ABSTRACT
This study examined the influence of rice husk (RH) particle size, carbonization temperature, and their interaction on the availability of Silicon (Si) in RH biochar obtained from the carbonization of different particle sizes of RH (≤1 mm, >1 mm, and the original size, i.e., the unmodified RH biomass (O_RH)) at varying carbonization temperatures from 300 to 700°C. Silicon content in the biochar was extracted with 0.01 M CaCl2. Results showed that the influence of RH particle size on available Si is modified by temperature. The maximum available Si from the RH biochar were 2145 mg kg−1 at 700°C for O_RH, 2428 mg kg−1 at 600°C for > 1 mm RH and 2562 mg kg−1 at 500°C for ≤ 1 mm RH. These were an increase of 171%, 207% and 224% respectively, compared to the uncarbonized original rice husk (790 mg kg −1). Smaller particle sizes attained the maximum values of available silicon at lower temperatures compared with the original RH probably due to the efficient heat transfer to the smaller RH particle surface because of the smaller packing/void volume between the particles. Thus, the carbonization of smaller particles of RH will produce higher available Si content at a lower carbonization temperature than that required to achieve the same effect in the original RH biomass, thereby saving energy and time.
1. Introduction
Rice is a major food crop for more than 50% of the world’s population and is grown continuously, leading to a depletion of soil nutrients. Tropical areas in Africa and Latin America are home to about 26% of the world population in 2022 and are projected to account for about 35% by the year 2050 (Worldometer Citation2022). Soils of these tropical regions are linked with a high degree of weathering primarily ‘desilication’ which subsequently leads to the development of soils low in nutrient bases and Si (Foy Citation1992; Juo and Sanchez Citation1986) and might be one of the unknown origins of lower rice yield of many tropical soils (Savant, Snyder, and Datnoff Citation1997).
To support this growing population, rice is intensively cultivated by growing high-yielding varieties for more than one cropping season in a year which may result in a further decrease in plant available Si in these soils (Miyake Citation1993), and part of this was shown by the low Si status of Sub-Saharan Africa (SSA) (Tsujimoto et al. Citation2014).
Silicon is an important element for rice production, especially under unfavorable environmental conditions for sustainable rice production (Luyckx et al. Citation2017; Wang et al. Citation2019). The literature is replete with projections on the benefits of Si in agriculture, however, the availability of Si and the exploration of easily soluble sources of Si is a daunting challenge (Abbas et al. Citation2018; Meunier et al. Citation2018). Inorganic and traditional Si sources used as fertilizers, mainly silicate slags are available in limited supply, expensive, bulky and constitute logistic problems and are not readily available to farmers, especially in developing countries (Haynes Citation2019; Sohail et al. Citation2020).
Rice husk (RH) as a by-product of rice cultivation and milling process accounts for about 20–25% by weight of the total paddy weight (Pode Citation2016). Globally, it amounts to about 220 million tons per year (FAOSTAT Citation2022). RH is a high-tonnage agro-industrial waste that is complicated to manage efficiently due to its inherent characteristics such as rigid surface, high resistance to decomposition by soil microorganisms, and poor feeding value, however, it has high silicon content (Zou and Yang Citation2019).
RH is majorly lignocellulose biomass comprising about 50% cellulose, 25–30% lignin and mainly 15–20% silicon as the mineral constituent (Singh Citation2018). The silicon content has been found to increase up to between 37 and 42% upon charring to ash (Majumder, Sharma, and Soni Citation2014). The Si found within RH tissues is usually present as amorphous SiO2 (Ma and Yamaji Citation2006), whose solubility is about 10 times higher than crystalline SiO2 minerals (Fraysse et al. Citation2009).
The importance of carbonization of the abundant RH residues is presently being examined as a potential alternate source of silica for rice cultivation in many parts of the world. Biochar technologies are especially applicable as far as developing countries are concerned and can be adapted to address global food production and security challenges. However, the optimal conditions, choice of materials, the choice of reactor and reactor conditions, the pyrolysis parameters in the reactors, the pre-treatment methods, and the post-treatment, for the design of candidate biochar that will meet these requirements, remain unclear.
Carbonization temperature has been identified by several studies as one of the defining conditions for producing Si-rich biochar from RH. Nwajiaku et al. (Citation2018) reported an increase in available Si from RH and sugarcane bagasse as the pyrolysis temperature increased from 300 to 700°C. The variation of carbonization products of RH between 300 and 1000°C showed that Si content increased with increasing temperature (Yefremova et al. Citation2019). However, Xiao et al. (Citation2014) reported a fluctuation in the amorphous form of Si during the transformation and dissolution of Si from rice straw-derived biochar at a temperature range of 150 to 700°C with a peak at 350°C. From the foregoing, finding an appropriate and optimum temperature for biochar production is a challenging task.
Another distinguishing parameter is the particle size of the biomass, whereas previous works on the influence of particle size on biochar production were mainly concerned with the yield and not the agronomic nutrient or silicon content of the biochar. Abbas et al. (Citation2018) found that decreasing the particle size of RH decreased the biochar yield but led to an increase in the liquid and gaseous products like the findings of Yousaf et al. (Citation2018) and Hasan et al. (Citation2017). Choi et al. (Citation2012) and Zhang et al. (Citation2009) and Dermirbas (Citation2004) reported an increase in biochar yield with increasing particle size during the carbonization of different biomass. A different effect was reported by Onay and Kockar (Citation2003) in that there was a decrease in the biochar yield of rapeseed when pyrolyzed up to a temperature of 550°C. There was no significant variation in the biochar yield on changing the particle size (Aysu and Küçük Citation2013). These inconsistent observations with different trends imply that the role of particle size in biochar product yields and nutrient composition remains unclear and requires further investigation.
In Nigeria and other Sub-Saharan African (SSA) countries where rice production has been on the increase (Wakatsuki and Iwashima Citation2022) arising from the booming demand for rice (GRiSP – Global Rice Science Partnership Citation2013), rice husk is continuously dumped in heaps around the milling sites. As more and more RH is being mounted, the RH could not maintain their original particle size as they are broken into smaller particle sizes. We assumed that these smaller particle sizes could influence the heating during the carbonization process and consequently influence the Si content of the resulting biochar. Illustrating silicon availability from rice husk biochar under different particle sizes and carbonization temperature, potential value and efficient processing method of the wasted RH as Si source could be discussed.
In the present study, we investigated how different particle sizes of RH and carbonization temperatures influence the amount of available silicon in RH biochar. The specific objectives were to (i) improve silicon availability in RH biochar, (ii) minimize the energy required to achieve optimum available silicon content in the carbonization of RH, and (iii) evaluate how the synergistic interactions of RH particle sizes and carbonization temperature influence the availability of Si from RH biochar.
2. Materials and Methods
2.1. Preparation of the RH biomass
The RH used in the experiment was from the harvested grains of the Koshihikari rice cultivar planted on sandy loam soil with 103 mg kg−1 soil 0.01 M CaCl2 extracted Si and no silicate fertilizer management. The dried RH biomass of the Koshihikari rice cultivar had an average particle size of 6 mm at the long axis. A laboratory scale mill (Wonder Crusher model WC-3, Osaka Chemical Co. Ltd) was used for the mechanical crushing of part of the dried RH biomass. The crushed sample was sieved by using 1 mm-mesh sieves for particle size separation and the other part was used directly without particle size separation. For ease of reference, the smaller and bigger samples after sieving and the original or as-received sample were designated as ≤1 mm (average particle size 0.5 -1 mm), >1 mm (average particle size 1.1–4 mm) and O_RH (average particle size 7 mm), respectively.
2.2. RH biochar preparation
An experimental programmable electric furnace (Model FO810, Yamato, Japan) equipped with a digital proportional-integral-derivative temperature controller was used for the carbonization. The different samples were put in stainless vessels and carbonized in the electric furnace at a heating rate of 5°C min−1 until the desired temperature of 300, 400, 500, 600, and 700°C was reached in each experimental run, and then kept at these temperatures for 2 h before the carbonized samples were cooled down to 100°C and kept in a desiccator before weighing. The pyrolysis was carried out in an inert atmosphere by supplying N2 gas at a rate of 5 L min−1 to avoid oxygen. The biochar yield was presented as the ratio of the biochar to that of the weighed uncarbonized RH biomass for each carbonization run.
2.3. Biochar laboratory analysis
Available Si content was extracted by 0.01 M CaCl2 with a ratio of 1:30 w/v and continuously shaken for 16 h (Haysom and Chapman Citation1975). The pH of the extract solution of RH and its biochar with 0.01 M CaCl2 was around 6.8 which is similar to that of flooding paddy soils (Ding et al. Citation2019). This is also in line with the proposition of Berthelsen et al. (Citation2001) and Houba et al. (Citation2000) on the suitability of 0.01 M CaCl2 as a single extractant for Si and available nutrients. Thus, silicon concentrations in the extracts were determined by colorimetry with the molybdenum blue method at a wavelength of 810 nm (Yanai, Yoshida, and Shimizu Citation1996) using UV-probe Spectrophotometer (Model UV-1800, Shimadzu, Kyoto, Japan).
The electrical conductivity (EC) and pH were determined in a 1:20 w/v biochar-water mixture with EC and pH meters (Horiba models D-24 and D-15, Horiba, Kyoto, Japan, respectively) (Ahmedna et al. Citation1997). The ash content was determined using the ASTM-D1752-84 protocol (ASTM Citation2011) Available phosphorus was extracted by NaHCO3 and the P in the supernatant was determined by the molybdenum blue method (Olsen and Sommer Citation1982). Extractable base nutrients (Ca, K, Mg, and Na) were extracted by 1:100 w/v of CH3COONH4 and quantitatively determined by Inductively Coupled Plasma Spectroscopy (Model ICPE-9000, Shimadzu, Kyoto, Japan).
All analyses were carried out in triplicates.
Selected initial properties of the uncarbonized RH biomass are presented in .
Table 1. Selected properties of the uncarbonized original Rice husk (O-RH).
2.4. Data analysis and presentation
The data were subjected to a two-way analysis of variance (2-way ANOVA) with 3 levels of RH Particle Sizes (‘≤1 mm,’ ‘>1 mm,’ and ‘O_RH’) and 5 levels of carbonization temperatures (300, 400, 500, 600, and 700°C) using the IBM® SPSS® Statistics package (Version 28) (IBM, SPSS Inc.). Post hoc analyses of significant differences accepted at the p < 0.05 level were tested using Tukey’s HSD test. Analyses of simple main effects for particle sizes and carbonization temperature were performed with statistical significance receiving a Bonferroni adjustment.
3. Results
3.1. Variation in Biochar available silicon under different RH particle sizes
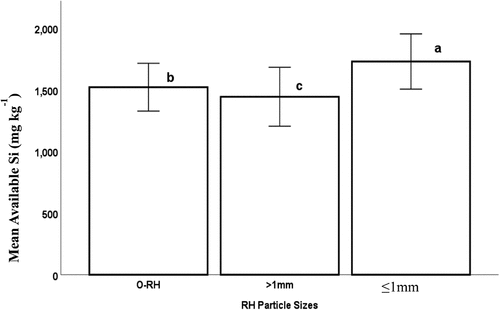
The mean available silicon content ranged from 1444 to 1730 mg kg−1 biochar. shows that the variation in the silicon concentration of the biochar was in the order of ‘>1 mm’ < ‘O_RH’ < ‘≤1 mm’ particles with average values of 1444, 1520, and 1730 mg kg−1. respectively. This main effect of rice husk particle size was significantly different (p < 0.001).
3.2. Effect of carbonization temperature on the mean available silicon in RH biochar
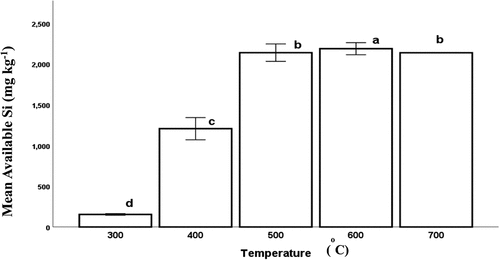
shows that the mean available silicon content of the produced biochar significantly increased (p < 0.001) with increasing carbonization temperature from 300 to 600°C before it decreased at 700°C. The mean available silicon content ranged from 154 to 2188 mg kg−1.
3.3. Interactive effect of rice husk particle size on Biochar’s yield and available si content moderated by carbonization temperature
shows the interactive effect of RH particle sizes and carbonization temperature on the yield and the mean available silicon content of the produced biochar.
Table 2. Interactive effects of RH particle size and carbonization temperature on RH biochar yield, and available Si (standard deviations are in parentheses).
Regarding the biochar yield, the simple main effect of particle size on the biochar yield was not statistically different among the three particle sizes. However, biochar from the same particle sizes shows that the simple main effect of carbonization temperature on the yield of biochar exhibits a progressive decrease in the yield as the temperature increases. Carbonization temperature of 300°C showed a statistically (p < 0.05) higher yield than those of carbonization temperatures of 500, 600, and 700°C for all the three particle sizes.
With respect to the available silicon, the simple main effect of RH particle sizes in the interaction revealed that biochar from RH with particle size ‘O_RH’ carbonized at 500 and 600°C had a statistically (p < 0.05) lower mean available Si than those from particle size ‘≤1 mm’ carbonized at the same temperatures as shown in .
In the same vein, simple main effects of temperature within each level of particle sizes of RH revealed that for the three particle sizes ‘O_RH,’ ‘>1 mm,’ and ‘≤1 mm,’ temperature 400°C had lower mean available Si with values of 1397, 670, and 1553 mg kg−1 compared to that of 500°C with values of 1986, 1869, and 2562 mg kg−1 respectively. For ‘>1 mm,’ the mean available silicon for 500°C (1869 mg kg−1) was lower than that for 600°C (2428 mg kg−1).
Thus, there was a statistically significant interaction between rice husk particle size and carbonization temperature on the mean available Si of the biochar, F (8, 30) = 3253.7, p < 0.001, and adjusted R-squared = 0.999 ().
Table 3. Summary ANOVA table for the main and interaction Effects of particle size and carbonization temperature on silicon availability in Rice husk biochar.
4. Discussion
4.1. Rice Husk (RH) biomass characteristics
As shown in , the RH biomass was slightly alkaline (pH 7.69) and saline in nature (EC 47.6 mS m−1) with high amount of available silicon (790 mg kg−1). Alkaline earth metals and silicates are the basic chemical constituents of the RH biomass, which makes it a good starting material for this investigation and a sustainable source of silicon-rich biochar. This RH feedstock physicochemical characteristics might have significantly influenced the quantity and quality of available silicon of the biochar produced. Thus, the choice of a specific biomass may have a considerable role in the attribute of biochar (Jindo et al. Citation2014) as well as in the efficient practicality of the carbonization process. This notion was also reported by Collett et al. (Citation2020) that biomass feedstocks with the most suitable characteristics can be selected to produce biochars for specific purposes.
4.2. Effect of Rice Husk (RH) particle size on available silicon content
Particle size has been identified as one of the process parameters which can be modified for biochar production. Previous works on the influence of particle size on biochar application are as a form of post-treatment and not as pretreatment. The RH particle sizes regulate the quantity of biochar produced in this experiment. As shown in , the change in the available silicon content of the biochar was in the order of ‘>1 mm’ < ‘O_RH’ < ‘≤1 mm’ particles. According to the results, a higher mean available silicon (1730 mg kg−1) was obtained from the smallest RH particles (‘≤1 mm’). This could be traced to the higher surface area and homogeneity of this category of RH biomass, which makes it to interact more efficiently with the carbonization medium. This confirms our hypothesis that smaller particle-sized RH biomass could improve the quantity of the available silicon from RH biochar as a sustainable renewable silicon source. Also, the biochar of the smaller particle-sized RH gave an improved available Si compared to the uncarbonized RH and biochar from O_RH with 119 and 14% increases, respectively. Thus, the objective to improve the available Si from RH was achieved.
On the other hand, the ‘>1 mm’ (average particle size 1.1–4 mm) sized RH particles produced a slightly lower amount of mean available silicon content compared to the ‘O_RH’ (average particle size 7 mm) particles due to the heterogeneity of the particle sizes after the sieving separation. Thus, to optimize the impact of particle size on available silicon in RH biochar, the homogeneity or near-homogeneity of the particle sizes should be considered.
4.3. Effect of carbonization temperature on available silicon content
The amount of available silicon held in high-tonnage agricultural wastes such as RH is greatly influenced by carbonization temperature during the carbonization process, keeping all other conditions constant. The effect of temperature could be seen in three stages.
The first stage is at 300°C. At this stage, the mean quantity of available silicon (154 mg kg−1, ) was less than that in the original feedstock (790 mg kg−1, ). This phenomenon was related to the inhibitory effect of the organic matrix (Anggria Citation2017) that covers Si in the RH Biochar. The organic matrix during carbonization refers to the organic material that is present in a substance before it undergoes the process of carbonization. The carbonization process results in the decomposition of the organic components and the formation of solid carbon-rich residues known as biochar (Chen et al. Citation2013). Thus, the amount of these solid residues (yield of the biochar) is directly related to the amount of the undecomposed organic matrix. This organic matrix was referred to as the unburned carbon as previously reported by Wada et al. (Citation1999). As shown in , this unburned organic matrix could be responsible for the higher biochar yield at this temperature. Similarly, Liu et al. (Citation2020) suggested that carbon is occluded in phytoliths, mainly silica bodies. Until carbonization and decomposition of the covering organic matrix starts at a faster rate and at a higher temperature than 300°C before silicon can be released. Thus, we agree with Ankyu et al. (Citation2017) that the 300°C temperature was not sufficient to produce silicon-rich RH biochar. Therefore, the appropriate carbonization temperature necessary to obtain silicon-rich biochar from RH should be higher than 300°C.
In the second stage, at the carbonization temperature from 400°C to 600°C, the mean available Si concentration increases as the carbonization temperature increases (). This result agrees with the literature (Ankyu, Kubota, and Noguchi Citation2017; Nwajiaku et al. Citation2018; Sun et al. Citation2017; Yefremova et al. Citation2019). The increase in the concentration of silicon with increasing carbonization temperature could be traced to the elimination of inhibitory organic matrix, or that from the silicon-carbon occluding bonds and an increase in the ash content (data not shown) of the biochar in which silicon content is about 80–90% (Majumder, Sharma, and Soni Citation2014).
Conversely, in the third stage, between 600 and 700°C, the mean amount of available silicon content started to decrease (from 2188.5 to 2138.6 mg kg−1), this might be associated with the change in the form of silica from the amorphous (soluble) to crystalline (insoluble) forms. Jindo et al. (Citation2014) previously noted that there is no need to make biochar at very elevated temperatures (above 700°C) so as to safeguard the stability nature of the biochar. Si exists in different forms namely gel, crystalline, amorphous, or polymerized form (Majumder, Sharma, and Soni Citation2014; Yoshida Citation1975; Yoshida, Ohnishi, and Kitagishi Citation1962) as the temperature increases, the Si changes in forms in which the Si gel, deposited, and concentrated in the rice husk changes to the amorphous form and then to crystalline form at certain temperatures (Todkar, Deshmukh, and Deorukhka Citation2016). There was a progressive increase in the mean available silicon content until the temperature of 600°C was reached. It seemed that beyond this temperature, the silicon started to crystallize, and this led to a decrease in the available silicon.
Hence, the mechanism of the effect of temperature on silicon availability (quantity and quality) in RH biochar was reflected to be the removal of the inhibitory effect of the covering organic matrix during carbonization at higher temperatures or the breaking of carbo-silicon occluding bonds (related to the biochar yield, ), the increase in mineral elements in the ash (data not shown), and the crystallization of silica as reported by Wada et al. (Citation1999). This increase in the concentration of available silicon could also be related to loss of volatile organic materials (reduction in the yield of biochar) as temperature increases (Ding et al. Citation2014) leading to the promotion of relative concentration of Si in the ash fraction.
4.4. Interactive influence of rice husk particle size on Biochar’s si availability moderated by carbonization temperature
The variability in the availability of Si from soil Si minerals and Si sources has been identified to be related to temperature, particle size, pH, and chemical composition (Savant, Snyder, and Datnoff Citation1997). Our results showed that reducing the particle size of RH before carbonization increased the availability of Si in biochar at a lower carbonization temperature compared to that of the unmodified RH. More specifically, ‘≤1 mm’ biochar available Si content has a maximum value of 2562 mg kg−1 at 500°C, ‘>1 mm’ reached a maximum of 2428 mg kg −1 at 600°C while ‘O_RH’ reached a maximum available Si content of 2145 mg kg−1 at 700°C (). The simple explanation for this observed result is that the interaction of carbonization temperature happens more with the reduced RH particle sizes because of their high surface area that generates more volatile products which were lost during the carbonization process and leave a solid matrix of biochar high in ash content and rich in Si.
This smaller particle size RH biomass produces higher Si (2562 mg kg −1) at a moderate temperature of 500°C probably due to the efficient heat transfer to the smaller RH particle surface because of the smaller packing/void volume (space) between the particles. These findings agree with the mechanisms of a higher bio-oil yield of biochar from smaller particles compared with bigger particles (Abbas et al. Citation2018; Hasan et al. Citation2017; Yousaf et al. Citation2018). With these results, the heat energy to produce a higher amount of available Si from RH during the carbonization process has been reduced compared to previous research as reported by Nwajiaku et al. (Citation2018) and Yefremova et al. (Citation2019). Thus, our second objective has been achieved. As shown in , since there is no significant difference in the yield of biochar among the three particle sizes between 500°C and 700°C, it will be more economical to use a particle size (≤1 mm) with higher available silicon at a lower carbonization temperature.
At 700°C, there was no difference in the available Si concentration among the three particle sizes. This could be because at this temperature, Si might have started changing from the amorphous form to the crystalline form (Parry and Smithson Citation1964; Todkar, Deshmukh, and Deorukhka Citation2016) whose solubility generally decreases.
5. Conclusion
This study has examined the influence of rice husk (RH) particle size, carbonization temperature, and their interaction on the availability of silicon in RH biochar obtained from the carbonization of different particle sizes of RH (‘≤1 mm,’ ‘>1 mm,’ and the original size, i.e., the unmodified RH biomass) at varying carbonization temperatures from 300 to 700°C. The maximum available Si from the RH biochar was 2145 mg kg−1 at 700°C for ‘O_RH,’ 2428 mg kg−1 at 600°C for ‘>1 mm’ RH and 2562 mg kg−1 at 500°C for ‘≤1 mm’ RH. These were an increase of 171%, 207% and 224% respectively, compared to the uncarbonized rice husk (790 mg kg −1). The results demonstrated that the particle size of the RH and carbonization temperature affect the availability of Si in RH biochar to a significant extent. Practically, RH biomass with smaller particle sizes as we found in Nigeria and other SSA countries could be processed as Si by relatively lower carbonization temperature thereby saving energy and time involved in the carbonization process. Systematic field application studies are suggested to further establish these facts.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abbas, Q., G. Liu, B. Yousaf, M. U. Ali, H. Ullah, M. A. M. Munir, and R. Liu. 2018. “Contrasting Effects of Operating Conditions and Biomass Particle Size on Bulk Characteristics and Surface Chemistry of Rice Husk Derived-Biochars.” Journal of Analytical and Applied Pyrolysis 134:281–292. https://doi.org/10.1016/j.jaap.2018.06.018.
- Ahmedna, M., M. M. Johns, S. J. Clarke, W. E. Marshall, and R. M. Rao. 1997. “Potential of Agricultural By-product-based Activated Carbons for Use in Raw Sugar Decolorization.” Journal of the Science of Food and Agriculture 75:117–124. https://doi.org/10.1002/(SICI)1097-0010(199709)75:1<117:AID-JSFA850>3.0.CO;2-M.
- Anggria, L. 2017. “Characterization of Indonesian Local Silicon Material and Evaluation of Controlling Factors for Soil Silicon Availability.” PhD diss., Tottori University
- Ankyu, E., Y. Kubota, and R. Noguchi. 2017. “Eluted Soluble Silica Content in Rice Husk Charcoal Produced by Rice Husk Burner.” Journal of the Japan Institute of Energy 96 (7): 217–227. https://doi.org/10.3775/jie.96.217.
- ASTM (American Society of Testing and Materials). 2011. “Standard Test Methods for Chemical Analysis of Wood Charcoal.” ASTM Int 84:1–2. https://doi.org/10.1520/D1762-84R07.2.
- Aysu, T., and M. M. Küçük. 2013. “Biomass Pyrolysis in a Fixed-Bed Reactor: Effects of Pyrolysis Parameters on Product Yields and Characterization of Products.” Energy 64:1002–1025. https://doi.org/10.1016/j.energy.2013.11.053.
- Berthelsen, S., A. Hurney, A. D. Noble, A. Rudd, A. L. Garside, and A. Henderson. 2001. “An Assessment of Current Silicon Status of Sugarcane Production Soils from Tully to Mossman.” In Proceedings of the Austra. Soc. Of Sugarcane Techn., May, 1-4, Mackay, Queensland 23: 289–296. http://hdl.handle.net/102.100.100/201926?index=1.
- Chen, Y., X. Shaoyi, C. Hui, and L. Hongbo. 2013. “The Effect of Catalyzer in the Matrix on the Structure and Performance of Carbon/Carbon Composite.” Journal of Composite Materials 47 (4): 409–417. https://doi.org/10.1177/0021998312440475.
- Choi, H. S., Y. S. Choi, and H. C. Park. 2012. “Fast Pyrolysis Characteristics of Lignocellulosic Biomass with Varying Reaction Conditions.” Renew Energy 42:131–135. https://doi.org/10.1016/j.renene.2011.08.049.
- Collett, C., O. Masek, N. Razali, and J. McGregor. 2020. “Influence of Biochar Composition and Source Material on Catalytic Performance: The Carboxylation of Glycerol with CO2 as a Case Study.” Catalysts 10 (9): 1067. https://doi.org/10.3390/catal10091067.
- Dermirbas, A. 2004. “Effect of Temperature and Particle Size on Biochar Yield from Pyrolysis of Agricultural Residues.” Journal of Analytical and Applied Pyrolysis 72:243–248. https://doi.org/10.1016/j.jaap.2004.07.003.
- Ding, C., S. Du, Y. Ma, X. Li, T. Zhang, and X. Wang. 2019. “Changes in the pH of Paddy Soils After Flooding and Drainage: Modeling and Validation.” Geoderma 337:511–513. https://doi.org/10.1016/j.geoderma.2018.10.012.
- Ding, Y., Y. Liu, W. Wu, D. Shi, M. Yang, and Y. Li. 2014. ““Effects of Pyrolysis Temperature on the Physicochemical Properties of Biochar Derived from Municipal Solid Waste.” Bioresource Technology 164:189–194. https://doi.org/10.1016/j.biortech.2014.05.008.
- FAOSTAT (Food and Agricultural Organization Statistics). 2022. Accessed September 20, 2022. https://www.fao.org/faostat/en/#data/QCL.
- Foy, C. D. 1992. “Soil Chemical Factors Limiting Plant Root Growth.” Advances in Soil Science 19:97–149. https://doi.org/10.1007/978-1-4612-2894-3_5.
- Fraysse, F., O. S. Pokrovsky, J. Schott, and J. D. Meunier. 2009. “Surface Chemistry and Reactivity of Plant Phytoliths in Aqueous Solutions.” Chemical Geology 258:197–206. https://doi.org/10.1016/j.chemgeo.2008.10.003.
- GRiSP – (Global Rice Science Partnership). 2013. Rice Almanac. 4th ed. Los Banos: International Rice Research Institute.
- Hasan, M. M., X. S. Wang, D. Mourant, R. Gunawan, C. Yu, X. Hu, S. Kadarwati, et al. 2017. “Grinding Pyrolysis of Mallee Wood: Effects of Pyrolysis Conditions on the Yields of Bio-Oil and Biochar.” Fuel Processing Technology 167:215–220. https://doi.org/10.1016/j.fuproc.2017.07.004.
- Haynes, R. J. 2019. “What Effect Does Liming Have on Silicon Availability in Agricultural Soils?” Geoderma 337:375–383. https://doi.org/10.1016/j.geoderma.2018.09.026.
- Haysom, M. B. C., and L. S. Chapman. 1975. “Some Aspects of Calcium Silicate Trials at Mackay.” In Proc. Queens. Soc. Sugarcane Technol., Mackay, Queensland 42: 117–122.
- Houba, V. J. G., E. J. M. Temminghoff, G. A. Gaikhorst, and W. van Vark. 2000. “Soil Analysis Procedures Using 0.01 M Calcium Chloride as Extraction Reagent.” Communications in Soil Science and Plant Analysis 31:9-10, 1299–1396. https://doi.org/10.1080/00103620009370514.
- Jindo, K., H. Mizumoto, Y. Sawada, M. A. Sanchez-Monedero, and T. Sonoki. 2014. “Physical and Chemical Characterization of Biochars Derived from Different Agricultural Residues.” Biogeosciences 11:6613–6621. https://doi.org/10.5194/bg-11-6613-2014.
- Juo, A. S. R., and P. A. Sanchez. 1986. “Soil Nutritional Aspects with a View to Characterize Upland Rice Environment.” In Upland Rice Research, edited by F. J. Shideler, 81–95. Los Banos: International Rice Research Institute.
- Liu, L., Z. Song, C. Yu, R. M. Eliam, H. Liu, B. P. Singh, and H. Wang. 2020. “Silicon Effects on Biomass Carbon and Phytolith-Occluded Carbon in Grasslands Under High-Salinity Conditions.” Frontiers in Plant Science 11:657. https://doi.org/10.3389/fpls.2020.00657.
- Luyckx, M., J. F. Hausman, S. Lutts, and G. Guerriero. 2017. “Silicon and Plants: Current Knowledge and Technological Perspectives.” Frontiers in Plant Science 8:8. https://doi.org/10.3389/fpls.2017.00411.
- Majumder, C. B., M. Sharma, and G. Soni. 2014. “A Simple Non-Conventional Method to Extract Amorphous Silica from Rice Husk.” International Journal of Offshore and Polar Engineering. https://www.academia.edu/9116091.
- Ma, J. F., and N. Yamaji. 2006. “Silicon Uptake and Accumulation in Higher Plants.” Trends in Plant Science 11 (8): 392–397. https://doi.org/10.1016/j.tplants.2006.06.007.
- Meunier, J. D., K. Sandhya, N. B. Prakash, D. Borschneck, and P. Dussouillez. 2018. “pH as a Proxy for Estimating Plant-Available Si: A Case Study in Rice Fields in Kamataka, (South India).” Plant and Soil 432:143–155. https://doi.org/10.1007/s11104-018-3758-7.
- Miyake, Y. 1993. “Silica in Soils and Plants.” Scientific Reports of the Faculty of Agriculture, Okayama University 81:61–79. https://ousar.lib.okayama-u.ac.jp/896.
- Nwajiaku, I. M., J. S. Olanrewaju, K. Sato, T. Tokunari, S. Kitano, and T. Masunaga. 2018. “Change in Nutrient Composition of Biochar from Rice Husk and Sugarcane Bagasse at Varying Pyrolytic Temperatures.” International Journal of Recycling of Organic Waste in Agriculture 7:269–276. https://doi.org/10.1007/s40093-018-0213-y.
- Olsen, S. R., and L. E. Sommer. 1982. “Determination of Available Phosphorus.” In Methods of Soil Analysis, edited by A. L. Page, R. H. Miller, and D. R. Keeney, 403. Vol. 2. Madison WI: American Society of Agronomy.
- Onay, O., and O. M. Kockar. 2003. “Slow, Fast, and Flash Pyrolysis of Rapeseed.” Renew Energy 28 (15): 2417–2433. https://doi.org/10.1016/S0960-1481(03)00137-X.
- Parry, D. W., and F. Smithson. 1964. “Types of Opaline Silica Deposition in the Leaves of British Grasses.” Annals of Botany London 28:169–185. https://doi.org/10.1093/oxfordjournals.aob.a083891.
- Pode, R. 2016. “Potential Applications of Rice Husk Ash Waste from Rice Husk Biomass Power Plant.” Renewable and Sustainable Energy Reviews 53:1468–1485. https://doi.org/10.1016/j.rser.2015.09.051.
- Savant, N. K., G. H. Snyder, and L. E. Datnoff. 1997. “Silicon Management and Sustainable Rice Production.” Advances in Agronomy 58:151–199. https://doi.org/10.1016/S0065-2113(08)60255-2.
- Singh, B. 2018. “Rice Husk Ash.” In Waste and Supplementary Cementitious Materials in Concrete: Characterization, Properties and Applications, 417–460. Elsevier. https://doi.org/10.1016/B978-0-08-102156-9.00013-4.
- Sohail, M. I., M. Z. Rehman, M. Rizwan, S. Ali, M. A. Ayub, T. Aziz, M. Saqib, and G. Murtaza. 2020. “Effect of Biochars, Biogenic, and Inorganic Amendments on Dissolution and Kinetic Release of Phytoavailable Silicon in Texturally Different Soils Under Submerged Conditions.” Arabian Journal of Geosciences 13:376. https://doi.org/10.007/s12517-020-05399-3.
- Sun, J., F. He, Y. Pan, and Z. Zhang. 2017. “Effects of Pyrolysis Temperature and Residence Time on Physicochemical Properties of Different Biochar Types.” Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 67 (1): 12–22. https://doi.org/10.1080/09064710.2016.1214745.
- Todkar, B. S., S. M. Deshmukh, and O. A. Deorukhka. 2016. “Extraction of Silica from Rice Husk.” International Journal of Engineering Research and Development 12 (3): 69–74 .
- Tsujimoto, Y., S. Muranaka, K. Saito, and H. Asia. 2014. “Limited Si-Nutrient Status of Rice Plants in Relation to Plant-Available Si of Soils, Nitrogen Fertilizer Application, and Rice-Growing Environments Across Sub-Saharan Africa.” Field Crops Research 155:1–9. https://doi.org/10.1016/j.fcr.2013.10.003.
- Wada, I., T. Kawano, N. Maeda, and M. Kawakami. 1999. “Process Technology and Development of Furnace for Production of High Reactive Rice Husk Ash.” Journal of the Japanese Society of Agricultural Machinery 61 (4): 125–132. https://doi.org/10.11357/jsam1937.61.4_125 .
- Wakatsuki, T., and N. Iwashima. (2022). “Rice Green Revolution Statistics of Sub-Saharan Africa and Asia During 1961 -2020.” Accessed June 4, 2023. http://www.kinki-ecotech.jp/download/sawahtech-1-17sep22.pdf/.
- Wang, Y., X. Xiao, Y. Xu, and B. Chen. 2019. “Environmental Effects of Silicon within Biochar (Sichar) and Carbon-Silicon Coupling Mechanisms: A Critical Review.” Environmental Science and Technology 53:13570–13582. https://doi.org/10.1021/acs.est.9b03607.
- Worldometer. 2022. “World Population by Region.” Accessed August 25, 2022 https://www.worldometers.info/world-population/population-by-region/.
- Xiao, X., B. Chen, and L. Zhu. 2014. “Transformation, Morphology and Dissolution of Silicon and Carbon in Rice Straw-Derived Biochar Under Different Pyrolytic Temperatures.” Environmental Science & Technology 48:3411–3419. https://doi.org/10.1021/es405676h.
- Yanai, M., Y. Yoshida, and Y. Shimizu. 1996. “Colorimetric Determination of Available Silicate in Soil Extracted by Acetate Buffer with Ascorbic Acid Powder.” Journal of Soil Science and Plant Nutrition 67 (3): 273–278. https://doi.org/10.20710/dojo.67.3_273.
- Yefremova, S., A. Zharmenov, Y. Sukharnikov, L. Bunchuk, A. Kablanbekov, K. Anarbekov, T. Kulik, et al. 2019. “Rice Husk Hydrolytic Lignin Transformation in Carbonization Process.” Molecules 24 (17): 3075. https://doi.org/10.3390/molecules24173075.
- Yoshida, S. 1975. “The Physiology of Silicon in Rice.” Technical Bulletin 25:1–27. https://nla.gov.au/nla.cat-vn4555774.
- Yoshida, S., Y. Ohnishi, and K. Kitagishi. 1962. “Chemical Forms, Mobility, and Deposition of Silicon in Rice Plant.” Soil Science & Plant Nutrition 8:107–111. https://doi.org/10.1080/00380768.1962.10430992.
- Yousaf, B., G. Liu, Q. Abbas, M. U. Ali, R. Wang, R. Ahmed, C. Wang, M. I. Al-Wabel, and A. R. A. Usman. 2018. “Operational Control on Environmental Safety of Potentially Toxic Elements During Thermalconversion of Metal-Accumulator Invasive Ragweed to Biochar.” Journal of Cleaner Production 195:458–469. https://doi.org/10.1016/j.jclepro.2018.05.246.
- Zhang, H., R. Xiao, H. Huang, and G. Xiao. 2009. “Comparison of Non-Catalytic and Catalytic Fast Pyrolysis of Corncob in a Fluidized Bed Reactor.” Bioresource Technology 100 (3): 1428–1434. https://doi.org/10.1016/j.biortech.2008.08.031.
- Zou, Y., and T. Yang. 2019. “Rice Husk Ash and Their Applications.” Rice Bran Rice Bran Oil 1:207–246. https://doi.org/10.1016/B978-0-12-812828-2.00009-3.