Abstract
Silica-supported sulfuric acid (H2SO4·SiO2) has been utilized as a heterogeneous recyclable catalyst for a highly efficient regio- and chemoselective condensation of hydrazines/hydrazides, diamines, and primary amines with various β-dicarbonyl compounds at room temperature to afford pyrazoles, diazepines, and β-enaminones/β-enamino esters under solvent-free conditions within 5–15 min.
INTRODUCTION
Pyrazoles and diazepines are valuable bio-active heterocycles, which are shown to possess important biological and pharmaceutical activities[ Citation 1 ] such as antimicrobial, antiviral, antitumor, anti-inflammatory, antifungal, antidepressant, and anticonvulsant activities. Meanwhile, β-enaminones/β-enamino esters are useful synthones for the synthesis of various pharmaceuticals[ Citation 2 ] and bioactive heterocycles.[ Citation 3 ]
Because of these prominent roles, the synthesis of these three kinds of compounds (pyrazoles, diazepines, and β-enaminones/β-enamino esters) has attracted considerable attention recently. By now, various synthetic approaches have been reported. For preparing pyrazoles, among the reported methods,[ Citation 4 ] the most preferred are the reactions of 1,3-dicarbonyl compounds with hydrazines in the presence of acid, such as polystyrene-supported sulfonic acid (PSSA),[4a] sulfuric acid,[4b] and so on. In regard to synthesis of diazepines, several routes have been reported, such as microwave heating of diazepine-diones in boiling POCl3,[5a] hydrogen transfer N-heterocyclization using catalyst [Cp∗IrCl2]2/K2CO3,[5b] condensation of 1,3-diketones with diamines using catalyst PSSA,[4a] and others.[5c–5e] Moreover, preparation of β-enaminones/β-enamino esters has been reported to proceed by condensation of β-dicarbonyl compounds with amines utilizing SiO2/microwaves,[6a] montmorillonite K-10,[6b] NaAuClO4,[6c] Bi(TFA)3,[6d] Zn(ClO4)2·6H2O,[6e] CeCl3·7H2O,[6f] SiO2/HClO4,[6g] and so on. However, most of the methods to prepare these three kinds of compounds suffer from certain drawbacks including long reaction times, unsatisfactory yields, higher temperatures, employment of organic solvents, and use of expensive nonreusable catalyst. Thus, there is still a need to develop greener and more efficient pathways for such synthesis.
Recently, carrying out organic reactions under solvent-free conditions has become highly desirable.[ Citation 7 ] Solventless organic reactions usually are rapid, are regio- or chemoselective, occur in high yields, and have environmental and economic advantages.[7a] These advantages become even more attractive if such reactions can be performed using reusable catalysts. Solid acid catalysts prepared by employing acid supported on oxides have been known and used for a long time; for instance, H2SO4·SiO2,[ Citation 8 ] HClO4·SiO2,[6g] and NaHSO4·SiO2 [ Citation 9 ] have been reported to efficiently catalyze some reactions. As we know, H2SO4·SiO2 can easily be prepared from readily available silica gel and H2SO4, which is much cheaper and more stable than HClO4, and H2SO4·SiO2 was also found to be a good protic acid source under milder and safer conditions than HClO4·SiO2 in some reaction systems.[ Citation 8 ] For these reasons, we studied synthesis of pyrazoles, diazepines, β-enaminones, and β-enamino esters by the solventless condensation of β-dicarbonyl compounds with hydrazines/hydrazides, diamines, and primary amines at room temperature using silica-supported sulfuric acid (H2SO4·SiO2) as a heterogeneous recyclable catalyst.
Preparation of H2SO4·SiO2: To a slurry of silica gel (10 g, 200–400 mesh) in dry diethyl ether (50 mL) was added commercially available concd. H2SO4 (3 mL) with shaking for 5 min. The solvent was evaporated under reduced pressure resulting in free-flowing H2SO4·SiO2, which was then dried at 110 °C for 3 h.
RESULTS AND DISCUSSION
Initially, condensation of hydrazines/hydrazides (1.1 mmol) and 1,3-dicarbonyl compounds (1 mmol) with H2SO4·SiO2 (20 mg) under solvent-free conditions was examined. Hydrazines/hydrazides reacted efficiently with various β-diketones to afford the desired pyrazoles in good to excellent yields (Table ). All of the reactions between hydrazines/hydrazides and symmetrical β-diketones gave single components 1a–10a (entries 1–10 in Table ). When an unsymmetrical β-diketone, 1-phenylbutane-1,3-dione, was employed to react with PhNHNH2, two regioisomers, 14a and 14b, were obtained in the ratio of 19:1 (entry 14 in Table ). Moreover, the β-ketoesters can also be used as a substitute for diketones in this synthesis (entries 11–13 in Table ). In such case, only single components were afforded, in low yields at room temperature and in high yields at 50 °C.
Table 1. Room temperature solventless synthesis of pyrazoles using H2SO4·SiO2
Footnote
a
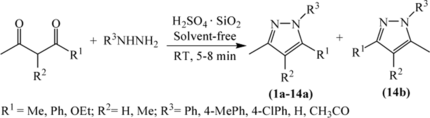
Next, reactions of diamines with β-dicarbonyl compounds were investigated (Table ). 1,2-Diaminobenzene reacted efficiently with pentane-2,4-diketone and 3-methylpentane-2,4-diketone to yield diazepines in a single step (entries 1 and 2 in Table ). The reaction proceeded at room temperature, delivering excellent yields. The reaction of 1,2-diaminobenzene with β-ketoesters, however, failed to give the desired products (entries 3 and 4 in Table ).
Table 2. Room-temperature solventless synthesis of diazepines using H2SO4·SiO2
Footnote
a
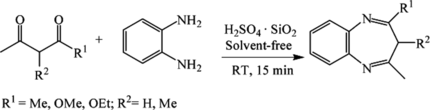
Finally, this protocol was also extended to the synthesis of various β-enaminones and β-enamino esters by the condensation of primary amines with various β-diketones and β-ketoesters at room temperature under solvent-free conditions, and the results are summarized in Table . All of the reactions between primary amines and symmetrical β-diketones and β-ketoesters gave single components in good to high yields (entries 1–13 in Table ). In the case of the reaction of unsymmetrical diketone 1-phenylbutane-1,3-dione with PhNH2, two regioisomers (14a and 14b) were obtained in the ratio of 27:1 (entry 14 in Table ).
Table 3. Room-temperature solventless synthesis of β-enaminone/β-enamino ester using H2SO4·SiO2
Footnote
a
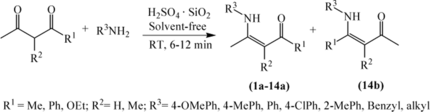
The method was found to be highly chemo- and regioselective. Groups –NH2 in RNH2 and RNHNH2 attack only at the ketone carbonyl for β-ketoesters (entries 11–13 in Table , entries 12 and 13 in Table ) and in priority at the certain ketone carbonyl connecting with a relatively weaker electron-donating group when using unsymmetrical diketone (entry 14 in Table , entry 14 in Table ). Moreover, the (Z)-selectivity in the products β-enaminones/β-enamino esters (entries 1–14 in Table ) derived from β-dicarbonyl compounds with primary amines was secured by intramolecular hydrogen bonding. In the 1H NMR spectra the proton of the –NH– group appeared in the region of δ 8.5–13.5.
The possibility of recycling the catalyst was examined. For this reason, the reaction of pentane-2,4-diketone and aniline at room temperature in the presence of H2SO4·SiO2 was studied (Table ). After completion of the reaction, the catalyst was recovered by simple filtration and reused in subsequent reactions with consistent activity.
Table 4. Recycle experiment of H2SO4·SiO2
Footnote
a

CONCLUSION
In conclusion, we have developed a green and efficient approach for room-temperature solventless synthesis of pyrazoles, diazepines, β-enaminones, and β-enamino esters by condensation of hydrazines/hydrazides, diamines, and primary amines with various β-dicarbonyl compounds in the presence of H2SO4·SiO2 as a heterogeneous catalyst, which may provide a useful route for drug discovery. The solvent-free conditions, simple experimental procedure, mildness of the conversion, clear reaction profiles, high yields and chemo- and regioselectivities, short reaction times, and low cost, stability, and reusability of the catalyst are the noteworthy advantages of the protocol.
EXPERIMENTAL
General Considerations
Silica-supported sulfuric acid was prepared by a reported method.[ Citation 8 ] All the reagents were obtained from commercial sources. 1H NMR spectra were recorded on a Bruker Avance DMX 500-MHz spectrometer in CDCl3solution. Low-resolution MS analyses were measured on a Bruke Esquire 3000 spectrometer using the ESI (electrospray ionization) technique.
General Procedure for the Synthesis of Pyrazoles, Diazepines, and β-Enaminones/β-Enamino Esters
To a mixture of a dicarbonyl compound (1 mmol) and a hydrazine/hydrazide (a diamine, a primary amine) (1.2 mmol), H2SO4·SiO2 (20 mg) was added. The mixture was stirred for 5–8 min (15 min, 6–12 min) at room temperature. After completion of the reaction, the reaction mixture was diluted with ethyl acetate (2 ml) and filtered. The catalyst was recovered from the residue. The filtrate was concentrated. The residue, on purification by column chromatography (silica gel, petroleum ether-ethyl acetate) afforded pure pyrazole (diazepine, β-enaminone/β-enamino ester).
Condensation of β-enamino esters (1 mmol) with hydrazine (1.2 mmol) was conducted at both room temperature and 50 °C.
When ethanediamine (1.2 mmol) was used as an amine, a dicarbonyl compound (2 mmol) was required.
Selected Data
5-Ethoxy-3-methyl-1-phenyl-1H-pyrazole (Table , Entry 12)
1H NMR (CDCl3): d 1.42–1.45 (t, J = 7.0 Hz, 3H, CH3), 2.28 (s, 3H, CH3), 4.11–4.16 (q, J = 7.1 Hz, 2H, CH2), 5.48 (s, 1H, CH), 7.21–7.72 (m, 5H, Ph). 13C NMR (CDCl3): d 14.77, 14.86, 68.01, 86.49, 121.97, 125.94, 128.96, 139.03, 148.94, 155.07. MS (ESI) m/z 203 ([M + H]+).
3-Methyl-1,5-diphenyl-1H-pyrazole (Table , Entry 14a)
1H NMR (CDCl3): d 2.39 (s, 3H, CH3), 6.55 (s, 1H, CH), 7.32–7.91 (m, 10H, Ph). 13C NMR (CDCl3): d 13.76, 107.97, 125.41, 127.40, 128.34, 128.63, 128.87, 129.09, 130.89, 140.27, 143.37, 149.70. MS (ESI) m/z 235 ([M + H]+).
5-Methyl-1,3-diphenyl-1H-pyrazole (Table , Entry 14b)
1H NMR (CDCl3): d 2.40 (s, 3H, CH3), 6.33 (s, 1H, CH), 7.22–7.32 (m, 10H, Ph). 13C NMR (CDCl3): d 12.75, 104.57, 125.16, 125.90, 127.79, 127.94, 128.75, 129.27, 133.52, 140.10, 140.36, 151.67. MS (ESI) m/z 235 ([M + H]+).
(1Z,4Z)-2,4-Dimethyl-3H-benzo[b][1,4]diazepine (Table , Entry 1)
1H NMR (CDCl3): d 2.34 (s, 6H, CH3), 2.81 (s, 2H, CH2), 7.20–7.22 (m, 2H, Ph), 7.35–7.38 (m, 2H, Ph). 13C NMR (CDCl3): d 27.87, 43.38, 125.09, 127.68, 140.43, 157.99. MS (ESI) m/z 173 ([M + H]+).
(Z)-Ethyl-3-(benzylamino)but-2-enoate (Table , Entry 13)
1H NMR (CDCl3): d 1.25–1.28 (t, J = 7.0 Hz, 3H, CH3), 1.92 (s, 3H, CH3), 4.09–4.13 (q, J = 7.1 Hz, 2H, CH2), 4.43–4.44 (d, J = 6.4 Hz, 2H, CH2), 4.55 (s, 1H, CH), 7.26–7.36 (m, 5H, Ph), 8.96 (s, 1H, NH). 13C NMR (CDCl3): d 14.83, 19.54, 47.00, 58.57, 83.45, 126.92, 127.54, 128.98, 138.98, 161.97, 170.79. MS (ESI) m/z 220 ([M + H]+).
(Z)-1-Phenyl-3-(phenylamino)but-2-en-1-one (Table , Entry 14a)
1H NMR (CDCl3): d 2.16 (s, 3H, CH3), 5.92 (s, 1H, CH), 7.19–7.95 (m, 10H, Ph), 13.13 (s, 1H, NH). 13C NMR (CDCl3): d 20.66, 94.48, 124.98, 125.99, 127.28, 128.50, 129.38, 131.12, 138.85, 140.23, 162.44, 188.88. MS (ESI) m/z 238 ([M + H]+).
(Z)-4-Phenyl-4-(phenylamino)but-3-en-2-one (Table , Entry 14b)
1H NMR (CDCl3): d 2.24 (s, 3H, CH3), 5.44 (s, 1H, CH), 6.77–7.35 (m, 10H, Ph), 9.71 (s, 1H, NH). 13C NMR (CDCl3): d 29.62, 100.33, 122.98, 123.79, 127.64, 128.20, 128.59, 129.18, 135.40, 139.60, 159.50, 197.36. MS (ESI) m/z 238 ([M + H]+).
Notes
a Reactions performed with 1.0 mmol of β-dicarbonyl compound, 1.1 mmol of hydrazine/hydrazide, and 20 mg of H2SO4·SiO2 mixed for 5–8 min under solvent-free condition, at room temperature.
b Isolated yields after column chromatography.
c Yields of reactions performed in 50°C.
a Reactions performed with 1.0 mmol of β-dicarbonyl compound, 1.1 mmol of diamine, and 20 mg of H2SO4·SiO2 mixed for 15 min under solvent-free conditions at room temperature.
b Isolated yields after column chromatography.
a Reactions performed with 1.0 mmol of β-dicarbonyl compound, 1.1 mmol of primary amines, and 20 mg of H2SO4·SiO2 mixed for 6–12 min under solvent-free conditions at room temperature.
b Isolated yields after column chromatography.
c Reactions performed with 2.0 mmol of β-dicarbonyl compound, 1.1 mmol of primary amines, and 20 mg of H2SO4·SiO2 mixed for 6–12 min under solvent-free conditions at room temperature.
a Reactions performed with 1.0 mmol of pentane-2,4-diketone, 1.1 mmol of aniline, and 20 mg of H2SO4·SiO2 mixed for 7 min under solvent-free condition at room temperature.
b Isolated yields after column chromatography.
REFERENCES
- (a) Hatheway , G. J. ; Hansch , C. ; Kim , K. H. ; Milstein , S. R. ; Schimidt , C. L. ; Smith , R. N. ; Quin , F. R. Antitumor 1-(X-aryl)-3,3-dialkyltriazenes, 1: Quantitative structure–activity relationships vs L1210 leukemia in mice . J. Med. Chem. 1978 , 21 , 563 – 574 ; (b) Katayama , H. ; Oshiyama , T. Preparation and bioactivity of pyrazole derivativies as potential cross-linking agent . Can. J. Chem. 1997 , 75 , 913 – 919 ; (c) Badawey , E. A. M. ; El-Ashmawey , I. M. Nonsteroidal antiinflammatory agents, part 1: Antiinflammatory, analgesic, and antipyretic activity of some new 1-(pyrimidin-2-yl)-3-pyrazolin-5-ones and 2-(pyrimidin-2-yl)-1,2,4,5,6,7-hexahydro-3H-indazol-3-ones . Eur. J. Med. Chem. 1998 , 33 , 349 – 361 ; (d) Bailey , D. M. ; Hansen , P. E. ; Hlavac , A. G. ; Baizman , E. R. ; Pearl , J. ; Defelice , A. F. ; Feigenson , M. E. 3,4-Diphenyl-1H-pyrazole-1-propanamine antidepressants . J. Med. Chem. 1985 , 28 , 256 – 260 .
- (a) Edafiogho , I. O. ; Moore , J. A. ; Alexander , M. S. ; Scott , K. R. Nuclear magnetic resonance studies of anticonvulsant enaminones. J. Pharm. Sci. 1994, 83, 1155–1170; (b) Foster , J. E. ; Nicholson , J. M. ; Butcher , R. ; Stables , J. P. ; Edafiogho , I. O. ; Goodwin , A. M. ; Henson , M. C. ; Smith , C. A. ; Scott , K. R. Synthesis, characterization, and anticonvulsant activity of enaminones, part 6: Synthesis of substituted vinylic benzamides as potential anticonvulsants. Bioorg. Med. Chem. 1999, 7, 2415–2425; (c) Wang , Y. F. ; Izawa , T. ; Kobayashi , S. ; Ohno , M. Stereocontrolled synthesis of (+)-negamycin from an acyclic homoallylamine by 1,3-asymmetric induction. J. Am. Chme. Soc. 1982, 104, 6465–6466; (d) Michael , J. P. ; De Koning , C. B. ; Hosken , G. D. ; Stanbury , T. V. Reformatsky reactions with N-arylpyrrolidine-2-thiones: Synthesis of tricyclic analogues of quinolone antibacterial agents. Tetahedron 2001, 57, 9635–9648; (e) Boger , D. L. ; Ishizaki , T. ; Wysocki , R. J. ; Munk , J. S. A. ; Kitos , P. A. ; Suntornwat , O. Total synthesis and evaluation of (±)-N-(tert-butoxycarbonyl)-CBI, (±)-CBI-CDPI1, and (±)-CBI-CDPI2: CC-1065 functional agents incorporating the equivalent 1,2,9,9a-tetrahydrocyclopropa[1,2-c]benz[1,2-e]indol-4-one (CBI) left-hand subunit. J. Am. Chem. Soc. 1989, 111, 6461–6463.
- Spivey , A. C. ; Srikaran , R. ; Diaper , C. M. ; Turner , D. J. Traceless solid phase synthesis of 2-substituted pyrimidines using an “off-the-shelf” chlorogermane-functionalised resin . Org. Biomol. Chem. 2003 , 1 , 1638 – 1640 .
- (a) Polshettiwar , V. ; Varma , R. S. Greener and rapid access to bio-active heterocycles: Room temperature synthesis of pyrazoles and diazepines in aqueous medium . Tetrahedron Lett. 2008 , 49 , 397 – 400 ; (b) Wang , Z. ; Qin , H. Solventless synthesis of pyrazole derivatives . Green Chem. 2004 , 6 , 90 – 92 ; (c) Mohamed Ahmed , M. S. ; Kobayashi , K. ; Mori , A. One-pot construction of pyrazoles and isoxazoles with palladium-catalyzed four-component coupling . Org. Lett. 2005 , 7 , 4487 – 4489 ; (d) Heller , S. T. ; Natarajan , S. R. 1,3-Diketones from acid chlorides and ketones: A rapid and general one-pot synthesis of pyrazoles . Org. Lett. 2006 , 8 , 2675 – 2678 ; (e) Deng , X. ; Mani , N. S. Reaction of N-monosubstituted hydrazones with nitroolefins: A novel regioselective pyrazole synthesis . Org. Lett. 2006 , 8 , 3505 – 3508 ; (f) Armstrong , A. ; Jones , L. H. ; Knight , J. D. ; Kelsey , R. D. Oxaziridine-mediated amination of primary amines: Scope and application to a one-pot pyrazole synthesis . Org. Lett. 2005 , 7 , 713 – 716 ; (g) Greenberg , S. ; Marcuccio , S. M. ; Leznoff , C. C. ; Tomer , K. B. Selective synthesis of binuclear and trinuclear phthalocyanines covalently linked by a one atom oxygen bridge . Synthesis 1986 , 409 ; (h) Siva , A. ; Murugan , E. A new trimeric cinchona alkaloid as a chiral phase-transfer catalyst for the synthesis of asymmetric α-amino acids . Synlett 2005 , 2927 .
- (a) Rault , S. ; Gillard , A. C. ; Foloppe , M. P. ; Robba , M. A new rearrangement under microwave heating conditions: Synthesis of cyclopenta[b][1,4]benzodiazepines and tetrahydrodibenzo[b,f][1,4]diazepines . Tetrahedron Lett. 1995 , 36 , 6673 – 6674 ; (b) Eary , C. T. ; Clausen , D. Preparation of substituted 1,2,3,4-tetrahydroquinoxalines and 2,3,4,5-tetrahydro-1H-benzo[b][1,4]diazepines from catalytic Cp∗Ir hydrogen transfer N-heterocyclization of anilino alcohols . Tetrahedron Lett. 2006 , 47 , 6899 – 6902 ; (c) Wang , Q. F. ; Hu , B. ; Luo , B. H. ; Hu , C. M. Synthesis of 5-per(poly)fluoroalkyl-2,3-dihydro-1,4-diazepines . Tetrahedron Lett. 39 , 2377 – 2380 ; (d) Bonacorso , H. G. ; Lourega , R. V. ; Deon , E. D. ; Zanatta , N. ; Martins , M. A. P. The first synthesis of dihydro-3H-pyrido[2,3-b][1,4]diazepinols and a new alternative approach for diazepinone analogues . Tetrahedron Lett. 2007 , 48 , 4835 – 4838 ; (e) Cairns , J. ; Clarkson , T. R. ; Hamersma , J. A. M. ; Rae , D. R. 11-(Tetrahydro-3 and 4-pyridinyl)dibenzo[b,e][1,4]diazepines undergo novel rearrangements on treatment with concentrated HBr . Tetrahedron Lett. 2002 , 43 , 1583 – 1585 .
- (a) Rechsteiner , B. ; Texier-Boullet , F. ; Hamalin , J. Synthesis in dry media coupled with microwave irradiation: Application to the preparation of enaminoketones. Synthesis in dry media coupled with microwave irradiation: Application to the preparation of enaminoketones . Tetrahedron Lett. 1993 , 34 , 5071 – 5074 ; (b) Braibante , M. E. F. ; Braibante , S. ; Missio , L. ; Andricopulo , A. Synthesis and reactivity of β-amino α,β-unsaturated ketones and esters using K-10 montmorillonite . Synthesis 1994 , 898 ; (c) Arcadi , A. ; Bianchi , G. ; Di Giuseppe , S. ; Marinelli , F. Gold catalysis in the reactions of 1,3-dicarbonyls with nucleophiles . Green Chem. 2003 , 5 , 64 – 67 ; (d) Khosropour , A. R. ; Khodaei , M. M. ; Kookhazadeh , M. A mild, efficient and environmentally friendly method for the regio- and chemoselective synthesis of enaminones using Bi(TFA)3 as a reusable catalyst in aqueous media . Tetrahedron Lett. 2004 , 45 , 1725 – 1728 ; (e) Bartoli , G. ; Bosco , M. ; Locatelli , M. ; Marcantoni , E. ; Melchiorre , P. ; Sambri , L. Zn(ClO4)2·6H2O as a powerful catalyst for the conversion of β-Ketoesters into β-enamino esters . Synlett 2004 , 239 ; (f) Khodaei , M. M. ; Khosropour , A. R. ; Kookhazadeh , M. Enamination of β-dicarbonyl compounds catalyzed by CeCl3·7H2O at ambient conditions: Ionic liquid and solvent-free media . Synlett 2004 , 1980 ; (g) Das , B. ; Venkateswarlu , K. ; Majhi , A. ; Reddy , M. R. ; Reddy , K. N. ; Rao , Y. K. ; Ravikumar , K. ; Sridhar , B. Highly efficient, mild and chemo- and stereoselective synthesis of enaminones and enamino esters using silica supported perchloric acid under solvent-free conditions . J. Mol. Catal. A: Chem. 2006 , 246 , 276 – 281 .
- (a) Cave , G. W. V. ; Raston , C. L. ; Scott , J. L. Recent advances in solventless organic reactions: Towards benign synthesis with remarkable versatility . Tetrahedron Lett. 2001 , 1 , 2159 – 2169 ; (b) Raston , C. L. ; Scott , J. L. Chemoselective, solvent-free aldol condensation reaction . Green Chem. 2000 , 2 , 49 – 52 ; (c) Yoshizawa , K. ; Toyota , S. ; Toda , F. Solvent-free Claisen and Cannizzaro reactions . Tetrahedron Lett. 2001 , 42 , 7983 – 7985 ; (d) Tanaka , K. ; Sugino , T. ; Toda , F. Selective Stobbe condensation under solvent-free conditions . Green Chem. 2000 , 2 , 303 – 304 ; (e) Yoshizawa , K. ; Toyota , S. ; Toda , F. Efficient solvent-free Thorpe reactions . Green Chem. 2002 , 4 , 68 – 70 .
- (a) Rajput , V. K. ; Roy , B. ; Mukhopadhyay , B. Sulfuric acid immobilized on silica: An efficient reusable catalyst for selective hydrolysis of the terminal O-isopropylidene group of sugar derivatives . Tetrahedron Lett. 2006 , 47 , 6987 – 6991 ; (b) Roy , B. ; Mukhopadhyay , B. Sulfuric acid immobilized on silica: An excellent catalyst for Fischer type glycosylation . Tetrahedron Lett. 2007 , 48 , 3783 – 3787 .
- Das , B. ; Veeranjaneyulu , B. ; Krishnaiah , M. ; Balasubramanyam , P. Synthesis of gem-dihydroperoxides from ketones using silica-supported sodium hydrogen sulfate as a heterogeneous catalyst. J. Mol. Catal. A: Chem. 2008, 284, 116–119.