Abstract
A simple, efficient, and environmentally benign method has been developed for the exclusive formation of biologically significant 2-aryl-1-arylmethyl-1H-benzimidazoles under the heterogeneous catalysis of Amberlite IR-120 in aqueous media in excellent yields. The catalyst is recyclable without loss of activity.
1,2-Disubstituted benzimidazoles are endowed with an extensive range of biological activities. They have emerged as potent nonnucleoside inhibitors of HIV-1 reverse transcriptase[ Citation 1a , Citation 1b ] and specific inhibitors of the NS5B polymerase of the hepatitis C virus (HCV).Citation [1c] Appropriately functionalized 1,2-disubstituted benzimidazoles are used as agonists against γ-butyric acid A receptor (GABAA).Citation [1d] Moreover, they display potent thrombin inhibitory activityCitation [2a] and antibacterial activity against gram-positive bacteria.Citation [2b]
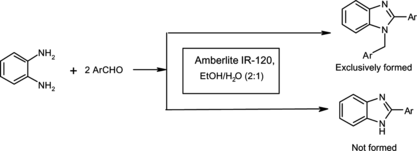
A number of improved methods have been developed for the synthesis of 1,2-disubstituted benzimidazoles, which include N-alkylation of 2-substituted benzimidazole in the presence of a strong base,[ Citation 3a , Citation 3b ] N-alkylation of o-nitroanilides followed by reductive cyclization,[ Citation 1a , Citation 1b ] cyclocondensation of N-substituted o-aminoanilides,Citation [2a] reductive cyclization of o-nitroanilides followed by N-alkylation in the presence of a strong base,Citation [1b] and condensation of N-substituted phenylenediamine with sodium salt of α-hydroxybenzylsulphonic acid.Citation [2b] In addition, 1,2-disubstituted benzimidazoles can also be accessed by direct one-step condensation of o-phenylenediamines with aldehydes under the influence of different acid catalysts.[ Citation 4 ] But one of the major limitations of these methodologies is that they show poor selectivity in terms of N-1 substitution, which results in the formation of two compounds (i.e., the formation of 2-substituted benzimidazole along with 1,2-disubstituted benzimidazole as a mixture[4a–c,4e]). We report the synthesis of 1,2-disubstituted benzimidazoles by the reaction of o-phenylenediamines and aldehydes in the presence of Amberlite IR-120 resin (Scheme ).
At the beginning of this work, to evaluate the catalytic efficiency of Amberlite IR-120, the reaction of o-phenylenediamine and benzaldehyde was studied by employing 0.100 g of the catalyst in water at room temperature. The resulting yield was not very good (entry 1, Table ). Optimization of the reaction condition was carried out next to increase the yield of the product, employing different catalyst loadings in a wide variety of solvents. The results are listed in Table . The conversion was dramatically increased to 95% within a much shorter time by adding 0.100 g of the catalyst in an ethanol/water (2:1) mixture (entry 3). It was found that a higher amount of the catalyst did not improve the yield at all (entries 6 and 7), whereas the yield was substantially reduced by decreasing the amount of Amberlite IR-120 in an ethanol/water (2:1) mixture (entries 4 and 5). Methanol/water (2:1) mixture was also found to be an effective reaction medium for this transformation (entry 12). Other solvents, such as CH3CN, CHCl3, CH2Cl2, toluene, tetrohydrofuran (THF), and dioxane (entries 13–18), rendered unfavorable results for this reaction. Otherwise, silica gel and alumina (entries 19 and 20) as heterogeneous catalysts also furnished high yield of products. Evidently, the reaction conditions in entry 3 of Table were found to be the most optimal.
Table 1. Optimization of the reaction conditions
b
To test the generality of this reaction, a series of aromatic aldehydes and o-phenylenediamines was subjected to the optimal reaction conditions. Almost all substrates could give their corresponding 1,2-disubstituted benzimidazoles exclusively as a single product (i.e., without the formation of 2-substituted benzimidazoles). The results are documented in Table .
Table 2. Synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoleFootnote b
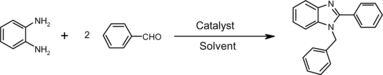
It is noteworthy that 2,4-dichlorobenzaldehyde represents as a single exceptional example by furnishing 2-substituted benzimidazole exclusively instead of 1,2-disubstituted benzimidazole (Scheme ).
In conclusion, the synthetic protocol described herein allows the formation of biologically significant 2-aryl-1-arylmethyl-1H-benzimidazoles exclusively under the heterogeneous catalysis of Amberlite IR-120 in aqueous media in excellent yields.
EXPERIMENTAL
General Experimental Procedure for the Synthesis of 2-Aryl-1-arylmethyl-1H-benzimidazoles
In a 50-ml, round-bottom flask, o-phenylenediamine (1 mmol) and aldehyde (2 mmol) were stirred in the presence of Amberlite IR-120 (0.100 g) in an EtOH/H2 O (2:1) mixture (10 ml) at room temperature for the stipulated time. The progress of the reaction was monitored by thin-layer-chromatography (TLC). After completion of the reaction, the solution was filtered to remove the catalyst. The filtrate was concentrated under reduced pressure to furnish the crude product, which was recrystallized from methanol to afford the pure product. The catalyst could be reused for fresh reactions without any loss of activity.
Characterization Data of the 2-Aryl-1-arylmethyl-1H-benzimidazoles
1-Benzyl-2-phenyl-1H-1,3-benzimidazole (1)
Mp 132 °C; 1H NMR (CDCl3, 300 MHz): δ 5.34 (s, 2H), 7.01–7.28 (m,8H), 7.34–7.40 (m, 3H), 7.62–7.64 (m, 2H), 7.83 (d, J = 8 Hz, 1H); FT-IR (KBr, cm–1): 1613.9; ESI-MS (m/z): 285.2 (M+ + 1). Anal. calcd. for C20H16N2: C, 84.48; H, 5.67; N, 9.85. Found: C, 84.41; H, 5.63; N, 9.89.
1-(4-Methylbenzyl)-2-(4-methylphenyl)-1H-1,3-benzimidazole (2)
Mp 128–130 °C; 1H NMR (CDCl3, 300 MHz): δ 2.47 (s, 3H), 2.53 (s, 3H), 5.54 (s, 2H), 7.11 (d, J = 7.9 Hz, 2H), 7.25 (d, J = 7.9 Hz, 2H), 7.33–7.37 (m, 2H), 7.35 (d, J = 8 Hz, 2H), 7.39 (m, 1H), 7.70 (d, J = 8.1 Hz, 2H), 7.97 (d, J = 8.1 Hz, 1H); FT-IR (KBr, cm–1): 1621.7; ESI-MS (m/z): 313.2 (M+ + 1). Anal. calcd. for C22H20N2: C, 84.58; H, 6.45; N, 8.97. Found: C, 84.50; H, 6.41; N, 8.90.
1-(4-Methoxybenzyl)-2-(4-methoxyphenyl)-1H-1,3-benzimidazole (3)
Mp 130 °C; 1H NMR (CDCl3, 300 MHz): δ 3.84 (s, 3H), 3.78 (s, 3H), 5.38 (s, 2H), 6.83 (d, J = 8.4 Hz, 2H), 6.95 (d, J = 8.5 Hz, 2H), 7.01 (d, J = 8.01 Hz, 2H), 7.20–7.30 (m, 3H), 7.61 (d, J = 8.6 Hz, 2H), 7.84 (d, J = 7.7 Hz, 1H); FT-IR (KBr, cm–1): 1619.3; ESI-MS (m/z): 345.3 (M+ + 1). Anal. calcd. for C22H20N2O2 : C, 76.72; H, 5.85; N, 9.29. Found: C, 76.77; H, 5.80; N, 9.22.
1-(4-Chlorobenzyl)-2-(4-chlorophenyl)-1H-1,3-benzimidazole (4)
Mp 135–136 °C; 1H NMR (CDCl3, 300 MHz): δ 5.35 (s, 2H), 7.14 (d, J = 7.1 Hz, 2H), 7.23 (d, J = 8.7 Hz, 1H), 7.27–7.56 (m, 8H), 7.81 (d, J = 7.2 Hz, 1H); FT-IR (KBr, cm–1): 1619.8; ESI-MS (m/z): 354.2 (M+ + 1). Anal. calcd. for C20H14 N2Cl2: C, 68.00; H, 3.99; N, 7.93. Found: C, 68.07; H, 3.91; N, 7.98.
1-(4-Bromobenzyl)-2-(4-bromophenyl)-1H-1,3-benzimidazole (5)
Mp 157–158 °C; 1H NMR (CDCl3, 300 MHz): δ 5.38 (s, 2H), 6.95 (d, J = 8.4 Hz, 2H), 7.20–7.59 (m, 9H), 7.86 (d, J = 7.2 Hz, 1H); FT-IR (KBr, cm–1): 1622.0; ESI-MS (m/z): 443.1 (M+ + 1). Anal. calcd. for C20H14N2Br2: C, 54.33; H, 3.19; N, 6.34. Found: C, 54.29; H, 3.12; N, 6.40.
1-(3,4-Dimethoxybenzyl)-2-(3,4-dimethoxyphenyl)-1H-1,3-benzimidazole (6)
Mp 171–173 °C; 1H NMR (DMSO-d6, 300 MHz): δ 3.76 (s, 3H), 3.78 (s, 3H), 3.84 (s, 3H), 3.93 (s, 3H), 5.44 (s, 2H), 6.61–6.68 (m, 2H), 6.80 (d, J = 8.2 Hz, 1H), 6.95 (d, J = 8.2 Hz, 1H), 7.22–7.32 (m, 4H), 7.55 (m, 1H), 7.79–7.91 (m, 1H); FT-IR (KBr, cm–1): 1613.1; ESI-MS (m/z): 405.1 (M+ + 1). Anal. calcd. for C24H24N2O4: C, 71.27; H, 5.98; N, 6.93. Found: C, 71.20; H, 5.93; N, 6.99.
1-(2-Chlorobenzyl)-2-(2-chlorophenyl)-1H-1,3-benzimidazole (7)
Mp 157–159 °C; 1H NMR (CDCl3, 300 MHz): δ 5.35 (s, 2H), 6.59 (dd, J = 8.6 and 1.3 Hz, 1H), 7.27–7.01 (m, 1H), 7.10–7.53 (m, 9H), 6.86 (d, J = 8.8 Hz, 1H); FT-IR (KBr, cm–1): 1615.9; ESI-MS (m/z): 354.2 (M+ + 1). Anal. calcd. for C20H14N2Cl2: C, 68.00; H, 3.99; N, 7.93. Found: C, 68.09; H, 4.03; N, 7.98.
1-(4-Hydroxy-3-methoxybenzyl)-2-(4-hydroxy-3-methoxyphenyl)-1H-1,3-benzimidazole (8)
Mp 184–186 °C; 1H NMR (DMSO-d6, 300 MHz): δ 3.72 (s, 3H), 3.76 (s, 3H), 5.41 (s, 2H), 6.48 (d, J = 8.1 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 6.94 (d, J = 8.1 Hz, 1H), 7.13–7.45 (m, 5H), 7.73 (d, J = 8.0 Hz, 1H), 7.77–7.89 (m, 1H), 9.25 (s, 1H), 9.32 (s, 1H); FT-IR (KBr, cm−1):1623.3, 3447.2; ESI-MS (m/z): 377.2 (M+ + 1). Anal. calcd. for C22H20N2O4: C, 70.20; H, 5.36; N, 7.44. Found: C, 70.16; H, 5.31; N, 7.49.
1-(3-Hydroxy-4-Methoxybenzyl)-2-(3-Hydroxy-4-Methoxyphenyl)-1H-1,3-benzimidazole (9)
Mp 229–231 °C; 1H NMR (DMSO-d6, 300 MHz): δ 3.82 (s, 3H), 3.90 (s, 3H), 5.35 (s, 2H), 6.77 (d, J = 8.1 Hz, 1H), 6.90 (d, J = 8.2 Hz, 1H), 7.11–7.27 (m, 5H), 7.62–7.73 (m, 3H), 8.60 (s, 1H), 8.82 (s, 1H); FT-IR (KBr, cm–1): 1621.8, 3436.9; ESI-MS (m/z): 377.2 (M+ + 1). Anal. calcd. for C22H20N2O4: C, 70.20; H, 5.36; N, 7.44. Found: C, 70.24; H, 5.39; N, 7.47.
1-(2-Methoxybenzyl)-2-(2-methoxyphenyl)-1H-1,3-benzimidazole (10)
Mp 151–153 °C; 1H NMR (CDCl3, 300 MHz): δ 3.72 (s, 3H), 3.76 (s, 3H), 5.31 (s, 2H), 6.71 (dd, J = 7.2 and 1.3 Hz, 1H), 6.76 (m, 1H), 6.84 (d, J = 8.3 Hz, 1H), 6.97 (d, J = 8.9 Hz, 1H), 7.05–7.48 (m, 6H), 7.53 (dd, J = 8.6 and 2.4 Hz, 1H), 7.91 (d, J = 8.6 Hz, 1H); FT-IR (KBr, cm–1): 1618.6; ESI-MS (m/z): 345.3 (M+ + 1). Anal. calcd. for C22H20N2O2: C, 76.72; H, 5.85; N, 9.29. Found: C, 76.79; H, 5.82; N, 9.28.
1-(4-Hydroxybenzyl)-2-(4-hydroxyphenyl)-1H-1,3-benzimidazole (11)
Mp 222 °C; 1H NMR (DMSO-d6, 300 MHz): δ 5.50 (s, 2H), 6.82–7.82 (m, 12H), 9.44 (s, 1H), 9.95 (s, 1H); FT-IR (KBr, cm–1): 1618.7, 3445.0; ESI-MS (m/z): 317.1 (M+ + 1). Anal. calcd. for C20H16N2O2: C, 75.93; H, 5.10; N, 8.86. Found: C, 75.99; H, 5.08; N, 8.82.
1-(3-Hydroxybenzyl)-2-(3-hydroxyphenyl)-1H-1,3-benzimidazole (12)
Mp 253 °C; 1H NMR (DMSO-d6, 300 MHz): δ 5.22 (s, 2H), 6.69–7.34 (m, 12H), 8.35 (s, 1H), 8.70 (s, 1H); FT-IR (KBr, cm–1): 1618.3, 3438.7; ESI-MS (m/z): 317.1 (M+ + 1). Anal. calcd. for C20H16N2O2: C, 75.93; H, 5.10; N, 8.86. Found: C, 75.97; H, 5.15; N, 8.90.
1-(2-Furylmethyl)-2-(2-furyl)-1H-1,3-benzimidazole (13)
Mp 94–96 °C; 1H NMR (DMSO-d6, 300 MHz): δ 5.58 (s, 2H), 6.19 (m, 1H), 6.25 (m, 1H), 6.62 (m, 1H), 7.17 (m, 1H), 7.24–7.29 (m, 3H), 7.46–7.53 (m, 1H), 7.62–7.82 (m, 2H); FT-IR (KBr, cm–1): 1622.0; ESI-MS (m/z): 265.1 (M+ + 1). Anal. calcd. for C16H12N2O2: C, 72.72; H, 4.58; N, 10.60. Found: C, 72.78; H, 4.52; N, 10.55.
5,6-Dimethyl-1-(4-methylbenzyl)-2-(4-methylphenyl)-1H-benzimidazole (14)
Mp 177–178 °C; 1H NMR (CDCl3, 300 MHz): δ 2.32 (s, 3H), 2.36 (s, 3H), 2.41 (s, 3H), 2.43 (s, 3H), 5.39 (s, 2H), 7.01–7.70 (m, 10H); FT-IR (KBr, cm–1): 1621.3; ESI-MS (m/z): 341.4 (M+ + 1). Anal. calcd. for C24H24N2: C, 84.67; H, 7.11; N, 8.23. Found: C, 84.62; H, 7.18; N, 8.19.
1-(4-chlorobenzyl)-2-(4-chlorophenyl)-5,6-dimethyl-1H-benzimidazole (15)
Mp 190–191 °C; 1H NMR (CDCl3, 300 MHz): δ 2.34 (s, 3H), 2.37 (s,3H), 5.42 (s, 2H), 7.04–7.81 (m, 10H); FT-IR (KBr, cm–1): 1618.8; ESI-MS (m/z): 382.4 (M+ + 1). Anal. calcd. for C22H18N2Cl2: C, 69.30; H, 4.76; N, 7.35. Found: C, 69.26; H, 4.71; N, 7.38.
2-(2,4-Dichlorophenyl)-1H-benzimidazole (Scheme 2)
Mp 227 °C; 1H NMR (DMSO-d6, 300 MHz): δ 7.27–7.78 (m, 6H), 8.00 (d, J = 8.4 Hz, 1H), 12.67 (s, 1H); FT-IR (KBr, cm–1): 1655.3, 3378.0; ESI-MS (m/z): 263.9 (M+ + 1). Anal. calcd. for C13H8N2Cl2: C, 59.34; H, 3.06; N, 10.65. Found: C, 59.39; H, 3.10; N, 10.60.
ACKNOWLEDGMENT
The authors acknowledge the director of the Northeast Institute of Science and Technology, Jorhat, for his help. Also, S. D. S. is grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, for the senior research fellowship.
Notes
a Isolated yield.
b Stirring at 25–30 °C.
a Isolated yield.
b Stirring at 25–30 °C.
REFERENCES
- (a) Roth , T. ; Morningstar , M. L. ; Boyer , P. L. ; Hughes , S. H. ; Buckheit Jr. , R.W. ; Michejda , C. J. Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase: 2-Aryl-substituted benzimidazoles . J. Med. Chem. 1997 , 40 , 4199 ; (b) Morningstar , M. L. ; Roth , T. ; Farnsworth , D. W. ; Smith , M. K. ; Watson , K. ; Buckheit Jr. , R. W. ; Das , K. ; Zhang , W. ; Arnold , E. ; Julias , J. G. ; Hughes , S. H. ; Michejda , C. J. Synthesis, biological activity, and crystal structure of potent nonnucleoside inhibitors of HIV-1 reverse transcriptase that retain activity against mutant forms of the enzyme . J. Med. Chem. 2007 , 50 , 4003 ; (c) Beaulieu , P. L. ; Bös , M. ; Bousquet , Y. ; Fazal , G. ; Gauthier , J. ; Gillard , J. ; Goulet , S. ; LaPlante , S. ; Poupart , M.-A. ; Lefebvre , S. ; McKerche , G. ; Pellerin , C. ; Austel , V. ; Kukolj , G. Non-nucleoside inhibitors of the hepatitis C virus NS5B polymerase: Discovery and preliminary SAR of benzimidazole derivatives . Bioorg. Med. Chem. Lett. 2004 , 14 , 119 ; (d) Falcó , J. L. ; Piqué , M. ; González , M. ; Buira , I. ; Méndez , E. ; Terencio , J. ; Pérez , C. ; Príncep , M. ; Palomer , A. ; Guglietta , A. Synthesis, pharmacology, and molecular modeling of N-substituted 2-phenyl-indoles and benzimidazoles as potent GABAA agonists . Eur. J. Med. Chem. 2006 , 41 , 985 .
- (a) Takeuchi , K. ; Bastian , J. A. ; Gifford-Moore , D. S. ; Harper , R. W. ; Miller , S. C. ; Mullaney , J. T. ; Sall , D. J. ; Smith , G. F. ; Zhang , M. ; Fisher , M. J. 1,2-Disubstituted indole, azaindole, and benzimidazole derivatives possessing amine moiety: A novel series of thrombin inhibitors . Bioorg. Med. Chem. Lett. 2000 , 10 , 2347 ; (b) Göker , H. ; Özden , S. ; Yıldız , S. ; Boykin , D. W. Synthesis and potent antibacterial activity against MRSA of some novel 1,2-disubstituted-1H-benzimidazole-N-alkylated-5-carboxamidines . Eur. J. Med. Chem. 2005 , 40 , 1062 .
- (a) Porcari , A. R. ; Devivar , R. V. ; Kucera , L. S. ; Drach , J. C. ; Townsend , L. B. Design, synthesis, and antiviral evaluations of 1-(substituted benzyl)-2-substituted-5,6-dichlorobenzimidazoles as nonnucleoside analogues of 2,5,6-trichloro-1-(β-D-ribofuranosyl)benzimidazoles. J. Med. Chem. 1998, 41, 1252; (b) Ries , U. J. ; Mihm , G. ; Narr , B. ; Hasselbach , K. M. ; Wittneben , H. ; Entzeroth , M. ; van Meel , J. C. A. ; Wienen , W. ; Hauel , N. H. 6-Substituted benzimidazoles as new nonpeptide angiotensin II receptor antagonists: Synthesis, biological activity, and structure–activity relationships. J. Med. Chem. 1993, 36, 4040.
- (a) Smith , J. G. ; Ho , I. Organic redox reactions during the interaction of o-phenylenediamine with benzaldehyde . Tetrahedron Lett. 1971 , 12 , 3541 ; (b) Nagata , K. ; Itoh , T. ; Ishikawa , H. ; Ohsawa , A. Synthesis of 2-substituted benzimidazoles by reaction of o-phenylenediamine with aldehydes in the presence of Sc(OTf)3 . Heterocycles 2003 , 61 , 93 ; (c) Itoh , T. ; Nagata , K. ; Ishikawa , H. ; Ohsawa , A. Synthesis of 2-arylbenzothiazoles and imidazoles using scandium triflate as a catalyst for both a ring closing and an oxidation steps . Heterocycles 2004 , 63 , 2769 ; (d) Perumal , S. ; Mariappan , S. ; Selvaraj , S. A microwave assisted synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles in the presence of montmorilloinite K-10 . Arkivoc. 2004 , 8 , 46 ; (e) Chakrabarty , M. ; Karmakar , S. ; Mukherji , A. ; Arima , S. ; Harigaya , Y. Application of sulfamic acid as an eco-friendly catalyst in an expedient synthesis of benzimidazoles . Heterocycles 2006 , 68 , 967 ; (f) Sun , P. ; Hu , Z. The convenient synthesis of benzimidazole derivatives catalyzed by I2 in aqueous media . J. Heterocycl. Chem. 2006 , 43 , 773 ; (g) Salehi , P. ; Dabiri , M. ; Zolfigol , M. A. ; Otokesh , S. ; Baghbanzadeh , M. Selective synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles in water at ambient temperature . Tetrahedron Lett. 2006 , 47 , 2557 ; (h) Varala , R. ; Nasreen , A. ; Enugala , R. ; Adapa , S. R. l-Proline-catalyzed selective synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles . Tetrahedron Lett. 2007 , 48 , 69 .