://doi.org/10.1016/j.cherd.2016.02.006.
- Liao, P. H., A. Chen, and K. V. Lo. 1995. “Removal of Nitrogen from Swine Manure Wastewaters by Ammonia Stripping.” Bioresource Technology 54 (1): 17–20. https://doi.org/10.1016/0960-8524(95)00105-0.
- Mingyu, L., Z. Xiaoqiang, Z. Fenghua, R. Gang, C. Gang, and L. Song. 2011. “Application of Modified Zeolite for Ammonium Removal from Drinking Water.” Desalination 271 (1–3): 295–300. https://doi.org/10.1016/j.desal.2010.12.047.
- Muche, H., T. Mucheye, and D. Tadesse. 2020. “Water Quality and Transpiration Responses of Eichornia Crassipes at Lake Tana, Ethiopia.” Sustainable Water Resources Management 6 (3): 1–7. https://doi.org/10.1007/s40899-020-00392-w.
- Önal, Y., C. Akmil-Başar, and Ç. Sarici-Özdemir. 2007. “Investigation Kinetics Mechanisms of Adsorption Malachite Green Onto Activated Carbon.” Journal of Hazardous Materials 146 (1–2): 194–203. https://doi.org/10.1016/j.jhazmat.2006.12.006.
- Qiangqiang, Y., D. Xia, L. Heng, K. Lanting, Y. Wang, H. Wang, Y. Zheng, and L. Qingbiao. 2016. “Effectiveness and Mechanisms of Ammonium Adsorption on Biochars Derived from Biogas Residues.” RSC Advances 6 (91): 88373–88381. https://doi.org/10.1039/c6ra16913a.
- Rajaniemi, K., H. Tao, E. Tuulia Nurmesniemi, S. Tuomikoski, and U. Lassi. 2021. “Phosphate and Ammonium Removal from Water Through Electrochemical and Chemical Precipitation of Struvite.” Processes 9 (1): 1–13. https://doi.org/10.3390/pr9010150.
- Rajapaksha, A. U., S. S. Chen, D. C. W. Tsang, M. Zhang, M. Vithanage, S. Mandal, B. Gao, N. S. Bolan, and O. Yong Sik. 2016. “Engineered/Designer Biochar for Contaminant Removal/Immobilization from Soil and Water: Potential and Implication of Biochar Modification.” Chemosphere 148:276–291. https://doi.org/10.1016/j.chemosphere.2016.01.043.
- Ruemei, F., C. Lung Chen, J. Yen Lin, J. Hua Tzeng, C. Pin Huang, C. Dong, and C. P. Huang. 2019. “Adsorption Characteristics of Ammonium Ion Onto Hydrous Biochars in Dilute Aqueous Solutions.” Bioresource Technology 272:465–472. (September 2018). https://doi.org/10.1016/j.biortech.2018.10.064.
- Shang, L., X. Hao, S. Huang, and Y. Zhang. 2018. “Adsorption of Ammonium in Aqueous Solutions by the Modified Biochar and Its Application as an Effective N-Fertilizer.” Water, Air, and Soil Pollution 229 (10). https://doi.org/10.1007/s11270-018-3956-1.
- Sica, M., A. Duta, C. Teodosiu, and C. Draghici. 2014. “Thermodynamic and Kinetic Study on Ammonium Removal from a Synthetic Water Solution Using Ion Exchange Resin.” Clean Technologies and Environmental Policy 16 (2): 351–359. https://doi.org/10.1007/s10098-013-0625-3.
- Simon, K., W. K. K. Shubiao Wu, M. Lei, L. Qimin, H. Bah, and R. Dong. 2015. “Evaluation of Slow Pyrolyzed Wood and Rice Husks Biochar for Adsorption of Ammonium Nitrogen from Piggery Manure Anaerobic Digestate Slurry.” Science of the Total Environment 505:102–112. https://doi.org/10.1016/j.scitotenv.2014.09.096.
- Song, H., J. Wang, A. Garg, X. Lin, Q. Zheng, and S. Sharma. 2019. “Potential of Novel Biochars Produced from Invasive Aquatic Species Outside Food Chain in Removing Ammonium Nitrogen: Comparison with Conventional Biochars and Clinoptilolite.” Sustainability 11 (24): 1–18. https://doi.org/10.3390/su11247136.
- Takaya, C. A., L. A. Fletcher, S. Singh, K. U. Anyikude, and A. B. Ross. 2016. “Phosphate and Ammonium Sorption Capacity of Biochar and Hydrochar from Different Wastes.” Chemosphere 145:518–527. https://doi.org/10.1016/j.chemosphere.2015.11.052.
- Takaya, C. A., K. R. Parmar, L. A. Fletcher, and A. B. Ross. 2019. “Biomass-Derived Carbonaceous Adsorbents for Trapping Ammonia.” Agriculture (Switzerland) 9 (1). https://doi.org/10.3390/agriculture9010016.
- Taşdemir, A., İ. Cengiz, E. Yildiz, and Y. Kemal Bayhan. 2020. “Investigation of Ammonia Stripping with a Hydrodynamic Cavitation Reactor.” Ultrasonics Sonochemistry 60. (August 2019). https://doi.org/10.1016/j.ultsonch.2019.104741.
- Thi Mai, V., V. Tuyen Trinh, D. Phuong Doan, H. Tap Van, T. Vinh Nguyen, S. Vigneswaran, and H. Hao Ngo. 2017. “Removing Ammonium from Water Using Modified Corncob-Biochar.” Science of the Total Environment 579:612–619. https://doi.org/10.1016/j.scitotenv.2016.11.050.
- Van Drecht, G., A. F. Bouwman, J. Harrison, and J. M. Knoop. 2009. “Global Nitrogen and Phosphate in Urban Wastewater for the Period 1970 to 2050.” Global Biogeochemical Cycles 23 (3): 1–19. https://doi.org/10.1029/2009GB003458.
- Wahab, M. A., S. Jellali, and N. Jedidi. 2010. “Ammonium Biosorption Onto Sawdust: FTIR Analysis, Kinetics and Adsorption Isotherms Modeling.” Bioresource Technology 101 (14): 5070–5075. https://doi.org/10.1016/j.biortech.2010.01.121.
- Wang, Z., L. Jie, G. Zhang, Y. Zhi, D. Yang, X. Lai, and T. Ren. 2020. “Characterization of Acid-Aged Biochar and Its Ammonium Adsorption in an Aqueous Solution.” Materials 13 (10): 2270. https://doi.org/10.3390/ma13102270.
- Wang, B., J. Lehmann, K. Hanley, R. Hestrin, and A. Enders. 2015. “Adsorption and Desorption of Ammonium by Maple Wood Biochar as a Function of Oxidation and pH.” Chemosphere 138:120–126. https://doi.org/10.1016/j.chemosphere.2015.05.062.
- Wu, P., Z. Wang, H. Wang, N. S. Bolan, Y. Wang, and W. Chen. 2020. “Visualizing the Emerging Trends of Biochar Research and Applications in 2019: A Scientometric Analysis and Review.” Biochar 2 (2): 135–150. https://doi.org/10.1007/s42773-020-00055-1.
- Xiaojian, H., X. Zhang, H. Hao Ngo, W. Guo, H. Wen, L. Chaocan, Y. Zhang, and M. Chanjuan. 2020. “Comparison Study on the Ammonium Adsorption of the Biochars Derived from Different Kinds of Fruit Peel.” Science of the Total Environment 707:135544. https://doi.org/10.1016/j.scitotenv.2019.135544.
- Yang, X., S. Zhang, J. Meiting, and L. Liu. 2019. “Preparation and Modification of Biochar Materials and Their Application in Soil Remediation.” Applied Sciences (Switzerland) 9 (7): 1365. https://doi.org/10.3390/app9071365.
- Yang, Z., L. Zifu, and I. B. Mahmood. 2014. “Recovery of NH4+ by Corn Cob Produced Biochars and Its Potential Application as Soil Conditioner.” Frontiers of Environmental Science and Engineering 8 (6): 825–834. https://doi.org/10.1007/s11783-014-0682-9.
- Yin, Q., M. Liu, and H. Ren. 2019. “Removal of Ammonium and Phosphate from Water by Mg-Modified Biochar: Influence of Mg Pretreatment and Pyrolysis Temperature.” BioResources 14 (3): 6203–6218. https://doi.org/10.15376/biores.14.3.6203-6218.
- Yin, Q., R. Wang, and Z. Zhao. 2018. “Application of Mg–Al-Modified Biochar for Simultaneous Removal of Ammonium, Nitrate, and Phosphate from Eutrophic Water.” Journal of Cleaner Production 176:230–240. https://doi.org/10.1016/j.jclepro.2017.12.117.
- Zhang, Y., X. Jin, L. Bin, Z. Xie, L. Xuede, J. Tang, and S. Fan. 2021. “Enhanced Adsorption Performance of Tetracycline in Aqueous Solutions by KOH-Modified Peanut Shell-Derived Biochar.” Biomass Conversion and Biorefinery 0123456789. https://doi.org/10.1007/s13399-021-02083-8.
- Zhang, L., S. Tang, and Y. Guan. 2020. “Excellent Adsorption-Desorption of Ammonium by a Poly(acrylic Acid)-Grafted Chitosan and Biochar Composite for Sustainable Agricultural Development.” ACS Sustainable Chemistry and Engineering 8 (44): 16451–16462. https://doi.org/10.1021/acssuschemeng.0c05070.
- Zhao, T., P. Chen, L. Zhang, L. Zhang, Y. Gao, A. Shuo, H. Liu, and X. Liu. 2021. “Heterotrophic Nitrification and Aerobic Denitrification by a Novel Acinetobacter Sp. TAC-1 at Low Temperature and High Ammonia Nitrogen.” Bioresource Technology 339 (July): 125620. https://doi.org/10.1016/j.biortech.2021.125620.
- Zheng, Y., and A. Wang. 2009. “Evaluation of Ammonium Removal Using a Chitosan-G-Poly (Acrylic Acid)/Rectorite Hydrogel Composite.” Journal of Hazardous Materials 171 (1–3): 671–677. https://doi.org/10.1016/j.jhazmat.2009.06.053.
- Zhixiong, Y., L. Zhang, Q. Huang, and Z. Tan. 2019. “Development of a Carbon-Based Slow Release Fertilizer Treated by Bio-Oil Coating and Study on Its Feedback Effect on Farmland Application.” Journal of Cleaner Production 239:118085. https://doi.org/10.1016/j.jclepro.2019.118085.
- Zhong, Z., Y. Guowen, M. Wenting, C. Zhang, H. Huang, L. Shengui, M. Gao, L. Xiejuan, B. Zhang, and H. Zhu. 2019. “Enhanced Phosphate Sequestration by Fe(iii) Modified Biochar Derived from Coconut Shell.” RSC Advances 9 (18): 10425–10436. https://doi.org/10.1039/c8ra10400j.
- Zhu, K., F. Hao, J. Zhang, L. Xiaoshu, J. Tang, and X. Xinhua. 2012. “Studies on Removal of NH4+-N from Aqueous Solution by Using the Activated Carbons Derived from Rice Husk.” Biomass and Bioenergy 43:18–25. https://doi.org/10.1016/j.biombioe.2012.04.005.