Abstract
An efficient, mild, chemoselective, and convenient protocol for synthesis of isothiocyanate derivatives. This protocol was applied successfully in the novel synthesis of anthelmintic drug 4-isothiocyanato-4′-nitrodiphenyl ether and its analogs.
INTRODUCTION
Isothiocyanates are key intermediates in the synthesis of various heterocycles[ Citation 1 Citation 2 ] and agrochemicals that have antifungal and anthelmintic activities.[ Citation3–5 ] Isothiocyanates have exhibited antitumor and antiparasitic activities[ Citation 3 Citation 4 ] and are used in the preparation of irreversible inhibitors.[ Citation 6 ] Isothiocyanates[ Citation 7 ] exist in nature as marine sesquiterpenes and play important roles as antiproliferatives.[ Citation 8 ] They are also used in the therapy of blood cancer as enzyme inhibitors for the HIV virus[ Citation 9 ] and are used as herbicides.[ Citation 10 ]
Various methods have been reported for the synthesis of isothiocyanates from amines,[ Citation11–16 ] isocyanides,[ Citation 17 Citation 18 ] organic halides,[ Citation 19 Citation 20 ] olefins,[ Citation 21 Citation 22 ] nitrile oxides,[ Citation 23 ] and aldoximes.[ Citation 24 ] To obtain isothiocyanate from amines, the most widely used methods involve reaction of the amine with an activated thiocarbonyl such as thiophosgene,[ Citation 25 ] thiocarbonyl diimidazole,[ Citation 26 ] and di-2-pyridyl thiocarbonate.[ Citation 27 ] However, most of the reported literature methods suffered from one or more drawbacks such as use of toxic reagents, long reaction times, low yields, and inconvenient workup procedures. Therefore it is necessary to develop a facile synthetic procedure for the preparation of isothiocyanates.
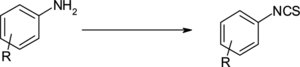
In the continuation of our ongoing program to develop efficient reagents for the conversion of amines to isothiocyanates,[ Citation 28 ] this article describes ferrous sulfate as a novel catalyst for the synthesis of isothiocyanates (Scheme ).
RESULTS AND DISCUSSION
Amine, carbon disulfide, and acetone were stirred at room temperature to form dithiocarbamate salt, which was subsequently treated with triethyl amine and ferrous sulfate to secure isothiocyanates. This reaction could not be completed without ferrous sulfate; therefore it acts as a catalyst. The pure product was isolated using n-hexane. A broad range of structurally diverse aromatic amines have been used (Table ).
Table 1. Synthesis of various isothiocyanates using ferrous sulfate
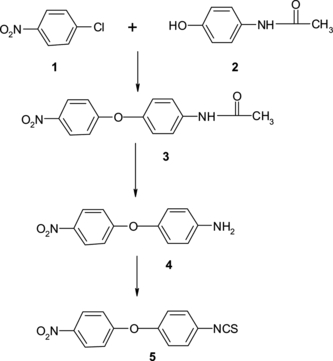
This novel methodology is used for the synthesis of anthelmintic drug 4-Isothiocyanato-4′-nitrodiphenyl ether (Scheme ).
CONCLUSION
In conclusion, this is an efficient, simple, and novel methodology for synthesis of isothiocyanates. Relatively short reaction times, utilization of cheap and readily available reagents, and high yields of products are some of the advantages of the present protocol. These advantages make the present method useful for large-scale operations.
EXPERIMENTAL
Melting points were taken in open capillaries. The IR spectra were recorded on a Perkin-Elmer 257 spectrometer using KBr discs. 1H NMR and 13C NMR spectra in CDCl3 were recorded on a VXR 300-MHz instrument using TMS as internal standard, and mass spectra were recorded on Shimadzu GC-MS. The homogeneity of the compounds was described by thin-layer chromatography (TLC) on silica-gel plates. The spots were developed in an iodine chamber.
4-Acetamindo-4′-nitrodiphenyl Ether (3)
4-Acetamidophenol (1.51 g, 0.01 mol; 1), p-nitrochlorobenzene (1.57 g, 0.01 mol; 2), potassium carbonate (3.2 g), and dimethylformamide (10.0 ml) were charged in a round-bottom flask under stirring. The reaction mass was heated at 153 °C for 5 h. The progress of the reaction was monitored by TLC. The reaction mass was quenched in 40.0 ml deionized water. The product was isolated by filtration and dried in an air oven at 50 °C. Purification of crude product was done using ethanol and activated charcoal. Yield: 120% w/w; melting point: 151 to 153 °C.
1H NMR (CDCl3) (δ ppm): 8.4 (s, 1H), 8.17 (d, 2H, J = 7.8 Hz), 7.2 (d, 2H, J = 7.8 Hz), 7.3 (d, 2H, J = 7.0 Hz), 7.6 (d, 2H, J = 7.0 Hz), 2.22 (s, 3H).
4-Amino-4′-nitrodiphenyl Ether (4)
4-Acetamindo-4′-nitrodiphenyl ether (2.72 g 0.01 mol) was treated with concentrated hydrochloride (8.0 ml) and deionized water (4.0 ml) in a round-bottom flask and slowly heated to reflux temperature for 3 h. The reaction was monitored by TLC using a dichloromethane–methanol (9:1) solvent system. The reaction mass was filtered through a Buckner filter and cooled at 40 to 50 °C. The reaction mass was basified slowly under stirring with 50% sodium hydroxide solution. The precipitate was isolated and purified by using charcoal treatment. Yield: 63.54% w/w; melting point: 135 to 137 °C.
1H NMR (CDCl3) (δ ppm): 8.10 (d, 2H, J = 7.6 Hz), 6.9 (d, 2H, J = 7.6 Hz), 7.1 (d, 2H, J = 7.1 Hz), 6.7 (d, 2H, J = 7.1 Hz), 3.71 (s, 2H).
4-Isothiocyanato-4′-nitrodiphenyl Ether (5)
4-Amino-4′-nitrodiphenyl ether (2.30 g, 0.01 mol), carbon disulfide (0.6 ml), triethylamine (7 ml), and acetone (10 ml) was charged into a round-bottom flask. The reaction mixture was stirred at room temperature for 1 h. to achieve triethylammonium dithiocarbamate salt. Then a pinch of ferrous sulfate and triethyl amine was added in triethylammonium dithiocarbamate salt, and the stirring was continued for 2 h. The reaction progress was monitored by TLC. After completion of the reaction, excess carbon disulfide was removed by distillation. The reaction mass was quenched in 50 ml ice-cold water with constant stirring. The product was isolated by filtration and dried under vacuum. The product was purified using n-hexane. Yield: 68% w/w; HPLC purity: 99%; melting point 122 to 124 °C.
IR (KBr): 2087, 1660, 1585, 1540, 1370, 1100, 870, 792. 1H NMR (CDCl3) (δ ppm): 8.13 (d, 2H, J = 7.8 Hz), 6.9 (d, 2H, J = 7.8 Hz), 7.02 (d, 2H, J = 7.4 Hz), 7.30 (d, 2H, J = 7.4 Hz). 13C NMR (CDCl3) (δ ppm): 162.9, 155.7, 140.8, 136, 125.2, 124, 120.6, 118.8, 118.1. Elem. anal. calcd.: C, 57.35; H, 2.96; N, 10.29; O, 17.63; S, 11.78. Found: C, 57.13; H, 2.98; N, 10.17; O, 17.68; S, 12.10.
Compound 10
IR (KBr): 2087, 1640, 1560, 1536, 1372, 1109, 868, 790. 1H NMR (CDCl3) (δ ppm): 7.3 (s, 1H), 7.18 (d, 1H, J = 6.8 Hz), 6.9 (d, 1H, J = 6.8 Hz), 7.0 (d, 2H, J = 7.0 Hz), 7.32 (d, 2H, J = 7.0 Hz). 13C NMR (CDCl3) (δ ppm): 157.08, 152, 136.03, 131.32, 128.70, 128.67, 127.03, 125.92, 125, 120.78, 120.43. Elem. anal. calcd.: C, 52.72; H, 2.38; Cl, 23.94; N, 4.73; O, 5.40; S, 10.83. Found: C, 52.87; H, 2.37; Cl, 24.03; N, 3.98; O, 6.32; S, 11.14.
Compound 11
IR (KBr): 2082, 1651, 1520, 1107, 873, 798. 1H NMR (CDCl3) (δ ppm): 7.1 (s, 1H), 7.19 (d, 1H, J = 6.8 Hz), 7.0 (d, 1H, J = 6.8 Hz), 6.85 (d, 2H, J = 7.4 Hz), 7.28 (d, 2H, J = 7.4 Hz). 13C NMR (CDCl3) (δ ppm): 157.82, 156.13, 135.43, 130.12, 129.80, 128.68, 127.15, 125.97, 120.05, 119.15, 118.90. Elem. anal. calcd.: C, 52.72; H, 2.38; Cl, 23.94; N, 4.73; O, 5.40; S, 10.83. Found: C, 53.10; H, 2.45; Cl, 23.57; N, 4.87; O, 5.13; S, 10.68.
Compound 12
IR (KBr): 2085, 1648, 1522, 1110, 868, 790. 1H NMR (CDCl3) (δ ppm): 7.9 (s, 1H), 8.3 (d, 1H, J = 7.6 Hz), 7.3 (d, 1H, J = 7.6 Hz), 7.1 (d, 2H, J = 7.2 Hz), 7.30 (d, 2H, J = 7.2 Hz). 13C NMR (CDCl3) (δ ppm): 161.30, 158.20, 141.45, 137.12, 134.80, 129.81, 125.60, 119.12, 117.90, 112.60. Elem. anal. calcd.: C, 49.21; H, 2.22; N, 13.24; O, 25.21; S, 10.11. Found: C, 50.43; H, 2.90; N, 14.01; O, 24.85; S, 11.04.
Calculation for the Purity of 4-Isothiocyanato-4′-nitrodiphenyl Ether
Preparation of Solutions
(a) n-Butyl amine (10 g) was dissolved in 500 ml 1,4-dioxane; (b) 0.4 g of sodium hydroxide was dissolved in 100 ml distilled water; (c) 2.72 ml of concentrated sulfuric acid (98%) was diluted to 1000 ml with distilled water.
Blank Titration
n-Butyl amine solution 25 ml and 50 ml distilled water were taken in a conical flask, and 2–3 drops of phenolphthalein indicator were added. This solution was titrated against 0.1 N sulfuric acid solution. The reading was noted at the endpoint (color changes from pink to colorless and stable for 10 to 15 min).
Back Titration Reading
Product 5 (0.272 g) was weighed, and 25 ml n-butyl amine solution were added to it. The mixture was stirred for 15 min. Then 50 ml distilled water and 2–3 drops of phenolphthalein indicator were added. This solution was titrated against 0.1 N sulfuric acid solution. The reading was noted at the endpoint (color changes from pink to colorless and is stable for 10 to 15 min).
Calculation for Purity of 4-Isothiocyanato-4′-nitrodiphenyl Ether
ACKNOWLEDGMENT
The authors gratefully acknowledge for the financial support from the University Grants Commission, New Delhi. The authors are thankful to Dr. S. T. Gadade, principal, C. K. Thakur College, for providing laboratory and other facilities. The authors are also thankful to Cipla Ltd. for help with the analytical analysis.
REFERENCES
- Mukerjee , A. K. ; Ashare , R. Isothiocyanates in the chemistry of heterocycles . Chem. Rev. 1991 , 91 , 1 – 24 .
- Sandler , S. R. ; Karo , W. Organic Functional Group Preparations, , 2nd ed. Academic Press : New York , 1983 ; vol. 12-I .
- Posher , G. H. S. ; Cho , C. G. ; Green , J. V. ; Zhang , Y. ; Talalay , P. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: Correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes . J. Med. Chem. 1994 , 37 , 170 – 176 .
- Arnold , J. T. ; Wilkinson , B. P. ; Sharma , S. ; Steele , V. E. Evaluation of chemopreventive agents in different mechanistic classes using a rat tracheal epithelial cell culture transformation assay . Cancer Res. 1995 , 55 , 537 – 543 .
- Lieber , E. ; Slutkin , R. Diisothiocyanates and derivatives . J. Org. Chem. 1962 , 27 , 2214 .
- Burke , T. R. ; Bajwa , B. S. ; Jacobson , A. E. ; Rice , K. C. ; Streaty , R. A. ; Klee , W. A. Probes for narcotic receptor mediated phenomena. 7: Synthesis and pharmacological properties of irreversible ligands specific for. µ. or. δ. opiate receptors . J.Med. Chem. 1984 , 27 , 1570 .
- (a) Guy , R. G. In The Chemistry of Cyanates and their Thio Derivatives ; S. Patai . (Ed.); John Wiley and Sons : New York , 1977 ; part 2 , pp. 819 – 886 ; (b) Gilmore , J. ; Gallagher , P. T. In Comprehensive Organic Functional Group Transformations, , 1st ed. ; A. R. Katritzky , O. Meth-Cohn , C. W. Rees , (Eds.); Elsevier Science Ltd. : New York , 1995 ; vol. 5 , pp. 1021 – 1051 .
- Nastruzzi , C. ; Cortesi , R. E. ; Menegatti , E. ; Leoni , O. ; Iori , R. ; Palmiery , S. In vitro antiproliferative activity of isothiocyanates and nitriles generated by myrosinase-mediated hydrolysis of glucosinolates from seeds of cruciferous vegetables . J. Agric. Food Chem. 2000 , 48 , 3572 – 3575 .
- Zhang , X. ; Neamati , N. ; Lee , Y. K. ; Orr , A. ; Brown , R. D. ; Whitaker , N. ; Pommier , Y. ; Burke , T. R. Arylisothiocyanate-containing esters of caffeic acid designed as affinity ligands for HIV-1 integrase . Bioorg.Med. Chem. 2001 , 9 , 1649 – 1657 .
- Lemin , A. J. U.S. Patent 3,449,112, 1969 . Chem. Abstr. 1969 , 71 , 3784w .
- Molina , P. ; Alajarin , M. ; Arques , A. Convenient improved syntheses of isocyanates or isothiocyanates from amines . Synthesis 1982 , 596 – 597 .
- Hodgkins , J. E. ; Reeves , W. P. The modified kaluza synthesis, III: The synthesis of some aromatic isothiocyanates . J. Org. Chem. 1964 , 29 , 3098 – 3099 .
- Prikle , W. H. ; Hoekstra , M. S. Automated liquid chromatography: Synthesis of a broad-spectrum resolving agent and resolution of 1-(1-naphthyl)-2,2,2-trifluoroethanol . J. Org. Chem. 1974 , 39 , 3904 – 3906 .
- Kim , J. N. ; Song , J. H. ; Ryu , E. K. A Convenient synthesis of isothiocyanates from primary nitroalkanes . Synth. Commun. 1994 , 24 , 1101 – 1105 .
- Kim , J. N. ; Jung , K. S. ; Lee , H. J. ; Son , J. S. A facile one-pot preparation of isothiocyanates from aldoximes . Tetrahedron Lett. 1997 , 38 , 1597 – 1598 .
- Sakai , S. ; Funinami , T. ; Aizawa , T. A convenient method for the preparation of aryl and alkyl isothiocyanates using amines, carbon bisulfide, and a grignard reagent . Bull. Chem. Soc. Jpn. 1975 , 48 , 2981 – 2982 .
- Tanaka , S. ; Uemura , S. ; Okano , M. The thallium (I) salt-catalyzed formation of isothiocyanates from isocyanides and disulfides . Bull. Chem. Soc. Jpn. 1977 , 50 , 2785 – 2788 .
- Fujiwara , S. ; Shin-Ike , T. Novel selenium catalyzed synthesis of isothiocyanates from isocyanides and elemental sulfur . Tetrahedron Lett. 1991 , 32 , 3503 – 3506 .
- Cho , C. G. ; Posner , G. H. Alkyl and aryl isothiocyanates as masked primary amines . Tetrahedron Lett. 1992 , 33 , 3599 – 3606 .
- Kulka , M. Synthesis of N-acyl-1,3-oxathiol-2-imines . Can. J. Chem. 1981 , 59 , 1557 – 1559 .
- Kitamura , T. ; Kobayashi , S. ; Taniguchi , H. Photolysis of vinyl halides: Reaction of photogenerated vinyl cations with cyanate and thiocyanate ions . J.Org. Chem. 1990 , 55 , 1801 – 1805 .
- Toshimitsu , A. ; Uemura , S. ; Okano , M. Reaction of olefins with a mixture of phenylselenenyl chloride and mercury(II) thiocyanate: Selective syntheses of β-(phenylseleno)alkyl isothiocyanates as precursors of vinylic isothiocyanates . J. Org. Chem. 1983 , 48 , 5246 – 5251 .
- Kim , J. N. ; Ryus , E. K. A convenient synthesis of isothiocyanates from nitrile oxides . Tetrahedron Lett. 1993 , 34 , 8283 .
- Kim , J. N. ; Jung , K. S. ; Lee , H. J. ; Son , J. S. A facile one-pot preparation of isothiocyanates from aldoximes . Tetrahedron Lett. 1997 , 38 , 1597 .
- Haugwitz , R. D. ; Angel , R. G. ; Jacobs , G. A. ; Mauer , B. V. ; Narayanan , V.L. ; Cruthers , L. R. ; Szanto , J. Antiparasitic agents, 5: Synthesis and anthelmintic activities of novel 2-heteroaromatic-substituted isothiocyanatobenzoxazoles and -benzothiazoles . J. Med. Chem. 1982 , 25 , 969 – 974 .
- Sato , M. ; Stammer , C. H. Synthesis of N-hydroxythiourea. J. Med. Chem. 1976, 19, 336–337.
- Kim , S. ; Yi , K. Y. Di-2-pyridyl thionocarbonate: A new reagent for the preparation of isothiocyanates and carbodiimides . Tetrahedron Lett. 1985 , 26 , 1661 – 1664 .
- Chaskar , A. C. ; Yewale , S. ; Bhagat , R. ; Langi , B. P. Triphosgene: An efficient catalyst for synthesis of isothiocyanates . Synth. Commun. 2008 , 38 , 1972 – 1975 .