ABSTRACT
This research intends to enhance our understanding of the tin-glaze technology employed in seventeenth- and eighteenth-century Delftware and other tin-glazed earthenware in the Netherlands. The study provides a detailed description of the raw materials utilised in Dutch tin glazes and offers a schematic overview of the production process. Additionally, 39 dated historical recipes for tin glazes are used to compare and interpret changes in the quantities of raw materials within a temporal context. Weight percentages of raw materials – estimated for the final glaze – reveal significant changes over time. The study shows that changes over time mirror the rise and decline of the Dutch tin-glazed earthenware industry, reflecting economic shifts. Most tin - the costliest glaze material – was utilised between c. 1680 and c. 1720, and the highest glaze qualities were reported during this period, marking the peak of the Dutch earthenware production. Towards the end of the heyday, a decrease in tin usage coincides with mentions of lower glaze qualities. The superior glaze quality of Delftware tin glazes compared to tin-glazes of other tin-glazed earthenware in the Netherlands could not be traced back to specific formulations. Other factors, such as the quality of raw materials, likely contributed to the perceived visual superiority.
Introduction
Antique Delftware, also known as Delft Blue or delftware with lowercase ‘d’, refers to tin-glazed earthenware produced during the seventeenth and eighteenth centuries. It was produced not only in Delft, a small city in the Netherlands but also in several other European cities. In Delft, however, it gained immense popularity and became a major industry. For the purpose of this research, we define the term ‘Delftware’ with capital ‘D’ for all tin-glazed earthenware originally produced in Delft, while tin-glazed earthenware made elsewhere is explicitly labelled differently.
Delftware is a type of high-quality, low temperature-fired fine earthenware (van Dam Citation2004). It consists of a pre-fired ceramic body covered with a milky-white lead glaze, to which tin has been added for a white appearance. This so-called ‘tin glaze’ is applied to both the outer surface or front and inner surface or back of the pre-fired ceramic body, and usually provided with bright blue decorative elements, or sometimes combined by other coloured decorations made from metal oxides. Even though tin glaze is basically a lead glaze with the inclusion of tin, the term ‘tin glaze’ is widely accepted and will be used throughout the subsequent text.
At the Rijksmuseum in Amsterdam, The Netherlands, the research on Delftware primarily focuses on decorative objects originally made in Delft. The museum holds one of the world’s largest collections of high-quality Delftware. In 2016, the museum began an extensive art historical and stylistic research project into its approximately 1600 Delftware pieces, as part of an effort to create an online catalogue (not yet published). This was accompanied by a comprehensive technical study to simultaneously investigate variations in the composition of the tin glaze and the blue decoration of pieces from c. 1600 to c. 1800. The aim was to establish connections with historical recipes and raw materials for a coherent interpretation.
The present research, which forms the first part of the technical study, mainly concentrated on comparing historical tin-glaze recipes and tracing changes over the period from c. 1600 to c. 1800. Only three recipes for Delftware tin glazes are known to have been preserved. Given the difficulty of describing temporal changes solely using three recipes, 33 recipes from other production centres in the Netherlands were included for comparison. These recipes cover the entire heyday of Delftware production. Additionally, one German recipe was included, and to broaden the timeframe, two sixteenth-century Italian tin-glaze recipes for majolica were added. Majolica, the precursor to Dutch tin-glazed earthenware, refers to a type of earthenware made with a thicker ceramic body and a tin glaze applied exclusively on the outer surface or front (Caiger-Smith Citation1973). Although comparable pieces from other Dutch production centres may vary in quality, Delftware stood out for a thinner ceramic body, a pleasing resonance, a clearer and glossier white glaze, and vibrant blue decoration.
Various authors have previously explored the historical and stylistic development of Delftware and other Dutch tin-glazed earthenware (Caiger-Smith Citation1973; van Aken-Fehmers et al. Citation1999; van Dam Citation1999; van Aken-Fehmers and Schledorn Citation2001; Eliëns Citation2003; van Dam Citation2004; Tichelaar Citation2005; van Aken-Fehmers Citation2007; van Aken-Fehmers et al. Citation2013; van Geenen Citation2013; Lambooy Citation2013a; Oskamp Citation2013; van Geenen Citation2017; van Erkel Citation2020). These authors describe the rise of Delftware at the beginning of the seventeenth century and its decline at the end of the eighteenth century. They highlight time-related developments; from objects with a thick ceramic body and a relatively matte white glaze, to elegantly modelled products with fine decoration in different colours and a glossy finish in the eighteenth century. Delft potters continuously strove for a superior quality to compete with the high demand for delicate Chinese porcelain in the beginning of the seventeenth century; and with the more affordable English creamware that emerged later.
Van Dam (Citation1999; Citation2004) outlines the interaction between various production centres in the Netherlands. The author notes that while potters closely followed technical developments and decorative styles from other factories, the production of high-quality decorative earthenware was typically separated from that of tiles and tableware. This separation stemmed from different demands in production scale, product size, and shape, which in turn required distinct kiln sizes and specialised painter skills. Van Dam also discusses the visual distinction between high-quality decorative wares such as Delftware and the more mass-produced tiles and tableware. Although tile plateaus could be of high quality, wall tiles typically display less vibrant colours and simpler decorations compared to the elaborate designs of individual Delftware pieces. Van Lookeren Campagne's dissertation (Citation2022) focuses on tin-glazed Dutch tiles from approximately 1600 to 1750. The author investigated the source and composition of the raw materials mentioned in historical recipes as well as the production techniques used for both the ceramic and glaze of Dutch tin glazed wares, with a special focus on tiles. Research into the physical and chemical characteristics of tin glazed tiles from the period was undertaken, with regard to both the tin glaze and the ceramic body. What distinguishes the present study from that of van Lookeren Campagne-Nuttall (Citation2022), which also includes recipe comparison of Dutch tin glazes, is the specific focus on the tin glaze of high-quality mainly decorative Delftware in the Rijksmuseum's collection and how these recipes changed over time.
Research aim
This research aims to improve our understanding of the glaze technology employed in the production of high-quality Delftware during the seventeenth and eighteenth centuries in Delft, the Netherlands. The research explores whether the superior quality of the tin glaze in Delftware can be deduced from historical recipes and the raw materials utilised. This was accomplished by comparing dated tin-glaze recipes from Delftware with those from other Dutch tin-glazed earthenware, considering both the quantity and quality of raw materials, as these factors contribute to the final appearance and properties of the glaze. Throughout the various production steps, weight percentages (wt.%) of raw materials were estimated based on the documented weights in the recipes, after which they were plotted against time. The results were then evaluated in the context of changes in glaze quality, as reported in the various historical sources.
Production technique
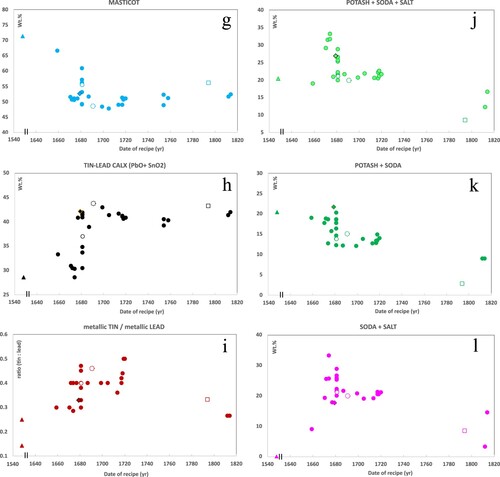
Tin-glazed earthenware traces its origin to Iraq, where a new glazing technique was developed in the eighth or ninth century to mask the colour of the fired clay body and to make it waterproof (Allan Citation1973; Mason and Tite Citation1997; Tite, Pradell, and Shortland Citation2008; Matin Citation2019; Pradell and Molera Citation2020). This section describes the production technique of tin-glazed earthenware in the Netherlands during the seventeenth and eighteenth centuries and provides a schematic overview in . It elaborates on the white tin glaze, and briefly touches on the blue glaze, the lead overglaze, and the ceramic body. The manufacturing processes and composition of the raw materials are presented within the context of the seventeenth and eighteenth centuries. It is important to note that the raw materials during these centuries differed from those used today. This difference arises from their high proportion of impurities and variable composition throughout the years.
Figure 1. A schematic overview of the preparation process for white tin glaze, ceramic body, blue glaze, and lead overglaze used in seventeenth- and eighteenth-century Dutch tin-glazed earthenware, as summarised from the handbooks of Sijbeda (Citation1712-Citation1720) and Paape (Citation1794). Raw materials of the seventeenth and eighteenth centuries are given as mentioned in the recipes and include impurities, having varying compositions. Their chemical composition is simplified here but detailed in . The approximate temperatures of kiln or furnace are given in red (Molera et al. Citation1999; Tichelaar Citation2005; Tite, Pradell, and Shortland Citation2008; Tite Citation2009; Pradell and Molera Citation2020). Illustrations of dipping the tin glaze and spattering the overglaze are sourced from Paape (Citation1794). Plate BK-1963-29 (Grieksche A, Rijksmuseum) dates to between 1690 and 1705. An asterisk (*) indicates that a raw material is not always used, (s) means solid.
The ceramic body
The process of preparing and modelling the clay is well-described in works by Paape (Citation1794) and van Lookeren Campagne-Nuttall (Citation2022). It involved mixing different local predominantly fluvial clays with imported calcium-rich dry marl or lime. The addition of the latter component was intended to reduce plasticity, enabling the creation of elegant and complex models with a thin body. Additionally, it contributed to making the ceramic body lighter in colour and to enhancing its strength. After cleaning, homogenising, and modelling the clay, it was slowly heated in a kiln and fired at temperatures between 950 and 1050°C (Tite Citation2009), followed by a gradual cooling. This resulted in a porous but hard yellowish ceramic body emerging from the kiln.
The white tin glaze
A tin glaze is primarily a melt of silicon dioxide (SiO2), sodium and potassium carbonate (Na2CO3 and K2CO3), and sodium chloride (NaCl), with the addition of lead(II) and tin(IV) oxide (; ). In terms of seventeenth- and eighteenth-century raw materials, the production of tin glaze involved the utilisation of ‘sand’, ‘soda’, ‘potash’, ‘salt’, and ‘tin-lead ash’. The white colour of the glaze results from the recrystallisation of small tin oxide particles in the lead-silicon oxide matrix upon cooling, inducing light scattering that imparts opacity. The making of a tin glaze was a labour-intensive process. It involved several heating and milling steps, along with a final step to fuse the tin-glaze suspension onto the pre-fired ceramic body. Two pre-processed components were involved: masticot (step 1) and tin-lead calx (step 2). provides a list of the raw materials as mentioned in historical tin-glaze recipes of the seventeenth- and eighteenth-century Delftware and other tin-glazed earthenware from the Netherlands by Kunckel (c. 1679; Appendix 3 in Lambooy Citation2013b), Sijbeda (Citation1712-Citation1720), and Paape (Citation1794). The information on composition, provenance, price, purity, and quality derives from references indicated in the Table. Raw materials of the blue glaze and lead overglaze have been added for completeness.
Table 1. List of raw materials used in the seventeenth and eighteenth centuries for making tin glaze, blue glaze, and overglaze, according to historical recipes of Delftware and other tin-glazed earthenware in the Netherlands. Recipes from Kunckel (c. 1679; Appendix 3 in Lambooy Citation2013b), Sijbeda (Citation1712-Citation1720), and Paape (Citation1794). Names of raw materials are given as mentioned in the recipes. Composition, synonyms, provenance, price, purity, and quality are obtained from references indicated in the last column. All seventeenth- and eighteenth-century raw materials contained impurities and varied in composition. The best quality is placed at the top of each material group. Prices of raw materials from Sijbeda’s recipes are selected from .3 in van Lookeren Campagne-Nuttall (Citation2022) and averaged. They are expressed in stu/lb, meaning stuivers (old Dutch currency unit) per pound (old Dutch weight unit). Brackets mean that prices are not specified for one particular variety of the raw material, but are indicative for the entire group. Aas is an old weight unit; ‘(s)’ means solid. Full descriptions of recipes are provided in Supplemental Table S1.
First step – masticot
The making of masticot largely involved the incomplete fusion of SiO2 (by adding sand in excess) and Na2CO3 and K2CO3 (by adding soda and potash) or NaCl (by adding salt) at temperatures below 1000°C (Tite, Pradell, and Shortland Citation2008; ). Soda, potash, and salt served as flux to reduce the melting point of the mixture. A good balance between the sodium and potassium salts was essential for a successful glaze, yet challenging due to the variable composition of the raw materials containing these salts, depending on their provenance and harvest time. The glassy substance produced primarily consisted of Na2SiO3, K2SiO3, and unreacted SiO2, while CO2 and FeCl2 were released during heating from the reaction with the carbonates and FeO in the sand.
Second step – tin-lead calx
The second step involved the melting of metallic lead and tin blocks together in a furnace, where the metals first melt and then gradually oxidise at c. 600°C (Pradell and Molera Citation2020; ). The resulting powder, existing of PbO + SnO2 known as tin-lead calx, could vary from pale to deep yellow in colour, depending on the lead-to-tin ratio used. Calx is defined as the fine powder that remains after a metal or mixture of metals has been calcined (oxidised) by heating in air to temperatures above its melting point (Matin, Tite, and Watson Citation2018). For producing a white glaze, a pale yellow tin-lead calx is required, achieved when the lead-to-tin weight ratio is below 3.5. With such a ratio, there is not enough lead available to complete the reaction to form the deep yellow Pb2SnO4 (Matin Citation2019), resulting in a powder composed of yellowish PbO, white SnO2, unreacted SnO, Pb(s), and Sn(s). The powder was then collected for further use.
Third step – the white glaze block
The third step regarded the fusion of the ground masticot, tin-lead calx (PbO + SnO2), and additionally added water soluble bases (alkali salts) (). In some cases, red copper filings were first calcined and then added as CuO, along with ‘smalt’ or blaauwzel (K-, Co-silicate), which give the final glaze a greenish and bluish hue, respectively. The reactions that occur during the fusion at c. 1000°C depend on the amount of each component present and the thermal path followed (Molera et al. Citation1999; Tite, Pradell, and Shortland Citation2008). The thermal path includes the duration for which the object is maintained at the firing temperature and the rates of heating and cooling. Upon cooling, the least soluble compound (SnO2) recrystallises. Newly formed tiny crystals (less than 1 µm) are responsible for the opacity and are smaller than unreacted SnO2 particles remaining from the tin-lead calx powder. After melting, the resulting hard, whitish glaze block was milled, suspended in water, and applied onto the ceramic body on both sides by immersion.
Fourth step – the white tin glaze
The fourth and final step involved fusing the dried tin-glaze suspension onto the ceramic body at approximately 950°C (Lightbrown and Caiger-Smith 2007 in Tite Citation2009; ). In this step the melting glaze adheres firmly to the body through the formation of an interface. The interface is created through the migration of free lead cations from the melting glaze to the body, and the diffusion of freely moving cations of K, Al, Ca, Fe, and Si from the body into the glaze. This process allows for the formation of K-Pb feldspar crystallites upon cooling (Molera et al. Citation2001). The crystalline phases constitute the interface but are rarely found within the glaze itself. During the fourth step, the white powdery surface ideally transforms into a shiny, glassy white, and waterproof glaze. However, the texture of the glaze may vary, ranging from smooth and glossy to uneven with irregularities. A ‘poor’ glaze can result from various variables, such as impurities in raw materials, tin loss, overfiring, and significant differences in expansion coefficients.
The blue glaze
To make the greyish vitreous glaze suspension for the blue decoration, zaffer (CoO, made by roasting cobalt minerals) and/or ‘smalt’ (K-, Co-silicate) were mixed with sand or masticot, potash, soda, or salt (; ). This mixture was heated to approximately 1000°C in rough pots or closed tubes, milled, and then suspended in water (Paape Citation1794). Following the third step of the tin glaze, but before the final fourth, painters used this suspension to paint the decoration on the raw white powdery surface applied to the ceramic body. Finally, both the white glaze and the greyish ‘paint’ were fired again, with the latter transforming into a bright blue hue. Proportions of the less viscous zaffer and the more viscous smalt were adjusted to the viscous properties and structure of the tin-glaze surface to minimise colorant diffusion. Each workshop and painter developed their own favourite mixtures in this way. For drawing contours, a darker blue was sourced from pots in which brushes had been washed or iron rust had been added (Paape Citation1794).
The lead overglaze
Before the final step, but after painting the decoration, a thin transparent lead overglaze (kwaart or coperta) was sometimes applied to improve the final finish (Sijbeda Citation1712-Citation1720; Paape Citation1794). Due to the low viscosity of a lead overglaze, it fills irregularities, enhances the shine due to its high refractive index, deepens colours, and counteracts any dull or greyish appearance in the white glaze (Sijbeda Citation1712-Citation1720; Paape Citation1794; Tite Citation2009; Lambooy Citation2013b). The lead overglaze was made of a mixture of sand or masticot, goudglette (red tetragonal lead(II) oxide, litharge, αPbO), potash, and salt (Paape Citation1794), heated at c. 1040°C (Tite, Pradell, and Shortland Citation2008; ; ). It was applied by dipping or spattering with a brush until the decoration became invisible (Paape Citation1794; Rackman Citation1955; Tite Citation2009). In the final melting, the overglaze suspension fuses to a clear transparent shiny layer. While the advantages of an overglaze have been known since the fifteenth century, it was not always chosen due to the risk of damaging delicate decoration (Tite Citation2009).
Results and discussion
Raw materials
Numerous raw materials were needed for the making of a seventeenth- and eighteenth-century tin glaze. Following on from the research undertaken by (van Lookeren Campagne, Megens and van Bommel Citation2018; van Lookeren Campagne-Nutall Citation2022), a comprehensive literature study was conducted to create a schematic overview of the raw materials documented in historical Dutch tin-glaze recipes, incorporating the three Delftware recipes. The overview combines information dispersed across various references. It intends to improve the readability of the paper and serves as a valuable resource for further research on tin-glazed earthenware. presents the overview, covering information on raw materials, their composition, impurities, provenance, quality, and associated costs.
Sand
Sand, the cheapest raw material used in the tin-glaze production, comprises particles larger than 63 µm and primarily consists of quartz (SiO2), iron compounds, and calcareous particles (). Iron compounds are undesirable as they could cause discoloration of the glaze (van Lookeren Campagne, Megens, and van Bommel Citation2018). In contrast, calcareous particles enhance glaze hardness and stability (Dungworth Citation2012). Hence, sand with shell fragments was preferred. Natural sources of the different sands used are provided in .
Potash
Potash includes various water-soluble potassium salts, mainly potassium carbonate (K2CO3) and its hydrates. Potassium carbonate serves as a flux and improves the strength, chemical resistance, and clarity of the glaze. Potash was commonly obtained from wine lees or ashes of continental plants and wood, which contain potassium by uptake from the soil (Wisniak Citation2003). Various qualities of potash were available. The most expensive potash, with the fewest impurities, was made from wine lees. Inexpensive potash was made from the wood of broad-leaved trees and ferns, from which it was extracted by evaporation. The most impure potash was produced from unrefined local plants (van Lookeren Campagne-Nuttall Citation2022).
Soda
Soda serves as the primary flux, lowering the melting temperature of a mixture. In addition, it is useful as bleaching agent for a glaze. In the seventeenth and eighteenth centuries, soda was extracted from ashes of marine and salt-tolerant plants. It comprised various compounds, such as Na-, K-, Mg-, and Ca-carbonates, sometimes sulphates, chlorides, and their hydrates (). The composition varied based on the provenance and harvesting season of the plants (Tite et al. Citation2006). Until the invention of synthetic soda in 1760, the purest form, in terms of Na2CO3 quantity, derived from the plant commonly known as barilla. In Alicante (SE Spain) barilla was cultivated for soda production. The extract contained a maximum of 40% Na2CO3 and hardly any other carbonates (Wisniak Citation2003). A more affordable type of soda was produced in England and Scotland, but not earlier than around 1660 (Dungworth Citation2009). It was made by burning cold-water seaweeds collectively known as ‘kelp’. The ash composition varied due to factors like fluctuations in the seawater composition. The main difference between soda from barilla and soda from kelp was that the former was purer and hardly contained carbonates other than Na2CO3 (Tite et al. Citation2006). Soda from kelp contained an approximately equal amount of Na2CO3 and K2CO3. The incorporation of both Na- and K-carbonates is essential for strengthening the glaze; when soda from kelp was used, the need for additional potash was reduced compared to soda from barilla.
Salt
The salt employed for Dutch tin glazes primarily consisted of sodium chloride (NaCl). While NaCl serves as a less effective flux compared to Na2CO3 and K2CO3, it excels at bleaching by removing unwanted colorations, e.g. iron compounds in sand, through chlorination. In 1828, it was asserted that, for achieving superior white glaze quality, the bleaching action induced by NaCl holds greater significance than that achieved by Na2CO3 and K2CO3 (Bastenaire-Daudenart Citation1828). Other researchers have documented the successful release of gaseous ferric chlorides (FeCl2 and FeCl3) when NaCl was added to the glaze mixture (Zhou et al. Citation2016).
In the Dutch tin-glaze recipes, salt is generally referred to as ‘ordinary’ salt. Only once has ‘mountain’ salt been mentioned, which is halite or sylvite, c. 100% pure NaCl and KCl respectively, deriving from Salzburg (Austria) (). During the flourishing period of Delftware, salt, predominantly sourced from sea salt pans, was imported for preserving fish and producing ceramics. Good-quality salt (rich in chlorides) came from Spain, Cape Verde, or Portugal. The best-quality salt was imported from St Ubes (Sebutal, Portugal). However, during the Spanish wars (1568–1648), salt was sourced from the Caribbean islands (Harreld Citation2004).
Lead (metallic)
Galena, which is lead(II) sulphide (PbS), stands as the primary ore source for metallic lead (Pb(s)). After Pb(s) is extracted from the ore through smelting, it undergoes various manufacturing processes before being sold for applications such as the ceramic and glass industries. In the seventeenth and eighteenth centuries, the main production centres of metallic lead in Europe were in Poland, Germany, and England (D’Imporzano Citation2021). England was the most consistent producer of high-quality lead. Lower-quality lead was imported from Germany in the early seventeenth century (van der Meulen and Smeele Citation2012). The use of processed ‘flat’ lead has been reported by Sijbeda (Citation1712-Citation1720). Local smelting factories produced flat lead by recycling used metallic lead into plates. Recently, lead isotope analyses of the glaze of a selected group of seventeenth- and eighteenth-century Delftware shards showed that the provenance of all bound lead in the glazes was England (D’Imporzano et al. Citation2023).
Tin (metallic)
The primary natural source of metallic tin (Sn(s)) is the tin oxide mineral cassiterite (SnO2). After smelting and refining the metal from the ore, Sn(s) is traded in various forms and qualities. According to Piccolpasso (c. 1558 in Rackman Citation1955), Sijbeda (Citation1712-Citation1720), Paape (Citation1794), and Dony (Citation1978) the highest quality came from Cornwall (UK).
This grade allowed for a maximum impurity level of 2% lead in the tin, which contributes to increased brittleness. Additionally, it permitted the addition of copper, bismuth, or antimony, which enhance hardness. In the western and southern provinces, a lower quality ‘Holland fine tin’ with a maximum of 8% lead was permitted. Even less strict requirements were set in the north for ‘Frisian tin’, which Sijbeda clarified by comparing the qualities with aas, an old unit of weight (). The use of tin in tin glaze was related not only to cost, being the most expensive raw material, but also to its availability.
Zaffer
The dark earthy zaffer is coarsely powdered impure cobalt oxide (CoO). It was used to make the powder for the blue glaze. Zaffer is obtained by roasting cobalt minerals such as cobaltite (CoAsS), erythrite (Co3(AsO4)2.8H2O), and skutterudite ((Co, Ni, Fe)As3) (Henderson Citation2013; Dehaine et al. Citation2021). The composition of impurities depends on the mineral used. In the case of skutterudite, zaffer contains compounds with Ni, Fe, and As. Zaffer was imported to the Netherlands from Saxony (Germany) in the seventeenth and eighteenth centuries (Mimoso Citation2015).
Smalt
Smalt (basically consisting of K- and Co- silicates) is produced by fusing zaffer (CoO) with quartz (SiO2) and potash (K2CO3) at approximately 1000°C. This process results in glassy pieces with an intense deep blue colour after a thermal shock in water. The composition is variable and depends on the amount of CoO, SiO2, and K2CO3 used, the purity of the raw materials, and the success of the production. Similar to zaffer, smalt was used to make the powder for the blue glaze, but a small amount of smalt was also added to the white glaze when potters wanted it to appear whiter (Paape Citation1794). Blaauwzel has the same composition as smalt but was ground in Dutch windmills ().
Red copper filings
In the preparation of the white glaze for Delftware, Paape (Citation1794) reports on the use of red copper filings or scraps of red copper, which were first calcined to copper oxide before mixing it with masticot and tin-lead calx to make the white glaze block (; ). Earlier, Sijbeda (Citation1712-Citation1720) writes about needle filings in one of his undated recipes, which he named ‘East-Indian white’. Red copper (Cu(s)) after calcination turns into black Cu(II) oxide (CuO). Both Coentro et al. (Citation2012) and Peng et al. (Citation2022) confirm that black CuO in ceramic glazes turns into green after firing if temperatures exceed 850°C, due to the formation of new Cu2+ complexes resulting from interaction with the glass matrix.
Goudglette
A transparent lead overglaze was sometimes applied for extra lustre or to fill in imperfections. From the recipes it is known that a special type of lead oxide was used to make a lead overglaze: the reddish form of lead(II) oxide, tetragonal polymorph litharge (αPbO) (Tite, Pradell, and Shortland Citation2008; Tite Citation2009; , ). Litharge, referred to in historical recipes as ‘goudglette’ or ‘goudglit’ by Sijbeda (Citation1712-Citation1720) and Paape (Citation1794), is the most stable polymorph of lead(II) oxide and is formed when metallic lead is heated above 600°C in oxidising conditions and then left to slowly cool down. It is a waste product in the cupellation of silver or roasting of galena (Krause et al. Citation2023). Sometimes, lead ash (impure PbO) was used instead of litharge in the production of a lead overglaze (Sijbeda Citation1712-Citation1720). It was made from roasting old lead sheets or galena (Walton Citation2004). Although the chemical distinction is not clarified, litharge is considered the most stable form of lead oxide and likely underwent heavily processing to maximise precious silver extraction, resulting in a purer product compared to lead ash. Both lead oxides were readily available, litharge (goudglette) from local pharmacies (Weege Citation1773) and lead ash from plumbers (Paape Citation1794).
Recipes
For this research, multiple sources containing recipes for Dutch tin glazes from the seventeenth and eighteenth centuries were utilised (). The selection of recipes was based upon the availability of two key factors: a date and sufficient data. The latter enabled the calculation of how much of each raw material was used to make the final tin glaze, the former allowed regarding the use of raw materials within a temporal framework. The selected (dated) recipes cover the period from 1659 to 1814. The batch, a total of 39 recipes, consists of three recipes from Delft, 33 from other Dutch production centres, one from Germany, and two from an Italian majolica factory. Two recipes were undated but were included for their specific raw material additions. The primary source for the Dutch tin-glaze recipes is the handwritten notebook of the Frisian potter Petrus Sijbeda. This notebook contains recipes not only from the production centre in Harlingen (Buiten de Kerkpoort), but also from other centres in the Netherlands, including two from Delft factories. Additionally, it includes contributions from Sijbeda’s parents and his successor Spannenburg. Other sources are from Paape (Delft, 1794) and from glassmaker Kunckel (Germany, c. 1679; Appendix 3 in Lambooy Citation2013b). The latter recipe represents the German interpretation of high-quality Dutch tin glaze. To provide a broader historical context for the Dutch recipes, two sixteenth-century recipes by Piccolpasso, dated around 1558, have also been included (in Rackman Citation1955). The Italian recipes refer to majolica, considered the precursor of tin-glazed earthenware in the Netherlands. Full descriptions of all selected recipes are given in Supplemental Table S1, both in original old-Dutch language and translated into English.
Table 2. List of studied Dutch tin-glaze recipes with source, author, origin, date, reference used, and type of earthenware for which they were applied. Two early Italian recipes are included for comparison, as well as one German recipe, along with recipes for blue glaze and overglaze. The list is alphabetically ordered by the origin of the recipes. Recipe labels: ‘W’ for white (tin glaze), ‘B’ for blue (blue glaze), and ‘O’ for overglaze (lead overglaze). Full descriptions of the recipes are available in Supplemental Table S1, and the data in Supplemental Table S2 with corresponding labels. Symbols indicate the source of the recipes in the figures; open symbols for Delft recipes. WMP = factory the Wildemanspoort (Delft); App. = appendix.
To ensure a fair comparison of the 39 tin-glaze recipes in the temporal context, weight percentages of raw materials were calculated over the entire production process for the final white glaze powder, using the documented quantities in each step. Data and calculations are provided in Supplemental Table S2. Crucial to note is that the weight percentages do not account for the weight and chemical changes that occur during the various melting steps, which introduce unavoidable inaccuracies (Molera et al. Citation2001). Therefore, the weight percentages should be interpreted as the percentage of raw materials (regardless their purity) used to produce the final glaze, rather than representing the actual composition of the glaze.
shows the weight percentages, ratios, and sums of raw materials, calculated from the 39 dated recipes, over time. illustrates the wt.% of raw materials for the three Delftware tin-glaze recipes through schematic box plots, allowing for mutual comparison. It is important to emphasise that in and , the terms ‘sand’, ‘lead (metallic)’, ‘tin (metallic)’, ‘potash’, ‘soda’, and ‘salt’ refer to the (impure) raw materials as documented in the seventeenth- and eighteenth-century recipes, and not to individual chemical compounds (, composition). This means that for example ‘soda from kelp’ represents a mixture of several compounds, not solely Na2CO3, and that impurities are included in the weight percentage. In the raw material weight percentages are averaged for the tin-glaze recipes of Delftware, other Dutch earthenware, Kunckel, and Piccolpasso. This averaging allows for the comparison of recipes within each group, irrespective of their date.
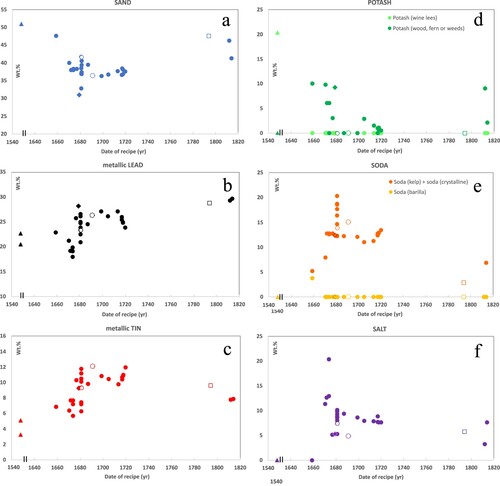
Figure 2. Time plots of raw material weight percentages (wt.%) versus the date of historical tin-glaze recipes (yr), based on data from the 39 selected recipes outlined in . Raw material names are given as mentioned in the recipes (). In plots (a–f), wt.% calculated for the final tin glaze, in plots (g–l) sums of wt.% and the ratio of metallic tin to metallic lead. Historical recipes are sourced from Piccolpasso (triangles 2x), Kunckel (rhombus 1x), Sijbeda (circles 35x), and Paape (square 1x). Recipes originating from Delft are indicated with open symbols. Symbol and reference overview in . Full descriptions of recipes and calculations of wt.% in Supplemental Tables S1 and S2. Scale break at 1560–1630.
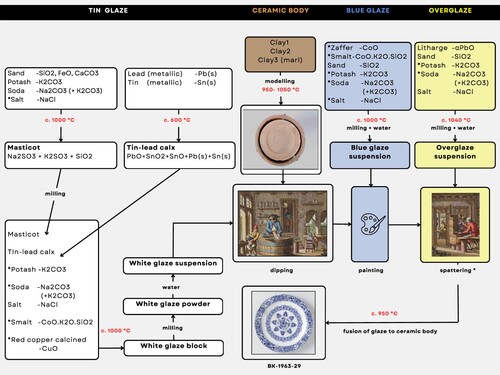
Figure 3. Box plots of raw material weight percentages from three historical tin-glaze recipes originating from Delft, calculated for the final glaze. Raw material names are given as mentioned in the recipes. Two recipes (W16a-b, open circle; W22a-b, open dashed circle) are documented in Sijbeda (Citation1712-Citation1720) and one (W35, open square) is from Paape (Citation1794). Symbol and reference overview in . Full descriptions of recipes and calculations of wt.% in Supplemental Tables S1 and S2. The dating of W16a-b is estimated from the recipe order in the handbook of Sijbeda. Additional notes for recipe W16a-b are ‘ordinary or English kelp soda of good quality in masticot and fine tin’; for recipe W22a-b ‘ordinary or English kelp soda of good quality in masticot and crystalline soda’; for recipe W35 ‘ordinary white, ordinary sand, English tin, red copper filings, smalt’. Added for comparison a blue glaze ‘Van het Blaauw’ (B1) and a lead overglaze ‘Van het Kwaart’ (O1) from Paape (Citation1794). Oxygen is not drawn but makes the total 100%. WMP = Wildemanspoort (factory in Delft).
Table 3. Average weight percentages (upper part) and ratios (lower part) of raw materials used in tin-glaze recipes for Delftware, other Dutch earthenware, Kunckel, and Piccolpasso. Averages wt.% are calculated in relation to the final glaze powder (Supplemental Table S2). Information on raw materials is given in , recipe sources and symbols in . In green, highest value of the particular raw material after horizontal comparison, in red lowest value. SD = standard deviation; soda total = soda from barilla + soda from kelp + crystalline soda; potash total = potash from wine lees, wood, fern, weeds. Soda total + potash total is regarded the total carbonates; potash total + soda total + salt = total flux; n = number of recipes.
Piccolpasso’s ‘ordinary white’ in context
The two ‘ordinary white’ tin-glaze recipes from Piccolpasso around 1558 (in Rackman Citation1955) stand out distinctively from all other recipes. These Italian recipes for majolica exhibit the highest amounts of potash, sand, and masticot, the lowest amounts of tin (metallic), lead (metallic), and tin-lead calx, and notably they lack any soda (, ). Furthermore, the type of potash differs: Piccolpasso documented potash from wine lees, a raw material not used in Northern Europe where grapes do not grow. While the purity of this potash was high and a substantial amount was used, the total flux (potash + soda + salt) remained relatively low due to the absence of soda.
Kunckel’s ‘Holland fine white’ in context
Kunckel’s German tin-glaze recipe for ‘Holland fine white’ shows only minor differences from Dutch tin-glaze recipes of the same period (, ). It shows slightly higher amounts of potash and lead (metallic), along with slightly lower amounts of soda, salt, and sand. Although all is relative, the fact that the recipe does not stand out among the Dutch recipes from that period may suggest that Kunckel or the German factory acquired knowledge from Dutch potters.
Delftware in context
A comparison of all 39 recipes reveals distinct differences in the wt.% of raw materials over the two centuries (). The same holds true for the three Delftware recipes (dates <1681, 1691, and 1794) (). However, when these three recipes are compared with other Dutch recipes from the same period, differences are minimal. Although this conclusion is drawn from only three observations (open symbols in ), the alignment of Delftware tin glazes within the broader temporal context of other Dutch tin glazes is apparent. The similarity within the same periods implies that potters within the Netherlands probably exchanged information. This exchange is evident from the inclusion of two recipes from Delft (<1681 and 1691) in the Frisian handbook from Sijbeda (Citation1712-Citation1720).
When considering Delftware and other Dutch tin-glazed earthenware as separate groups and when averaging the wt.% over the two centuries, subtle deviations emerge (). Notably, none of the three Delftware recipes mention potash, in contrast to half of the other Dutch recipes. Additionally, all three Delft recipes describe a slightly lower amount of soda and salt. The use of a lower total flux (potash + soda + salt) could have been a conscious choice, as it leads to a more viscous glaze. This higher viscosity proves beneficial when applying decoration, reducing the risk of diffusion. Alternatively, low quantities of soda and salt might result from the use of higher-quality soda and salt, requiring less weight. Future technical analyses capable of determining the glaze composition, will help understand which of the two scenarios is more plausible.
Delftware ‘evolution’
When comparing the three tin-glaze recipes of Delftware (<1681, 1691, 1794) with each other, significant differences emerge (). Contrary to the notion that they remained similar over the two centuries (van Dam Citation1999; van Aken-Fehmers and Schledorn Citation2001; van Dam Citation2004; Tichelaar Citation2005; Lambooy Citation2013a), these recipes ‘evolved’. The most notable change occurs between the recipes of 1691 and 1794. The recipe W22a-b (1691) features the highest amount of metallic tin and utilises crystalline soda. In Paape’s ‘ordinary white’ recipe (W35, 1794), the flux is low, the tin-to-lead ratio is the lowest, and smalt is included, as well as calcined red copper filings. Additionally, it describes a lead overglaze for increased gloss. The incorporation of smalt could potentially contribute to enhanced whiteness of the glaze (van Dam Citation1999; Citation2004). Somewhere during the 103-year gap between the last two Delftware recipes decisions must have been made for the adjustments described. Likely changes were taken gradually. Economic factors may have played a role, as the two earliest recipes were written during the peak of the heyday of Delftware production, while Paape wrote his during the fall, when the production was in decline. The lower demand and earnings during the late eighteenth and early nineteenth centuries could possibly justify the distinctive composition of recipes from this period.
Glaze quality and metallic tin quantity
The quality of the tin glazes, ranging from ‘bad’ to ‘excellent’, is documented in most recipes by the authors, and for this research converted into numerical values from 1 to 6, where 6 represents the highest quality. The conversion is based on the original text (Supplemental Tables S1 and S2) and summarised in . The quality ratings for the 39 tin glazes were plotted against time, using the recipe dates (a). As the temporal pattern showed similarities with the wt.% of metallic tin over time (c), the quality of the tin glaze was also plotted against the wt.% of metallic tin (b). The latter figure illustrates a weak positive relationship between the glaze quality and the quantity of metallic tin in wt.% (coefficient of determination R2 = 0.30), suggesting that, in general, higher-quality glazes were achieved with the use of more tin. Over time, the quality of the glazes, as documented in the recipes, shows a similar trend to that of metallic tin, with highest ratings in the early eighteenth century (c and a). The best-rated glazes at the beginning of the eighteenth century used high amounts of metallic tin (10.9 and 11.9 wt.%, b).
Figure 4. A time plot of (a) the quality of tin glazes as documented in 39 historical tin-glaze recipes by the recipes’ authors versus the date of the recipes (yr) and in (b) versus the quantity of metallic tin (wt.%) used to make the final glaze. Glaze quality conversion from text descriptions to numerical values in . Historical recipes are sourced from Piccolpasso (triangles 2x), Kunckel (rhombus 1x), Sijbeda (circles 35x), and Paape (square 1x). Recipes originating from Delft = open symbols. Two variants for W16 and W22 show the effect of using different types of soda (open circles and open dashed circles). CS = crystalline soda, ES = good quality English soda, S = ordinary soda. Symbol and reference overview in . Full descriptions of recipes and calculations of wt.% in Supplemental Tables S1 and S2. Scale break at 1560–1630.
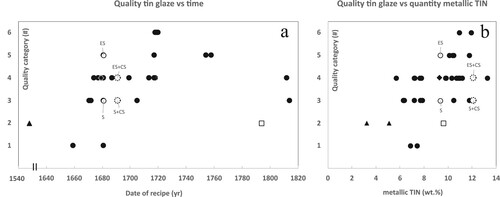
Table 4. Conversion of the tin-glaze quality, as described in historical recipes by the recipes’ authors (text in italic), into numerical quality categories ranging from 1 to 6, with 6 representing the highest quality. ‘White’ refers to the white tin glaze.
While Paape (Citation1794) notes that a higher quantity of metallic tin results in a better (whiter) final glaze, recent authors argue that this correlation might not hold true due to inaccuracies in the calculated amount of metallic tin, arising from losses during processes and impurities in raw materials (Molera et al. Citation2001). Nevertheless, a weak relationship between the quality of the glaze and the quantity of metallic tin is apparent from the recipes in this research (b). Reflecting on this and assuming that a higher quality generally indicates a whiter glaze raises the following suggestion. More metallic tin does not necessarily yield a whiter, higher-quality glaze when the recrystallisation of SnO2 during cooling is ineffective. Efficient recrystallisation is crucial for achieving a whiter appearance, as it promotes the formation of smaller and more numerous SnO2 crystals, contributing to the opacity of the glaze (Tite Citation2009). Two examples of glazes not documented as ‘excellent white’, while more than 12 wt.% metallic tin was used, are Delft W22a (‘white’ = rating 3) and Delft W22b (‘fine white’ = rating 4). They are indicated in b with open dashed circles. These examples demonstrate that other factors besides the amount of tin also influence the quality of a tin glaze.
Glaze quality and soda
Another influential factor on glaze quality is the type of soda used. Sijbeda wrote: ‘a fine tin and weak soda result in a glaze as white as ordinary tin and strong soda’. While the best-quality soda (barilla soda, made from ashes of a salt tolerant plant, deriving from Alicante) is rarely mentioned in Dutch recipes, the second and third best grades (crystalline and good-quality English soda, made from ashes of seaweeds, produced in Scotland and England) were commonly used, alongside lesser grades like ordinary and weak soda. Good-quality soda contains higher percentages of Na- and K-carbonates compared to weak soda () and is more capable of lowering the melting temperature of a glassy mixture, resulting in a better fusion of the glaze at lower temperatures (Wisniak Citation2003; Tite et al. Citation2006; Dungworth Citation2009).
Sijbeda, who reproduced two Delftware tin-glaze recipes (W16a-b; < 1681 and W22a-b; 1691), experimented with two different qualities of soda for making masticot; ordinary soda (S) and English soda (ES). When English soda (higher amounts of Na- and K-carbonates) was used instead of ordinary soda, alongside equal amounts of metallic tin, the glaze quality improved significantly. b illustrates the positive impact of good-quality soda on the glaze quality. For recipe W16a-b (open circles) the glaze received rating 5 (‘excellent fine white’) instead of 3 (‘white’), and for recipe W22a-b (open dashed circles) it received rating 4 (‘fine white’) instead of 3 (‘white’).
Conclusions
Recipes
The research offers further insights into the use of raw materials in seventeenth- and eighteenth-century tin-glazed earthenware from the Netherlands, as documented in historical recipes, despite variations in composition. By estimating wt.% of raw materials over the entire production process for the final glaze and situating them in a temporal context, the research provides a distinctive perspective on changes over time related to availability, economic situation, and product development. Significant differences emerged when comparing the recipes of Piccolpasso (‘ordinary white’), Kunckel (‘Holland fine white’), Delftware (‘white-white’, ‘double gloss’, ‘ordinary white’), and other Dutch tin-glazed earthenware (from ‘bad white’ to ‘excellent white’).
Tin and quality
The quality of the tin glaze, as documented in the historical recipes used, is weakly positively related to the quantity of metallic tin, as calculated from these recipes. A similar relationship between both variables is also observed when considering the quality of the glaze and the quantity of metallic tin over time. The best glaze quality and highest amounts of metallic tin used are reported in recipes from around 1680 to 1720. This period is regarded as the peak of the heyday of Dutch earthenware production. Over a broader timeframe, the quality of the tin glaze and the quantity of metallic tin roughly mirror the rise and decline of the tin-glazed earthenware industry in the Netherlands. They possibly reflect the economic conditions that affected potters’ ability to acquire expensive tin. Lower-quality glazes apparently gained acceptance in the late eighteenth century, coinciding with slightly lower metallic tin quantities. While it is possible that the tin was purer (requiring less weight), this would likely have resulted in a good glaze quality, which is not reported in the recipes from this period. In Delft, potters may have compensated for the presumed lower quality of the tin glaze (‘ordinary white’) by applying an overglaze. A lead overglaze enhances shine and simultaneously fills in pores.
Lead and overglaze
Although the inclusion of extra metallic lead in glaze preparation increases gloss, lead-to-tin ratios never exceeded 3.5 in the Dutch recipes. Glazes tend to turn yellow at ratios greater than 3.5 (Matin Citation2019). The interest in achieving increased gloss persisted through the centuries. This interest is well reflected in a Delft recipe from 1691 documented by Sijbeda, which notes the addition of overglaze powder to the white glaze powder to obtain a double gloss effect. Additionally, Delft potters sometimes applied overglazes directly on top of the tin glaze to enhance shine.
Soda and potash
Soda from kelp became the primary fluxing agent from c. 1670 onwards, instead of soda from barilla and potash from wood, ferns, or weeds. Following this shift, a significant decline is evident in the weight percentage of total carbonates (potash + soda) in the eighteenth century. This decline may be attributed to the use of higher-quality soda or potash, but it could also be a conscientious choice, aiming for a sturdier glaze, which proved beneficial in the decorating process.
Paape
Paape’s recipe, dating from the late eighteenth century, is remarkable not only for the addition of smalt and calcined red copper but also for its distinct combination characterised by a minimal amount of flux, a substantial quantity of sand, and a surprising inclusion of a significant amount of salt in masticot. This formulation yields an impractically rigid, highly viscous, and matte glaze (van Lookeren Campagne, personal communication, September 2023). The reason for this contradictory combination of abundant sand and low flux – the mixture will not easily melt – is currently under investigation.
Considerations
Delftware recipes did change over time
The prevailing belief is that Delft workshops underwent minimal technical changes, as knowledge was passed down orally through generations and between workshops (van Dam Citation1999; van Aken-Fehmers and Schledorn Citation2001; van Dam Citation2004; Tichelaar Citation2005; Lambooy Citation2013a). This is cited as the reason for the scarcity of technical documentation on the production process. Indeed, the technical equipment used does imply consistency over centuries; similar kilns were documented from inventories of early seventeenth-century workshops to late eighteenth-century workshops (van Dam Citation2004). Regarding recipes, Dutch potters generally followed the approach used by Piccolpasso for sixteenth-century majolica, involving a distinct preparation of masticot and tin-lead calx, alongside a pre-fired ceramic body (Matin Citation2019). But just as most comparisons are relative and must be placed in a specific context, the current research suggests that raw materials and quantities did change within Delftware (and other Dutch earthenware) in the context of time.
Delftware recipes were shared with other potters within The Netherlands
The recipes from other tin-glazed earthenware in the Netherlands, dated from c. 1600 to c. 1800, exhibit changes over time. The good matching of the Delftware tin glaze and the other Dutch earthenware in the context of time implies that there were parallel changes for both with regard to the raw materials used. It suggests that communication among potters in the Netherlands occurred, and that decisions were probably influenced by the availability of raw materials and on experiments with alternatives. Material availability was likely driven by political circumstances in the seventeenth and eighteenth centuries characterised by (sea) wars.
Why only three Delftware recipes?
One of the explanations for the scarcity of written documents from Delft is the belief that Delft potters maintained confidentiality. Can this be true, or is it necessary to regard this in a historical timeframe? Van Dam (Citation2004) noted that Delft factory owners organised themselves in a guild to coordinate matters like ensuring fair wages for their employees, emphasising collaboration over competition. Delft potters from the various factories operated more as colleagues than competitors, while they externally functioned as one unified large-scale industry, with the aim of remaining the market leader. The need to unify arose due to the growing popularity of European porcelain in other European countries. By around 1740, the export of Delftware ceased (van Dam Citation2004) as porcelain gained dominance. While historical records indicate that Delft potters initially travelled to, for example Portugal, France, and England, and shared knowledge with outsiders, they may have stopped sharing when foreign potters started to become severe competitors. That outsiders in question were not the potters from production centres elsewhere in the Netherlands, at least until around 1700, is evident from the inclusion of two Delftware tin-glaze recipes (<1681, 1691) in the handbook of the Frisian potter Sijbeda (Citation1712-Citation1720). This indirectly suggests that these potters, primarily producing tiles and tableware, were not perceived as competitors, likely due to their distinct sales markets (van Dam Citation1999; Citation2004).
Do these factors adequately explain why only three recipes from Delftware, with just one originating in Delft itself, have been preserved, while other production centres in the Netherlands and abroad boast a greater number of historical sources? The notion of confidentially may make sense for the eighteenth century, but not for the seventeenth century. Could there be other factors responsible for the limited preservation of sources on Delftware production techniques? From this perspective, calamities such as (factory) fires, the gunpowder mill incident (1742), and the gunpowder factory explosion (1654) may better explain the lack of preserved sources. The latter event devastated much of the Delft centre where most earthenware factories were located.
Note on weight percentages
To determine the relative contribution of each raw material used to make the final glaze, weight percentages were calculated at each stage of production, starting from the initial addition of raw materials as specified in the recipes and continuing until the final glaze was achieved (, Supplemental Tables S1 and S2, including formulas). Only recipes that provided information on weights for all stages were selected to ensure comparability. However, weight changes resulting from losses, oxidation, and other chemical reactions could not be accounted for due to numerous factors, partly unknown variables. The only weight change considered was due to the uptake of oxygen by metallic lead and tin in the production of tin-lead calx (PbO + SnO2, step 2, ).
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the authors used ChatGPT3.5 (OpenAI, Microsoft Corporation) in order to improve the readability and language of the text. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
20240502_Supplemental_TableS2_DATA_1
Download MS Excel (84.2 KB)20240502_Supplemental_TableS1_RECIPES_1
Download MS Excel (28.3 KB)Acknowledgements
The authors express their gratitude to the Loudon family for enabling the early phase of this research through the Fund for Dutch Tin-glazed Earthenware. Special thanks to Annelies van Hoesel (former Rijksmuseum) and Neha Verma (former Rijksmuseum) for setting up the research. Arie Pappot (Rijksmuseum) and Haske Reiling (Vrije Universiteit Amsterdam) are appreciated for their collaboration and invaluable discussions on the subject.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allan, J. W. 1973. “Abu‘l-Qasim’s Treatise on Ceramics.” Iran 11 (1): 111–120. http://www.jstor.org/stable/4300488?origin=JSTOR-pdf.
- Bastenaire-Daudenart, F. 1828. L’art de fabriquer la faïence, recouverte d’un émail opaque blanc et coloré; suivi de quelques notions sur la peinture au grand feu et a réverbère, et d’un vocabulaire des mots techniques. Paris: De Malher et Cie.
- Caiger-Smith, A. 1973. Tin-glaze Pottery in Europe and the Islamic World: The Tradition of 1000 Years in Maiolica, Faience and Delftware. London: Faber.
- Coentro, S., J. M. Mimoso, A. M. Lima, A. S. Silva, A. N. Pais, and V. S. F. Muralha. 2012. “Multi-analytical Identification of Pigments and Pigment Mixtures Used in 17th Century Portuguese Azulejos.” Journal of the European Ceramic Society 32 (1): 37–48. https://doi.org/10.1016/j.jeurceramsoc.2011.07.021.
- Dehaine, Q., L. T. Tijsseling, H. J. Glass, T. Törmänen, and A. R. Butcher. 2021. “Geometallurgy of Cobalt Ores: A Review.” Minerals Engineering 160, 106656: 1–28. https://doi.org/10.1016/j.mineng.2020.106656.
- D’Imporzano, P. 2021. "Implications of Lead Isotope Variation in Lead White From 17th-Century Dutch Paintings." PhD Thesis. Vrije Universiteit Amsterdam.
- D’Imporzano, P., H. M. Reiling, J. van Iperen, I. Garachon, K. Keune, and G. R. Davies. 2023. “Lead Isotope Analysis of Delftware via Portable Laser Ablation.” In Book of Abstracts, Technart2023. Non-destructive and Microanalytical Techniques in Art and Cultural Heritage, edited by M. Manso, V. Antunes, and M. L. Carvalho, 344. Lisbon: Universidade Nova de Lisboa – Faculdade de Ciências e Tecnologia.
- Dony, F. 1978. Handboek antiek. 5th ed. Baarn: Tirion.
- Dungworth, D. 2009. Innovations in the 17th-century glass industry: the introduction of kelp (seaweed) ash in Britain. Deuxième Colloque International de l’Association Verre et Histoire, pp. 119-123.
- Dungworth, D. 2012. “Three and a Half Centuries of Bottle Manufacture.” Industrial Archaeology Review 34 (1): 37–50. https://doi.org/10.1179/0309072812Z.0000000002.
- Eliëns, T.M. 2003. Delfts aardewerk: geschiedenis van een nationaal Product. Volume III: De Porceleyne Fles. Zwolle: Waanders.
- Harreld, D. J. 2004. “The Dutch Economy in the Golden Age (16th - 17th Centuries).” In EH.Net Encyclopedia, edited by R. Whaples. EHA. Accessed November 15, 2023. https://eh.net/encyclopedia/the-dutch-economy-in-the-golden-age-16th-17th-centuries/.
- Henderson, J. 2013. Ancient Glass: An Interdisciplinary Exploration. Cambridge: Cambridge University Press.
- Krause, P., S. Klein, C. Domergue, C. Berthold, and N. Jöns. 2023. “Litharge from El Centenillo and Fuente Espi: A Geochemical and Mineralogical Innovation of Spanish Silver Processing in the Sierra Morena.” Metallography, Microstructure, and Analysis 12 (2): 262–275. https://doi.org/10.1007/s13632-023-00940-8.
- Kunckel, J. 1679. Ars vitraria experimentalis: oder Vollkommene Glasmacher-Kunst. Leipzig: Christoph Riegels.
- Lambooy, S. M. R. 2013a. “The Spread of White Faience Within Europe from the Sixteenth Century to the Present day.” In Dutch Delftware. History of a National Product. Volume V: White Delft. Not Just Blue, edited by T. M. Eliëns, 13–44. Den Haag/Zwolle: Gemeente Museum Den Haag / Waanders.
- Lambooy, S. M. R. 2013b. “A Glaze That is ‘White, Pure and Glistening’. The Preparation and Composition of tin Glazes in the Northern Netherlands.” In Dutch Delftware. History of a National Product. Volume V: White Delft. Not Just Blue, edited by T. M. Eliëns, 125–147. Den Haag/Zwolle: Gemeente Museum Den Haag/Waanders.
- Mason, R. B., and M. S. Tite. 1997. “The Beginnings of Tin-Opacification of Pottery Glazes.” Archaeometry 39 (1): 41–58. https://doi.org/10.1111/j.1475-4754.1997.tb00789.x.
- Matin, M. 2019. “Tin-based Opacifiers in Archaeological Glass and Ceramic Glazes: A Review and new Perspectives.” Archaeological and Anthropological Sciences 11 (4): 1155–1167. https://doi.org/10.1007/s12520-018-0735-2.
- Matin, M., M. Tite, and O. Watson. 2018. “On the Origins of tin-Opacified Ceramic Glazes: New Evidence from Early Islamic Egypt, the Levant, Mesopotamia, Iran, and Central Asia.” Journal of Archaeological Science 97: 42–66. https://doi.org/10.1016/j.jas.2018.06.011.
- Mimoso, J. M. 2015. “Origin, Early History and Technology of the Blue Pigment in Azulejos.” In Proceedings of the GlazeArch2015 International Conference, edited by J. Delgado Rodrigues, and J. M. Mimoso, 357–375. Lisbon: LNEC.
- Molera, J., T. Pradell, N. Salvadó, and M. Vendrell-Saz. 1999. “Evidence of tin Oxide Recrystallization in Opacified Lead Glazes.” Journal of the American Ceramic Society 82 (10): 2871–2875. https://doi.org/10.1111/j.1151-2916.1999.tb02170.x.
- Molera, J., T. Pradell, N. Salvadó, and M. Vendrell-Saz. 2001. “Interaction Between Clay Bodies and Lead Glazes.” Journal of the American Ceramic Society 84 (5): 1120–1128. https://doi.org/10.1111/j.1151-2916.2001.tb00799.x.
- Oskamp, S. 2013. “White Delft as Part of the Range of Faience Produced in the Dutch Republic of the Seventeenth and Eighteenth Century.” In Dutch Delftware. History of a National Product. Volume V: White Delft. Not Just Blue, edited by T. M. Eliëns, 77–123. Den Haag/Zwolle: Gemeente Museum Den Haag / Waanders.
- Paape, G. 1794. De plateelbakker of Delftsch aardewerkmaaker, volledige beschrijving van alle konsten, ambachten, handwerken, fabrieken, trafieken, derzelver werkhuizen, gereedschappen, enz. Part 12. Dordrecht: A. Blusse en Zoon.
- Peng, I., K. Hills-Kimball, I. M. Lovelace, J. Wang, M. Rios, O. Chen, and L.-Q. Wang. 2022. “Exploring the Colors of Copper-Containing Pigments, Copper(II) Oxide and Malachite, and Their Origins in Ceramic Glazes.” Colorants 1 (4): 376–387. https://doi.org/10.3390/colorants1040023.
- Pradell, T., and J. Molera. 2020. “Ceramic Technology. How to Characterise Ceramic Glazes.” Archaeological and Anthropological Sciences 12 (8): 189. https://doi.org/10.1007/s12520-020-01136-9.
- Rackman, B. 1955. “Italian Majolica: Notes on its Materials from Piccolpasso.” Studies in Conservation 2 (1): 41–44. https://doi.org/10.1179/sic.1955.004.
- Sijbeda, P. 1712-1720. Een boekien, waar in geschreven sijn, alle soorten van verwen, welke tot een gleijbakkerij nodig sijn, ‘t gebruijken, en de maniere hoe men deselve moet praepareren. Inv. no. 872 of Museum Hannemahuis Museum Harlingen.
- Tichelaar, P. J. 2005. “Het werk van geleibakkers aan de hand van drie documenten.” In Fries Aardewerk. Harlingen, Bedrijfsgeschiedenis 1610-1933 & Producten tot 1720, edited by A. J. Gierveld, and J. Pluis, 71–82. Leiden: Primavera Pers.
- Tite, M. S. 2009. “The Production Technology of Italian Maiolica: A Reassessment.” Journal of Archaeological Science 36 (10): 2065–2080. https://doi.org/10.1016/j.jas.2009.07.006.
- Tite, M., T. Pradell, and A. Shortland. 2008. “Discovery, Production and use of tin-Based Opacifiers in Glasses, Enamels and Glazes from the Late Iron Age Onwards: A Reassessment.” Archaeometry 50 (1): 67–84. https://doi.org/10.1111/j.1475-4754.2007.00339.x.
- Tite, M. S., A. Shortland, Y. Maniatis, D. Kavoussanaki, and S. A. Harris. 2006. “The Composition of the Soda-Rich and Mixed Alkali Plant Ashes Used in the Production of Glass.” Journal of Archaeological Science 33 (9): 1284–1292. https://doi.org/10.1016/j.jas.2006.01.004.
- van Aken-Fehmers, M. S. 2007. Dutch Delftware. History of a National Product. Volume IV: Vases with Spouts. Three Centuries of Splendour, edited by T. M. Eliëns. Zwolle: Waanders.
- van Aken-Fehmers, M. S., F. Burghout, N. L. Jaspers, S. M. R. Lambooy, L. Megens, S. Ostkamp, and G. Verhaar. 2013. Dutch Delftware. History of a National Product. Volume V: White Delft. Not Just Blue. T.M. Eliëns, ed. Den Haag / Zwolle: Gemeente Museum Den Haag / Waanders.
- van Aken-Fehmers, M. S., and L. A. Schledorn. 2001. Delfts aardewerk: geschiedenis van een nationaal product. Volume II. T.M. Eliëns, ed. Zwolle / Den Haag: Waanders / Gemeente Museum Den Haag.
- van Aken-Fehmers, M. S., L. A. Schledorn, A.-G. Hesselink, and T. M. Eliëns. 1999. Delfts aardewerk: geschiedenis van een nationaal product. Volume I. Zwolle / Den Haag: Waanders / Gemeente Museum Den Haag.
- van Dam, J. D. 1999. “Delfts’ uit de provincie. Aardewerk uit Hollandse tegelfabrieken. Mededelingenblad Nederlandse Vereniging van vrienden van Ceramiek en Glas.” Vormen uit vuur 168/169 (3-4): 3–107.
- van Dam, J. D. 2004. "Delfts Aardewerk: Een proeve tot her-ijking." PhD Thesis. Radboud University Nijmegen. Available at http://hdl.handle.net/2066/61999.
- van der Meulen, A., and P. Smeele. 2012. De pottenbakkers van Gouda 1570-1940: en hun betekenis voor de geschiedenis van de Nederlandse keramiek. Leiden: Primavera Pers.
- van Erkel, J. 2020. Royal Delft, Cahier Series Part 7. 4th ed. Amersfoort: Bekking & Blitz.
- van Geenen, L.-P. 2013. The Timeless Beauty of White Delft. Hoorn: Poldervondsten.
- van Geenen, L.-P. 2017. Dutch Delftware. Marks and Factories. Hoorn: Poldervondsten.
- van Lookeren Campagne, K. E., L. Megens, and M. van Bommel. 2018. “Understanding 17-18th Century Dutch tin-Glaze Through the Interpretation and Reconstruction of Historical Recipes.” In Proceedings of GlazeArt2018 International Conference, edited by S. Pereira, M. Menezes, and J. Delgado Rodrigues, 150–164. Lisbon: LNEC.
- van Lookeren Campagne-Nuttall, K. E. 2022. "Understanding Dutch Tin-Glazed Tiles (1600-1750) Through the Interpretation of Texts, Analysis and Recipe Reconstruction." PhD Thesis. University of Amsterdam. Available at https://dare.uva.nl/search?identifier = ee16b189-fbfd-4348-955d-b380a1368a1b.
- Walton, M. 2004. "A Materials Chemistry Investigation of Archaeological Lead Glazes." PhD Thesis. University of Oxford.
- Weege, D. 1773. Kweekschool der artzenykunde: zynde verzamelingen en waarneemingen uyt de beste en nieuwste schryvers getrokken. Amsterdam: Ex Legato Verrijst.
- Wisniak, J. 2003. “Sodium Carbonate: From Natural Resources to Leblanc and Back.” Indian Journal of Chemical Technology 10: 99–112.
- Zhou, S., Y. Wei, B. Li, H. Wang, B. Ma, and C. Wang. 2016. “Mechanism of Sodium Chloride in Promoting Reduction of High-Magnesium low-Nickel Oxide ore.” Scientific Reports 6, 29061: 1–12. https://doi.org/10.1038/s41598-016-0001-8.