ABSTRACT
Rattan, a climbing palm of the tropical region of Southeast Asia (SEA), supplied material for objects of myriad purposes including ceremonial, religious, utilitarian, and artistic as exemplified by the collections at the National Museum of Singapore, Asian Civilization Museum, and the Singapore Art Museum. The aim of this analysis of rattan is to further the understanding of rattan, in particular its surface, and to propose a protocol-enabling positive identification of rattan in artifacts, thus guiding conservation decisions. The rattan surface defines an artifacts esthetic appearance and stability, and understanding its characteristics is essential to the development of an informed preservation strategy. Rattan epidermis morphology, topography, and cellular structure were characterized by a multi-scale and a multi-sensory approach using optical microscopy, confocal laser scanning microscopy, and field-emission scanning electron microscopy (FESEM). The chemical composition was analyzed with energy dispersive X-ray spectroscopy and macro-X-ray fluorescence spectroscopy (m-XRF) and indicated the presence of a siliceous compound, biogenic silica, in the epidermis and phytoliths in vascular tissue. A parallel analytical protocol was applied to samples extracted from museum artifacts (nineteenth-twentieth century) and contemporary objects acquired during field studies in Indonesia, Malaysia, Myanmar, and Cambodia. The methods of processing rattan have a direct impact on the siliceous layer thus leading to a better understanding of surface patterns found on artifacts. An attempt to find correlations between the percentage of silica and thickness of the siliceous layer did not produce conclusive results; both varied greatly in all studied examples. Darkening of rattan was clearly associated with aging but did not indicate any distinct effect on the chemical composition of the outer layer. The chemical analysis of the biogenic silica layer combined with recording methods of processing rattan observed during field studies in SEA provide a basis for a better understanding of rattan collections in the museum context.
Introduction
This study of rattan is part of a larger project, which is a survey of materials that once played a prominent role in the cultural landscape of Southeast Asia (SEA) and now are vanishing for a variety of reasons. The cultural importance of rattan in the SEA was so great due to its multitude of applications that a phrase ‘rattan culture’ was coined. Rattan was used for structural support of houses and suspended bridges in the rain forests; comprised baskets, mats, and furniture; and in the nineteenth century was constructed into war gear, shields, and helmets. Lack of recorded methods of processing rattan combined with rapidly diminishing rain forests, a natural habitat for rattan, and the aggressive introduction of commercially lucrative oil palms resulted in the destruction of rattan along with its usages, calling for an in-depth study of artifacts made of rattan. Museum collections may be soon the only evidence of this once-indispensable material of the SEA region.
Substantial bibliographies of rattan focus on its botanical aspect as well as the cultivation and propagation of rattan plants. Among scarce references of museum relevance, a monograph of basketry on Borneo Island offers extensive analyses of styles and materials, among them rattan, decorations, and traditions of baskets on this Malaysian and Indonesian island (Sellato Citation2012). The technical information on how rattan, the plant, was transformed into artifacts and what processes were applied during their making is scattered in field reports produced for the Food and Agricultural Organization (FAO) and interwoven into agriculturally important monographs (Weiner and Liese Citation1991; Wan Razali, Dransfield, and Manokaran Citation1992; Peluso Citation2001; Palisoc Citation2005; Meijaard et al. Citation2014).
The general aim of this project was to gain an understanding of rattan, the material, methods used in its transformation into objects, and impacts of working techniques on rattan appearance. In particular, the outer layer on rattan’s cane was the focus of analysis because historically it was this lustrous, glassy surface that dictated the price and esthetic value of rattan objects. Positive identification of rattan in artifacts, presence or absence of the glassy, biogenic silica layer, an integral epidermis of the plant, have direct impact on the development of a conservation strategy, permitting use of a wide array of solvents if the biogenic silica is present or in case of its absence, application of very limited treatment. When present, the biogenic silica layer is not soluble in organic solvents besides hydrochloric acid. In the second case, when the biogenic silica layer is stripped and the surface is covered with synthetic lacquers, naturally the lacquers can be dissolved in a wide array of solvents.
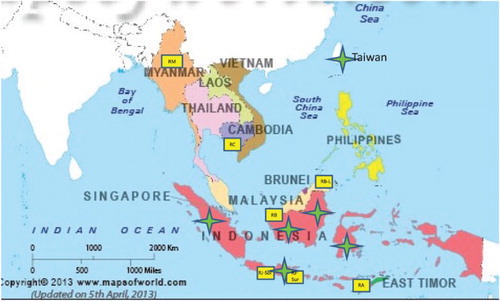
The methodology used in this study relied on the analysis of historic artifacts produced in the SEA region in the collection of the National Heritage Board in Singapore and modern products made of rattan collected during field work in the SEA region during a two-year period, 2015–2017 ().
Figure 1. Provenance of the study material. Map of SEA with the marked provenance of the cultural heritage (CH) artifacts used in sampling and location of the new rattan objects (RR). The yellow squares indicate the location of rattan references (RR). The green stars indicate the attributed provenance of CH artifacts.
Both groups, the cultural heritage (CH) artifacts and modern objects (RR), underwent the same protocol of microanalysis, including characterization of surface topography and morphology, and identification of elemental composition of rattan epidermis.
Rattan, plant morphology, and biogenic silica
Rattan morphology, an overview
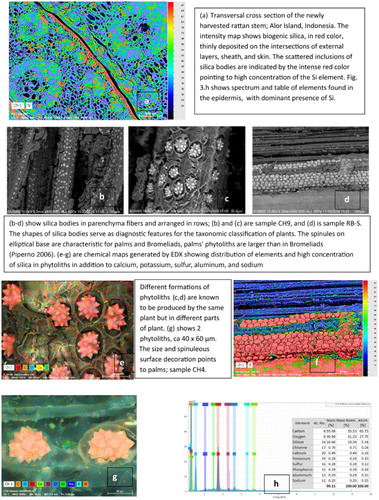
Rattan is a climbing palm growing predominantly in tropical and subtropical Asia and the Pacific rainforests, where 10 out of the 13 known genera are endemic (Uhl and Dransfield Citation1987; Weiner and Liese Citation1988, Citation1990, Citation1991). There are approximately 600 species but only 20 or so are reported to be used commercially. The greatest diversity of rattans is on the island of Borneo, both Indonesian Kalimantan and Malayan Sarawak and Sabah, where it is harvested in the wild. Other areas of rattan growth are in equatorial Africa and in New Guinea, however, represented by a smaller number of genera (Weiner and Liese Citation1990; Dransfield and Manokaran Citation1994; Ebanyenle and Oteng-Amoako Citation2003; Johnson, Rattan, and Sunderland Citation2004). Rattan grows apogeotropically, meaning upwards, climbing on other trees, by the means of hooks ((a–c)); hence the primary rain forest is essential to provide the physical support for rattan palms.
One of the identifying features of rattan is the characteristic open cellular structure of vascular bundles visible on the transverse cross section, as seen in (g) and (a). The configuration of large, so-called sieve tubes and phloem tissue around them are one of the classifying features for botanists in establishing its phylogenetic nomenclature. Similarly, the interface and cellular structure of the biogenic silica and phytoliths, small siliceous inclusions in vascular tissue, reveal the characteristic features of species. Based on that, the micrograph in (i) allowed Prof. G. Wiener to identify the species from the object Child’s Cot, as Calamus laevigatus Mart. (personal communication, January 2017).
High content of sugar and polysaccharides make fresh rattan prone to fungal and insect attacks; one causes discoloration and the other weakens the structure by internal holes. Susceptibility of rattan to biodegradation has been always a concern for users as well as manufacturers. Various methods of preventing insect and fungi infestation are applied at different stages of processing and are elaborated on in the subsequent sections.
Biogenic silica in rattan
Biogenic silica is produced by rattan in two forms: one in the epidermis, deposited on the surface, and the other as inclusions in the internal plant tissue (). The epidermal deposit is the main focus of this study; it produces the desired glassy, lustrous layer. The inclusions in the plant tissue are referred to in the literature as silica bodies or opal phytoliths. They serve as one of the diagnostic features distinguishing rattans on the genus level (Kealhofer and Piperno Citation1998; Prychid, Rudall, and Mary Citation2003; Piperno Citation2006). While the epidermal siliceous deposit varies in chemical composition in relation to soil characteristics and plant senescence, the diagnostic phytoliths are not affected by the soil chemistry or plant maturity. In lack of biogenic silica on the surface, when for example it was removed, the presence and shape of phytoliths provide diagnostic clues about the plant.
The siliceous deposits are known to be produced by plants in a form of oxide or silicate (Prychid, Rudall, and Mary Citation2003; Piperno Citation2006; Currie and Perry Citation2007; Perry Citation2009). Silica, a common name referring to silicon dioxide (SiO2) at times is also interchangeably referred to as ‘silicon’. For consistency with the published sources the biogenic silica will be referred to in this article as ‘silica dioxide’.
Other forms of siliceous deposits in rattan are silica bodies or so-called phytoliths. Although not a main concern regarding preservation, acknowledging their presence contributes to a general knowledge of rattan’s morphology, especially because their shapes may be mistakenly regarded as, for example, fungal fruiting structures or other inclusions. Furthermore, in lack of the outer layer of silica dioxide in a sample, the presence of a particular shape of phytoliths may be the only indication that the plant fiber was derived from palms. The silica bodies may be positioned in axial files ((b)), or rows, as sheets ((d,f)); individual inclusions are of various shapes in different plants – spherical, conical, or resembling druses, with spinulose or a nodular surface ((b,c,e,g)). However, their shapes as mentioned earlier are indicative of specific plant families. In the case of palms, the ellipsoidal-based phytoliths are covered with protruding projections, referred to in the literature as spinules (Piperno Citation2006) ((c,e,g)). Considering the chemistry of silica bodies, Prychid, Rudall, and Mary Citation2003 reported a significant concentration of nitrogen and carbon within their structures and on surfaces. Other elements reported by these authors and others (Piperno Citation2006) included aluminum, chlorine, copper, iron manganese, phosphorous, and titanium. The same group of elements was also detected in the energy dispersive X-ray spectroscopy (EDX) analysis of silica bodies on the artifacts and in the new rattan samples in this study ((g,h)).
How the silica layer is formed is not entirely known. Recent genetic studies indicate that the transport of Si in the plant is most likely energy- and temperature-dependent, based on the fact that metabolic inhibitors and low temperature retard Si transport (Ma and Yamaji Citation2006). What is known about silica is that in plant tissue it serves an array of functions, such as structural support, improving resistance to diseases, protecting against pathogens and predators by stimulating plants’ defense mechanisms (Kaufman et al. Citation1971; Prychid, Rudall, and Mary Citation2003; Fauteux et al. Citation2005; Hodson et al. Citation2005; Ma and Yamaji Citation2006; Piperno Citation2006). As such, the means of increasing percentages of silica in plant tissue has been the subject of studies in agriculture to increase the resilience of commercially viable plants. References to silica in rattan, however, are only marginal and even scarcer when the siliceous surface is concerned.
The rattan’s sap which carries water and nutrients to the plant reflects the chemical composition of the soil. Based on that one may hypothesize that by establishing the soil contents of a particular area the provenance of the plant can be established. That could, however, not necessarily indicate a provenance of an artifact made of that plant. As to solidification of sap, some researchers of silica deposits in grass proposed that the development of such deposits occurs early on during plants’ growth, initially an amorphous substance with age becomes crystalline (Prychid citing Wilding, Smek, and Dress Citation1977). However, the crystallinity of silica dioxide has not been confirmed by experts studying phytoliths (Piperno Citation2006). Based on the observation of the fresh sample of rattan, parallel hypotheses may be proposed as to the transformation of a colloidal amorphous layer into a glassy, translucent, crystalline-like material. However, further investigation is necessary to validate that concept.
Fresh samples of rattan revealed that before the epidermal layer changes into solid, light yellowish, glassy sheeting it is green in color and appears colloidal in consistency ((e,f)). The change in color and consistency occurs within a few days after harvesting ((h)), most likely as a result of polymerization or other chemical changes once the layer is exposed to air. Once the outer layer solidifies it does not react with any solvent except hydrochloric acid (Yoshida, Ohnishi, and Kitagishi Citation1962; personal communication, PC2). Establishing that an artifact is made of rattan which retained its original siliceous layer has a significant impact on how such an object could be handled and cared for. For example, the surface can be cleaned without fear of removing this naturally produced top layer.
With time, the light yellow, straw-like color darkens uniformly to saturated rich brown as shown in a 1970 child’s cot, sample CH4 ((d)) and plaited rattan mat, sample CH5 ((e)) to nearly black in the oldest artifacts, nineteenth and twentieth century shield and helmet from Sumatra, sample CH1–CH3, and a Toraja basket CH7 (). It is worth noting that color change is not easily recognized in artifacts made of composite fibers, such as rattan, grasses, and roots, as in the Malayan food basket shown in (h).
Harvesting and processing rattan
Harvesting at a village level and commercially
Rattan is harvested in the wild for commercial purposes in larger quantities and for local use in aboriginal communities, usually by single stems; each dictates the course of processing methods. The extraction of rattan strands from the forest, however, is the same whether for commercial usage or at the village level ((a)).
In general, commercially harvested rattan undergoes several stages of processing which is not practiced at the village level, as summarized in .
Figure 4. Diagrammatic illustration of the main processing steps of rattan by an aboriginal community, at a village level and in a commercial, large-scale operation. (1) Village-level harvesting was observed on Aolor Island, Timore Lesser Sunda. (2): Reporting of commercial harvesting is based on bibliographic sources.
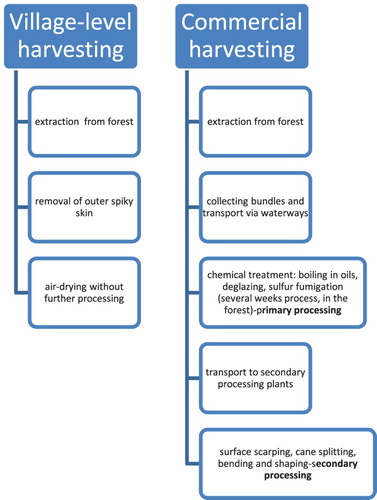
At a village level, the outer spiky skin is separated, usually at the time of pulling the rattan strand out of the plant cluster, to facilitate extraction and transport ((b)). The outer skin is carefully separated from the core first by twisting the stem to break the outer layer, and next, by peeling it off ((b)). On a commercial level, the researchers report transporting canes via waterways in bundles for further chemical processing, usually carried out in forests, and referred to as primary processing. Other researchers reported that it takes two–three weeks before rattan leaves the forest. Once the canes are distributed to specialized plants, they undergo further processing, referred to as secondary processing or fabrication (Bhat Citation1996; Peluso Citation2001; Dransfield Citation2003).
Processing of rattan
Commercially harvested rattan undergoes both a chemical treatment carried out first in the forest, immediately after harvesting, and ‘mechanical’ processing, usually in secondary processing plants. Chemical treatment involves ‘seasoning’, or ‘curing’ which is boiling in oil, followed by drying, fumigation, bleaching, and deglazing. Deglazing refers to the mechanical removal of the heavy silicified epidermis, leaving the thinner one underneath. The fumigation with sulfur and boiling in oil, kerosene, or coconut (whichever is available) aims to protect rattan against biological damage and disfiguring fungal stains. When discoloration occurs due to fungal infestation, the canes are bleached, as reported by Dransfield (Citation2003) for Kalimantan region, Palisoc (Citation2005) for Philippines, and by Mr. Liem L. for Java, (personal communication, PC1). Palisoc cited the use of sodium hydroxide and sodium hypochlorite in combination with hydrogen peroxide for the bleaching purpose. Bleaching leaves residue in the outer layer of rattan that can be detected in the course of chemical analysis. Such trace elements may indicate the types of processes applied to the raw material before it was transformed into an object.
The mechanical treatment depends on the cane quality, such as its surface evenness, lack or presence of blemishes on the surface, and the type of final product into which rattan is intended to be transformed.
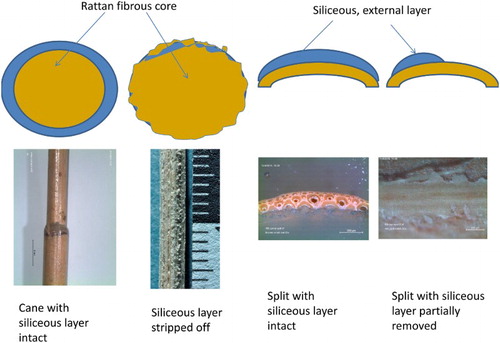
Rattan can be used generally in two forms, one as an intact cane, or as so-called ‘split’, which is a narrow strip, typically 2–5 mm wide, removed from the cane ().
The cane may retain the siliceous layer, which is seen in the early twentieth-century rattan furniture, or the surface may be mechanically stripped, as practiced in modern processing plants. The stripping of the outer layer is carried out by so-called stripping machines (h,i) or by hand (layer removed partially, (b,f,g)). Once the siliceous layer is removed, or its thickness reduced, bending the cane into desired shapes is easier. After shaping, the surface-stripped cane is coated with synthetic lacquers. The process of stripping and coating was observed in Solo, near Jogjakarta, and in six plants on the outskirts of Surabaya (January and July 2016, respectively), both in Indonesia.
In the first instance, when natural siliceous layer remains intact, the rattan is protected from mechanical damage and insect infestation; the surface layer is insoluble in organic solvents. In the second instance, when the natural layer is replaced by man-applied coating (if it has not undergone cross-linking) the coating may be dissolved and removed by several types of organic solvents.
In the manual cleaning of rattan cane, the knife leaves a specific pattern of ragged ‘island’ of silica, as seen in (d). A similar pattern of silica remnants (‘islands’) was observed on historic artifacts, indicating that what may be perceived as loss, and attributed to material aging or wear, may, in fact, be an indication of how the cane was processed ((e)).
In another form in which rattan is used, as ‘splits’, the external lustrous layer is retained on one side while the other, where the split was attached to cane, is fibrous. Splits are used in weaving fine baskets, mats, seats for furniture, and contemporary art pieces. In addition to splits, artists use small diameter strands, which are cut out of a larger diameter cane and therefore do not have the silicified outer layer showing only fibrous roughness of the plant core ((j)). Such small diameter (3 mm) strands were used by the Javanese artist Nindityo Adipurnomo in his 1997 sculpture, sample CH6 ((f)).
Experimental work
Materials
The samples from artifacts, artworks, newly produced objects, and raw stems used in this study can be classified into two groups: Group I, CH and Group II: RR ( and ). Group (I) consisted of 10 samples extracted from the artifacts selected after a preliminary database survey of 382 entries of NHB’s collection of objects made entirely from rattan. The artifacts were dated from the nineteenth to mid-twentieth century, constructed for different purposes and in various geographical locations in the SEA: Eight utilitarian objects, including four baskets, one mat, a child’s cot, a medicinal bottle, and one contemporary artwork created by a Javanese artist, Nindityo Adipurnomo (). The samples were labeled CH1–CH10, each one identified with a museum accession number that indicated the artifact from which the sample was extracted and abbreviation of the museum to which the artifact belongs: the National Museum of Singapore (NMS), the Asian Civilization Museum (ACM), and the Singapore Art Museum (SAM).
Table 1. Group (I) CH artifacts used for sampling.
Table 2. Group (II), RR, new artifacts made of rattan used for sampling.
Group (II) consisted of 10 new RR samples of raw material and extracted from recently made objects acquired during field studies in four SEA countries: Malaysia (Borneo’s Sarawak and Sabah); Indonesia (Java, Surabaya, and Solo near Jogjakarta); Myanmar, Inle Lake; Cambodia, Angkor Village; and in Alor Island, Lesser Sunda, near West Timor, Indonesia (). The samples were labeled with prefix ‘R’ for ‘Reference’ and the first letter of the places where the samples were collected, such as RB (Borneo), RJ-Sur (Java-Surabaya), RM (Myanmar), RA (Alor), and so on. Their provenance is indicated on , location of CH and RR sampling material.
All new reference samples with the exception of one, collected on Alor Island, underwent commercial, secondary processing. The total number of analyzed samples for Groups I and II was 20.
Analytical methods
A surface morphology and topography
The morphology and topography of surfaces and cross sections of each sample were analyzed first with a stereo optical microscope Olympus Digital Stereo SZX7 in reflected illumination to determine the overall features of samples. Next, the topography of the siliceous layer and morphology of rattan cross sections were imaged and characterized in a low vacuum field-emission scanning electron microscope (FESEM) SU5000 Hitachi, using ultra-variable pressure detection (UVD) and backscattered electron (BSE) mode in accelerating voltage 5–15 kV, HV 50 Pa, spot intensity 50 kV, and observation distance 5–8 mm.
The topography, thickness, and surface features of the siliceous layer were captured with an imaging system using confocal laser scanning microscopy (CLSM), Olympus LEXT Model. The thickness of the siliceous layer on each sample was measured in five areas. The conditions of CLSM analysis were: laser source 405 nm, average number of scan steps 900–4000, CCD camera, 16.6-pixel scan, 0.03 µm per pixel. The surface data acquisition was in ‘fine’ mode. The images were acquired in color, in white light, and as laser scans; 3D topography mapping was used for precision measurements of the siliceous layer thickness. The digital separation of siliceous layers was carried out with the multi-layer measurement function of CLSM.
Chemical composition of rattan
The qualitative and semi-quantitative elemental analyses of the outer layer, silica bodies, and rattan stem were carried out using EDX; data were collected and processed with a Bruker X-Flash6|60. The data were collected with macro-X-ray fluorescence spectroscopy (m-XRF), M6 JetStream was used to evaluate the silica intensity in the upper layer on eight rattan samples, four from each group. The maximum magnification of images in SEM used for data acquisition for all samples was 300×, to ensure comparative conditions of all surfaces; as for elemental data acquisition for all samples in EDX the conditions were as follows: 15 kV, 5–8 mm working distance, time of spectral acquisition 3 min, and for m-XRF, time 37–60 min per set of four samples assembled for one scan, pixel time 10 ms/pixel; tube condition HV 50 kV anode current near 598 µA.
Results
Morphology and topography of biogenic silica
The analysis of the SiO2 layer and its interface with the fibrous core evaluated in UVD and BSE mode showed a glassy, smooth surface well attached to the core ( and ). The pattern of cracks, running across the stem, or split, was observed in most of the CH samples, and only in some from the RR group. The fine cracks were randomly located in new rattan (RR group), present on one strand or split and absent on other strands within the same object. In one case, on the sample from a plaited chair seat (RJ_Sur1) reddish discoloration along the cracks was observed ().
Figure 8. Analytical protocol applied to the examination of the siliceous layer on rattan in CH artifacts, illustrated on sample CH4.
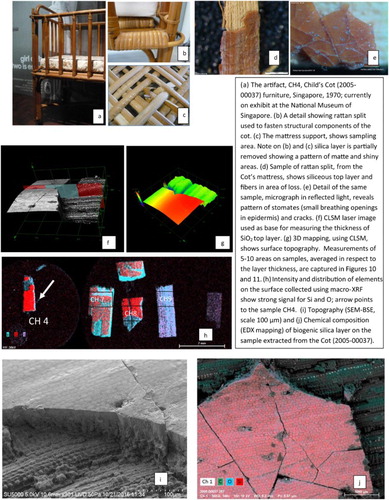
Figure 9. Analytical protocol applied to the examination of RR illustrated on samples: RB-L split and RC-stem.
The cellular pattern of the siliceous layer, unique for each sample, was clearly pronounced on few samples extracted from CH group and visible in all new rattan RR samples ( and ). Regarding the interface of the siliceous layer and fibrous core, observed in eucentric position in SEM, the SiO2 layer was well attached to the fibers in both new and historic, naturally aged samples; no internal separation or splitting was observed on CH or RR samples.
Table 3. Summary of elemental analysis, CH samples.
Table 4. Summary of elemental analysis, RR samples.
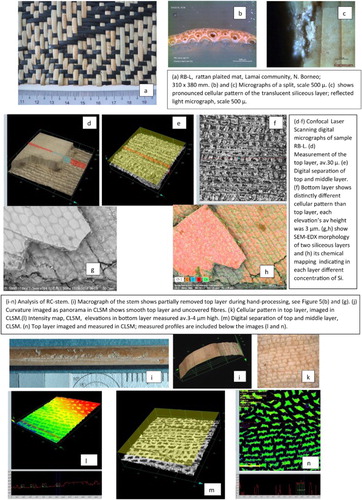
A distinct, two-layer structure within the siliceous epidermis was detected in most new reference samples (RR Group II); the lower layer, typically thinner, showed a repeated pattern of elevations ranging from 2 to 4 µm in height. The top layers and bottom layers showed distinctly different cellular patterns in each sample. The thickness of the upper one ranged from 70 to 140 µm, while the lower one showed thickness up to 20 µm. The thickness of both siliceous layers in the new samples (RR, Group II) was measured using the digital layer-separation function in CLSM. The digital separation of layers was not possible on historic materials (CH, Group I) most likely due to the layers increased opacity, dark pigmentation, and build-up of deposits over the years. The average thickness of the siliceous layer in CH was 49.4 µm and in RR 54.39 µm.
The attempt to correlate the SiO2 thickness with artifact date did not produce conclusive results. It was clear that neither age of the artifacts nor their provenance had any impact on the thickness of the siliceous layer. For example, a very thin layer was detected on an artifact from Sumatra, dated to the later nineteenth century and an equally thin layer on a much younger artifact from Malaysia dated to the mid-twentieth century ( and ).
Figure 10. The percentage of elemental Si and thickness of the biogenic silica layer showed no correlation; the thicker layer not necessarily contained a greater percentage of Si element, as seen in sample CH8 and CH9 and a thinner layer contained larger percentage of Si, as seen in sample CH2. The sample CH6 was stripped of the siliceous layer, and therefore indicated the minimal percentage of Si.
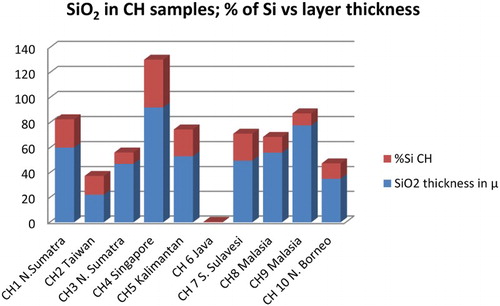
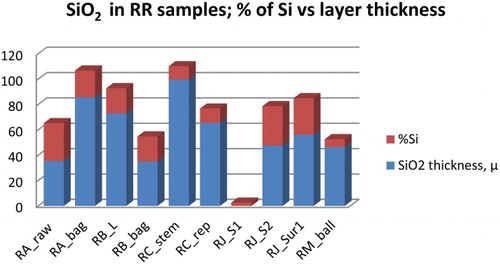
Figure 11. The percentage of elemental Si and thickness of the biogenic silica layers in RR also showed no correlation. As seen in samples RC, the thickest layer contained a relatively small percentage of elemental Si when compared to samples RA raw and RJ_S2 which showed nearly 1:1 ratio of Si and the layer thickness.
Chemical composition
The qualitative and semi-quantitative X-ray microanalysis of the outer layer on rattan carried out with EDX and m-XRF analysis indicated high percentages of silica and oxygen of biogenic silica. The high percentage of Si was recorded also on the surface of silica bodies in the stems’ transverse sectioning. The intensity of Si mapped with m-XRF in the outer layer varied within two studied groups; some historic samples (CH4 Child’s Cot and CH8 basket) showed high intensity, others very low. Similarly, within the group of Reference Rattan only one, RC, showed a uniform Si intensity throughout the outer layer. The results collected with m-XRF were confirmed with EDX data. (Application of XRF to rattan analysis is undergoing further investigation.) Other elements detected in silica bodies included Al, P, Ca, Na, and Mg which confirmed findings of other researchers. The average silica percentage content in CH samples was 18% (with the exclusion of 2.6% in one, stripped sample) and in RR 19.89% of the total mass in the analyzed area.
Discussion and conclusion
The morphology, topography, and interfaces of the siliceous layer in all samples showed that the epidermal layer of rattan is an integral part of the plant; therefore, spontaneous separation of this layer is unlikely to occur. No separation of the epidermis was observed in new rattan and aged cultural heritage samples, leading to the conclusion that losses observed on artifacts are not necessarily caused by aging of the material. ‘Loss’ of the siliceous layer can be achieved mechanically and deliberately, as it was observed during mechanical surface cleaning in the processing plants. Working practices observed during field studies showed similarities in the patterns left on the surfaces cleaned by hand to patterns observed on CH samples leading to the conclusion that what is perceived as losses attributed to material aging or ware could be possibly caused by working methods.
The micrographs generated in FESEM showed that morphology of biogenic silica layer on historic samples (CH) and new rattan (RR) varied within each group and showed distinct cellular patterns unique for each sample (see and ). The natural variations of cellular pattern in the epidermis within each group point out to the diversity of features found in natural plant material.
The thickness of the siliceous layer as well as its chemical composition analyzed in both groups showed great and random variations of both components. No correlation could be established in either group between the thickness and percentage of silica in these layers, or association between the features of the layer with age or geographical location of artifacts and the origin of new samples; in other words the thickness of the layers was independent of the percentage of Si in their content.
The intensity and percentage of silica element measured with EDX in all samples can be considered only as semi-quantitative in evaluating elemental composition, even though the conditions of data acquisition were identical for all samples. The approximation is suggested mainly because of the variations within the natural plant material which create a multitude of potential variables. The data maybe also affected by the location from which the sample was collected on the artifacts.
The typical methods of evaluating the elemental content in plant material used by botanists require analysis of ash after pyrolysis. However, the limited amount of material that can be extracted from the CH artifacts precludes that technique. Therefore, even though the EDX results may not be completely accurate they provide approximate numbers and a general understanding of the differences that can be found in the analyzed material.
The new contribution not reported in other bibliographic sources was the discovery of two layers within siliceous epidermis on new rattan. The translucency and light, straw-like color of the epidermis on new rattan permitted digital separation of layers with CLSM revealing the different cellular pattern of each layer. Therefore, the mechanical removal of the upper siliceous layer not necessarily exposes fibrous core underneath, rather it may reveal a thinner, yet still smooth, siliceous surface underneath the thicker one.
The distinct changes of color between new light color rattan and darker, old samples, point to the dependence of coloration on the age of objects. Interestingly, however, there was no change in the chemistry of the siliceous layer that could be associated with these age-related alterations. The uniformity of darkening of most of the CH artifacts, from light brown to deep, saturated brown and nearly black suggests that the change in color is internal and in bulk rather than triggered by light or other external factors, in which case it would be spotty and uneven.
The most significant factor of practical value for the design of a preservation protocol is the proposed set of analytical tests to positively identify rattan and the presence of the siliceous layer. That in turn will ensure establishing a sound conservation process for rattan artifacts broadening the gamut of applicable surface treatment methods.
Many variables affect the features of SiO2 on surfaces of museum objects requiring therefore taking into consideration the context from which the samples were extracted and the artifacts themselves. Although these preliminary findings contribute to a better understanding of biogenic SiO2 layer on artifacts, until now little known, they also show the need for further investigation. Two areas in particular need further clarification, the process of transformation of sap into a solid glassy layer, and the efficiency of chemical mapping carried out with m-XRF.
Personal communications
PC1 – Liem Laurentius, Director, Indonesian Furniture Industry & Handicraft Association, ASMINDO, Java, Indonesia. Meeting in July 19, 2016, Surabaya.
PC2 – Dr. G. Weiner and Dr. W. Liese, former Chairman of Wood Biology, Hamburg University, Germany, corresponded in 2015 through 2017.
Acknowledgements
The author appreciates the support extended by the NHB, especially Sean Lee, Director of the Heritage Conservation Centre, Singapore that enabled presentation of the preliminary results as a poster at the Gordon Scientific Conference at Sunday River ME, USA, 2016. The gratitude is extended to Prof. Dr. Piotr Targowski, Institute of Physics, University of Nicolaus Copernicus UMK, Torun, Poland, for macro-XRF data processing; to Mr D. Lizun, Painting Conservator at NHB-HCC, Singapore for preparing embedded samples of rattan; to Dr G. Weiner and Dr W. Liese, former Chairman of Wood Biology, Hamburg University, Germany, for their invaluable comments on rattan’s anatomy; ASMINDO Director L. Liem for kindly touring the rattan plants near Surabaya and sharing his knowledge of rattan production in the region. The author is indebted to Dr Frantisek Kratochvil, Nanyang University of Singapore, NTU, for the invitation to participate in his anthropological linguistic research expedition to Alor Island, Indonesia. I am indebted to Dr Carole Neves, V. Horie, two anonymous reviewers, and Dr S. Golfomitsou, Associate Editor, for their thoughtful comments. Thanks are due to the Singapore Art Museum, National Museum of Singapore, and the Asian Civilization Museum in Singapore for permission to use images of art and artworks in their collections.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Bhat, K. M. 1996. Grading Rules for Rattan. A Survey of Existing Rules and Proposal for Standardization. INBAR Working Paper No.6. Kerala Forest Research Institute.
- Currie, H. A., and C. Perry. 2007. “Silica in Plants: Biological, Biochemical and Chemical Studies. Botanical Briefing.” Annals of Botany 100 (7): 1383–1389. doi: 10.1093/aob/mcm247
- Dransfield, J. 2003. “General Introduction to Rattan – The Biological Background to Exploitation and the History of Rattan Research.” In Rattan: Current Research Issues and Prospects. FAO Corporate Document Repository. http://www.fao.org/docrep/003/y2783e/y2783e06.htm.
- Dransfield, J., and N. Manokaran, eds. 1994. Plant Resources of South-East Asia, No. 6. Rattan. Bogor: PROSEA.
- Ebanyenle, E., and A. A. Oteng-Amoako. 2003. “Anatomy and Identification of Five Indigenous Rattan Species in Ghana. Forestry Research Institute of Ghana.” Ghana Journal of Forestry II (2): 77–90.
- Fauteux, F., W. Remus-Borel, J. G. Menzies, and R. R. Belanger. 2005. “Silicon in Plant Disease Resistance Against Pathogenic Fungi. Mini Review, Elsevier.” FEMS Microbiology Letters 249: 1–6. doi: 10.1016/j.femsle.2005.06.034
- Hodson, M. J., P. J. White, A. Mead, and M. R. Broadley. 2005. “Phylogenetic Variation in the Silicon Composition of Plants.” Annals of Botany. 96: 1027–1046. IFAD. l99l. Research needs for bamboo and rattan to the year 2000. Rome. doi: 10.1093/aob/mci255
- Johnson, D. V., Rattan glossary, and T. C. H. Sunderland. 2004. Compendium Glossary with Emphasis on Africa. Non-wood Forest Products, 16. Rome: Food and Agricultural Organization of the United Nations (FAO).
- Kaufman, P. B., W. C. Bigelow, R. Schmid, and N. S. Ghosheh. 1971. “Electron Microprobe Analysis of Silica in Epidermal Cells of Equisetum.” American Journal of Botany. 58: 309–316. doi: 10.1002/j.1537-2197.1971.tb09978.x
- Kealhofer, L., and D. R. Piperno. 1998. Opal Phytoliths in Southeast Asian Flora. Contributions to Botany, No. 88. Washington, DC: Smithsonian Institution Press.
- Ma, J.-F., and N. Yamaji. 2006. “Silicon Uptake and Accumulation in Higher Plants.” Elsevier, Trends in Plant Science 11 (8): 392–397. doi: 10.1016/j.tplants.2006.06.007
- Meijaard, E., R. Achdiawan, M. Wan, and A. Taber. 2014. Rattan, the Decline of a Once-important Non-timber Forest Product in Indonesia. Center for International Forestry Research Occasional Papers 101. Bogot Barat: CIFOR.
- Palisoc, J. G. 2005. Bleaching and Finishing of Rattan. Technology Guide No. 05 ITTO-Philippines-ASEAN Rattan Project. Laguna: ERDB College. ISBN: 978-971-8831-26-7.
- Peluso, N. L. 2001. Case-study One, Rattan Industries in East Kalimantan. FAO Corporate Document Repository. Accessed June 23, 2015. www.fao.org/docrep/x5860e/x5860e04.htm.
- Perry, C. C. 2009. “An Overview of Silica in Biology: Its Chemistry and Recent Technological Advances.” Progress in Molecular and Subcellular Biology 47: 295–313. doi: 10.1007/978-3-540-88552-8_13
- Piperno, D. R. 2006. Phytoliths, A Comprehensive Guide for Archaeologists and Paleoecologists. Lanham, MD: AltaMira Press, A Division of Rowman & Littlefield.
- Prychid, C. J., P. J. Rudall, and Greogory Mary. 2003. “Systematics and Biology of Silica Bodies in Monocotyledons.” The Botanical Review 69 (4): 377–440. doi: 10.1663/0006-8101(2004)069[0377:SABOSB]2.0.CO;2
- Sellato, B. 2012. Plaited Arts from the Borneo Rainforest. Singapore: The Lontar Foundation/NTU Press.
- Uhl, N. W., and J. Dransfield. 1987. General Palmarum: A Classification of Palms Based on the Work of Harold E. Moor, Jr. Lawrence, KS: The L. H. Baily Hortorium and the International Palm Society/Allen Press.
- Wan Razali, W. M., J. Dransfield, and N. Manokaran. 1992. A Guide to the Cultivation of Rattan. Malayan Forest Record No. 35. Kepong: Forest Research Institute Malaysia (FRIM).
- Weiner, G., and W. Liese. 1988. “Anatomical Structures and Differences of Rattan Genera From Southeast Asia.” Journal of Tropical Forest Science 1 (2): 122–132.
- Weiner, G., and W. Liese. 1990. Rattans-stem Anatomy and Taxonomic Implications. IAWA Bulletin 11 (1): 61–70. doi: 10.1163/22941932-90001145
- Weiner, G., and W. Liese. 1991. Anatomical Comparisons of Commercial and Non-commercial Rattans. Seminar Proceedings, Oil Palm Trunk and Other Palmwood Utilization. Kuala Lumpur. Published in: Oil Palm Tree Utilization, Committee of Malaysia, Ministry of Primary Industries. 360–367 (copy courtesy of the authors).
- Wilding L. P., N. E. Smek, and L. R. Dress. 1977. “Silica in Soils: Quartz, Cristobalite, Tridymite and Nopal.” In Minerals in Soil Environments, 471–552. Madison, WI: Soil Science Society of America.
- Yoshida, S., Y. Ohnishi, and Kakuzo Kitagishi. 1962. “Histochemistry of Silicon in Rice Plant. The Presence of Cuticle-silica Double Layer in the Epidermal Tissue.” Soil Science and Plant Nutrition 8 (2): 1–5. Taylor and Francis Group. doi: 10.1080/00380768.1962.10430982